Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection
Abstract
Expulsion of the gastrointestinal nematode Trichuris muris is mediated by a T helper 2 type response involving IL-4 and IL-13. Here we show that Th1 response-associated susceptibilityis dependent on activation signals mediated by MyD88 and Toll-like receptor 4 (TLR4). TLR4- and MyD88-deficient mice are highly resistant to chronic T. muris infection and develop strong antigen-specific Th2 responses in mucosa-associated lymphoid tissues. Hence, TLR4 and MyD88 are involved not only in the development of pro-inflammatory responses against bacterial pathogens but are also crucially involved in responses against multicellular organisms such as helminths. These results provide the first demonstration of the critical role of TLR4 and MyD88 in bridging the innate and acquired immune response during gastrointestinal nematode infection.
Abbreviations:
-
- TLR:
-
Toll-like receptor
-
- MLN:
-
Mesenteric lymph node
-
- KO:
-
Knockout
-
- p.i.:
-
Post-infection
-
- WT:
-
Wild-type
1 Introduction
Gastrointestinal nematodes cause some of the most prevalent and chronic human diseases worldwide. The human whip worm (Trichuris trichiura) currently infects 1 billion people 1. The naturally occurring mouse counterpart T. muris has provided much information on the immunoregulatory mechanisms underlying resistance and susceptibility to this parasite. Trichuris lives partly embedded in the epithelium of the large intestine. Infection with T. muris results in expulsion of the worms and the development of resistance in the majority of inbred mouse strains (e.g. BALB/c and BALB/K). However, certain strains of mice (e.g. AKR/J) fail to expel this parasite and harbor chronic infections. A number of studies have shown that development of resistance or chronicity is dependent on Th2 and Th1 cells, respectively 2–5.
Antigen-presenting cells express a range of Toll-like receptors (TLR) that recognize specific patterns of microbial components and regulate the activation of both innate and adaptive immunity (reviewed in 6, 7). Mouse strains with a natural mutation in the TLR4 gene are hyporesponsive to LPS 8–10, and the degree of LPS responsiveness correlates with the level of TLR4 expression 11. The downstream signaling events involve the MyD88 adapter protein, which links members of the TLR family, including TLR2, TLR4 and TLR9, and the IL-1 receptor superfamily to the downstream activation of NF-κB and MAP kinases 7, 12. Thus, TLR4 and MyD88 signaling processes are important in the development of pro-inflammatory responses.
In this report, we provide new information on the initiation of chronic gastrointestinal nematode infection. We demonstrate that TLR4 and MyD88 knockout (KO) mice are highly resistant to chronic nematode infection and develop strong antigen-specific Th2 responses. This study provides, for the first time, conclusive evidence that TLR play a key pathogenic role in chronic gastrointestinal nematode infections.
2 Results
2.1 Mice with a natural defect in TLR4 are resistant to chronic T. muris infection
To evaluate the functional importance of TLR4 in the development of chronic gastrointestinal nematode infection, we infected C3H/HeJ mice, which have a natural mutation in the TLR4 gene, and TLR4-expressing C3H/FeJ controls with T. muris. We utilized the low-dose infection protocol, which allows establishment of chronic T. muris infection regardless of the genetic background of the mouse strain 5. C3H/HeJ and C3H/FeJ mice were infected with 25 embryonated T. muris eggs, and worm burdens were assessed on days 10, 18 and 35 post-infection (p.i.). C3H/FeJ mice developed chronic infection with full worm burdens by day 35 p.i. (Fig. 1A). Interestingly, the C3H/HeJ mice carrying a TLR4 mutation had already started to expel the worms on day 18 p.i. (p<0.03) and had completed expulsion by day 35 p.i. (p<0.01), demonstrating that TLR4 is critically involved in the development of chronic gastrointestinal nematode infection.
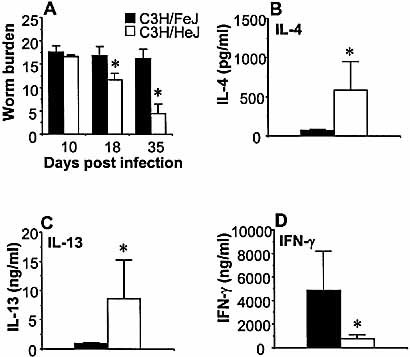
C3H/HeJ mice are resistant to chronic gastrointestinal nematode infection. Wild-type C3H/FeJ (black bars) and TLR4-deficient C3H/HeJ (white bars) mice were inoculated orally with 25 embryonated T. muris eggs. (A) Mice were sacrificed, and worms were counted at the time points indicated. MLN were removed from T. muris-infected mice on day 18 p.i., and cells were stimulated in vitro with T. muris antigen. Supernatants were analyzed by sandwich ELISA for the presence of (B) IL-4, (C) IL-13 and (D) IFN-γ. Mean and SEM for five to eight mice per group are shown in this and all subsequent figures. Representative data from one of three experiments are shown. Asterisks indicate statistically significant differences between mouse strains (p<0.05).
2.2 C3H/HeJ mice secrete high levels of Th2 cytokines during T. muris infection
To investigate the basis for the difference between TLR4-deficient and normal mice, we investigated the cytokine profiles from in vitro-stimulated mesenteric lymph node (MLN) cultures. Restimulation of MLN cells with T. muris antigen on day 18 p.i. revealed that, in comparison to susceptible C3H/FeJ controls, the resistant C3H/HeJ mice secreted significantly higher levels of IL-4 (Fig. 1B, C3H/HeJ 582.0±365.7 pg/ml vs. C3H/FeJ 74.2±16.6 pg/ml, p<0.03) and IL-13 (Fig. 1C, C3H/HeJ 8.52±6.7 ng/ml vs. C3H/FeJ 1.03±0.05 ng/ml, p<0.03). Corresponding to its resistant Th2-dominated phenotype, the C3H/HeJ mice secreted lower amounts of antigen-specific IFN-γ as compared to C3H/FeJ mice at day 18 p.i. (Fig. 1D, C3H/HeJ 760.6±299.7 ng/ml vs. C3H/FeJ 4825.7±3392 ng/ml, p<0.03).
2.3 Gene-targeted TLR4 KO mice are resistant to chronic T. muris infection
To confirm that resistance to chronic intestinal nematode infection was due to the absence of TLR4 in the C3H/ HeJ mice, we infected mice with a targeted disruption of the TLR4 gene. The TLR4 KO mice were on a C57BL/6 background, and we utilized the same low-dose infection protocol described above. TLR4 KO and C57BL/6 wild-type (WT) mice were infected with 25 embryonated T. muris eggs, and worm burdens were assessed on days 10, 18 and 35 p.i. WT mice developed chronic infection with full worm burdens by day 35 p.i. (Fig. 2A). The TLR4 KO mice, however, expelled the worms and had completed expulsion by day 35 p.i. (p<0.01), thus providing evidence that TLR4 is an absolute requirement for the development of chronic gastrointestinal nematode infection.
2.4 TLR4 KO mice secrete high levels of IL-13 but low IL-4 during T. muris infection
Restimulation of MLN cells with T. muris antigen on day 18 p.i. revealed that the resistant TLR4 KO mice secreted significantly higher levels of IL-13 (Fig. 2C, TLR4 KO 2.42±0.11 ng/ml vs. WT 1.06±0.15 ng/ml, p<0.01) and lower levels of IFN-γ (Fig. 2D, TLR4 KO 1380±92.01 ng/ml vs. WT 2036 ± 233 pg/ml, p<0.03) than the WT mice. Interestingly, the TLR4 KO mice also secreted significantly lower levels of IL-4 (TLR4 KO 133.4±40.3 pg/ml vs. WT 353.7±45.3 pg/ml, p<0.02) as compared to the WT mice (Fig. 2B), indicating that TLR4 signaling is important for the secretion of certain Th2 cytokines, such as IL-4, but not for others, i.e. IL-13.
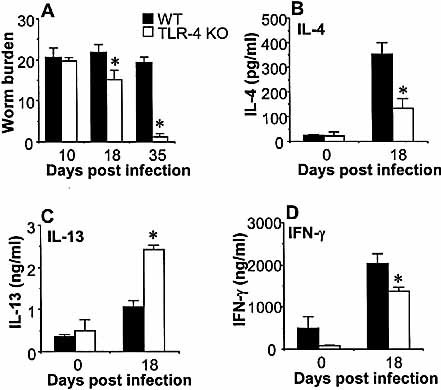
TLR4 is a crucial component in the development of chronic gastrointestinal nematode infection. Wild-type C57BL/6 (black bars) and TLR4 KO (white bars) mice on a C57BL/6 background were inoculated orally with 25 embryonated T. muris eggs. (A) Mice were sacrificed, and worms were counted at the time points indicated. MLN cells from T. muris-infected (day 18 p.i.) and naive (day 0) mice were harvested and stimulated in vitro with T. muris antigen. Supernatants were analyzed by sandwich ELISA for the presence of (B) IL-4, (C) IL-13, and (D) IFN-γ. Representative data from one of two experiments are shown. Asterisks indicate statistically significant differences between KO and WT mice (p<0.05).
2.5 MyD88 is a crucial component in the development of chronic intestinal nematode infection
To investigate the involvement of the MyD88 signaling pathway in the development of chronic T. muris infection, we infected mice that have a targeted disruption of the MyD88 gene. WT mice developed chronic infection with full worm burdens by day 35 p.i. (Fig. 3A). The MyD88 KO mice, however, had completed expulsion by day 35 p.i. (p<0.01), demonstrating that signaling through MyD88 is another crucial requirement for the development of chronic gastrointestinal nematode infection.
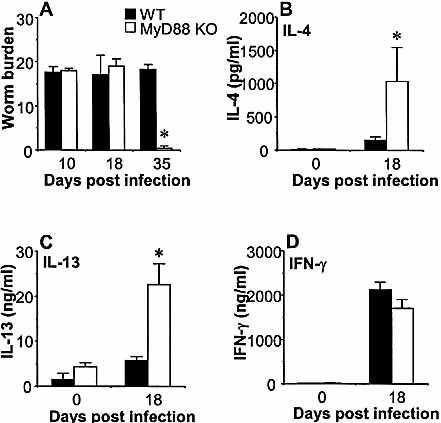
The development of chronic gastrointestinal nematode infection is MyD88-dependent. Wild-type C57BL/6 (black bars) and MyD88 KO mice (white bars) on a C57BL/6 background were inoculated orally with 25 embryonated T. muris eggs. (A) Mice were sacrificed, and worms were counted at the time points indicated. MLN cells from T. muris-infected (day 18 p.i.) or naive (day 0) mice were harvested and stimulated in vitro with T. muris antigen. Supernatants were analyzed by sandwich ELISA for the presence of (B) IL-4, (C) IL-13, and (D) IFN-γ. Representative data from one of three experiments are shown. Asterisks indicate statistically significant differences between KO and WT mice (p<0.05).
2.6 MyD88 KO mice secrete high levels of Th2 cytokines during T. muris infection
Restimulation of MLN cells with T. muris antigen on day 18 p.i. demonstrated that the resistant MyD88 KO mice secreted significantly higher levels of IL-13 (Fig. 3C, MyD88 KO 22.5±4.8 ng/ml vs. WT 5.7±0.8 ng/ml, p<0.03) and IL-4 (Fig. 3B, MyD88 KO 1040±508 pg/ml vs. WT 154±46 pg/ml, p<0.04) than WT mice. The MyD88 KO mice and WT controls secreted similar levels of IFN-γ (Fig. 3D, MyD88 KO 1697±204 ng/ml vs. WT 2117±174 ng/ml).
3 Discussion
The data presented in this report provide the first demonstration that the LPS receptor TLR4 and the adaptor protein MyD88 are crucial components in the development of chronic gastrointestinal infection. The data also clearly show that TLR4 and MyD88 signaling are not required for the generation of a protective host Th2 response, characterized by high IL-13 production.
Little is known about the role of the innate response in initiation of Th1-mediated chronic gastrointestinal nematode infection. To investigate the functional role of TLR4 in the T. muris infection model, we infected C3H/HeJ, which have a natural mutation in the TLR4 receptor, and control C3H/FeJ mice with the nematode. The spontaneous mutation displayed in C3H/HeJ mice is an exchange of proline for histidine in position 712 of the TLR4 protein 8. We successfully established chronic infection in the C3H/FeJ control mice and, consistent with a susceptible phenotype, these mice had high IFN-γ and low IL-4 and IL-13 responses in the MLN. Importantly, the TLR4-deficient C3H/HeJ mice rapidly expelled the worms. There was already a significant decrease in worm burden by day 18 p.i. in C3H/HeJ mice and almost complete expulsion by day 35. The requirement for TLR4 was confirmed using gene-targeted TLR4 KO mice. Thus, our data demonstrate that TLR4 is involved in the development of Th1-mediated susceptibility to chronic intestinal helminth infection.
Both TLR4 KO and MyD88 KO mice have previously been shown to display defective Th1 responses in response to various antigens 13, 14. Interestingly, cytokine analysis of cultures of antigen-stimulated MLN (the lymph nodes that drain the large intestine) from T. muris-infected MyD88 KO mice showed that in the absence of MyD88, the IFN-γ response on day 18 p.i. was equivalent to that seen in WT mice, demonstrating that IFN-γ induction in this infection model is independent of MyD88. In TLR4 KO mice, however, there was a significant reduction in IFN-γ production. It is notable that the normal levels of IFN-γ secretion in MyD88 KO mice and the low levels of IFN-γ seen in TLR4 KO mice did not reflect the speed of expulsion. Similar to findings in IL-18 KO mice that we reported previously 5, these results demonstrate that worm expulsion can take place even in the presenceof high levels of IFN-γ.
The Th2 cytokine analysis of antigen-stimulated MLN cultures from the different mouse lines revealed that neither TLR4 nor MyD88 is required for Th2 priming in this system. Recent reports suggest that Th2 responses in the lungs of TLR4-deficient C3H/HeJ mice are impaired 14, 15, while others suggest that Th2 polarization is normal in the absence of TLRsignaling 13. Our data demonstrate that both TLR4 and MyD88 KO mice are able to develop strong antigen-specific Th2 responses in the gut-associated lymphoid tissue. Interestingly, TLR4 KO mice displayed elevated IL-13 but reduced IL-4 secretion, indicating a role for TLR4, but not MyD88, in priming for IL-4 production. In contrast, the TLR4-deficient C3H/HeJ mice displayed elevated IL-4 secretion, indicating that the genetic background of the host may be important for TLR4-induced IL-4 secretion. This is interesting to note, as most studies performed with TLR4-deficient mice have used the C3H/HeJ strain. Taken together, it appears that the influence of the TLR4/MyD88 pathway on cytokine priming may depend on the disease, the genetic background of the host and/or the site of the immune response.
In addition to its involvement in the signaling pathways of several TLR, the adapter protein MyD88 has been shown to be essential for both IL-1 and IL-18 signaling 16. We demonstrated previously that IL-18 is required for the development of chronic T. muris infection 5. It is therefore possible that the resistance to chronic T. muris infection seen in MyD88 KO mice is due to impaired IL-18 signaling rather than a defect in TLR4 signaling. Furthermore, it has been shown that TLR4 can signal through a MyD88-independent pathway [17. It is therefore unclear whether the resistance to chronic infection in MyD88 KO mice is due to impaired IL-18 or TLR4 signaling. Nevertheless, our data from TLR4 KO and TLR4-deficient C3H/HeJ mice clearly demonstrate a crucial role of TLR4 in the initiation of chronic infection. Taken together with our previously published data, we can conclude that TLR4, MyD88 and IL-18 are all indispensable components of chronic T. muris infection.
In regard to the origin of the antigens involved in activation through TLR4, it is possible that T. muris secretes products that can bind to TLR4 and activate the innate response in order to promote chronic infection and parasite survival. This has recently been suggested for the parasitic trematode worm Schistosoma mansoni, which secretes a phosphatidylserine molecule capable of activating and altering dendritic cell function through TLR2 18. However, it is also possible that the molecules involved are not trichuroid in origin. Trichuris is a large roundworm, which burrows deep into the epithelial layer, digging syncytial tunnels as it moves through the large intestinal epithelium. The resulting damage to the epithelial surface may resultin leakage of LPS and other bacterial products into the subepithelial layers. It has recently been demonstrated that TLR4 is expressed in the large, but not the small, intestine of the mouse and isfurther up-regulated during colitis 19. Since the large intestine is the most heavily colonized region of the gut, it is possible that the combination of luminal bacteria and tissue-damaging worms results in leakage of LPS and subsequent activation of the innate response, leading to chronic infection. This raises the possibility that "bystander" responses that promote Th1 cytokine profiles (e.g. bacterial LPS) can influence the developing response to helminth infection, thus promoting worm survival. T. muris infection is certainly susceptible to such effects 3, 5, 20, 21.
The data presented in this paper provide new information about the influence of the innate immune system on the cytokine-mediated initiation of chronic T. muris infection. In summary,our studies provide conclusive evidence that TLR4 plays a key pathogenic role in chronic gastrointestinal nematode infection. This is the first report showing the importance of TLR4 during parasitic intestinal helminth infection, and these results extend our knowledge about the regulation of intestinal inflammation, providing information that may be important for the design of rational therapies against helminth infection, allergic reactions and inflammation of the gut and other mucosal sites.
4 Materials and methods
4.1 Animals and infections
Male 6–8-week-old C57BL/6 mice were purchased from Harlan Olac Ltd (Bicester, GB). TLR4 and MyD88 KO mice on a C57BL/6 background 10, 16 were kindly provided by Prof. Shizuo Akira and Dr Kiyoshi Takeda (Osaka University, Japan). All animals were bred at the University of Manchester animal unit under SPF conditions. Male 6–8-week-old C3H/HeJ and C3H/FeJmice were purchased from Jackson laboratories (Bar Harbor, Maine). All experiments were performed at least twice, with five to eight mice per group at each time point. All experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986). Infections, worm counts and T. muris antigen preparation were performed as previously described 5.
4.2 Cell culture and cytokine analysis
MLN were removed from uninfected and infected animals and resuspended in RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.05 mM β-mercaptoethanol (all from Invitrogen, Paisley, GB). MLN were cultured at 37°C and 5% CO2 in flat-bottomed 96-well plates (Nunc, Roskilde, Denmark) at a final concentration of 5×106/ml in 0.2 ml/well. Cells were stimulated with T. muris ES antigen (50 μg/ml), plate-bound anti-CD3 antibody (mAb 145–2C11, 10 μg/ml, ATCC) or LPS (1 μg/ml, Sigma, Gillingham, GB). Anti-IL-4 receptor mAb (clone M1, 5 μg/ml, from Dr. C. Maliszewski, Immunex, Seattle, WA) was added to cultures to increase detection of IL-4. Cell-free supernatants were harvested after 48 hours and stored at –80°C.
4.3 Cytokine ELISA
Cytokine analyses were carried out using sandwich ELISA for IL-4 (mAb 11B11 and BVD6–24G2, Mabtech AB, Nacka, Sweden), and IFN-γ (AN18 and R46A2, Mabtech). IL-13 was analyzed using an antibody pair from R&D Systems (Abingdon, GB).
4.4 Statistics
Significant differences (p<0.05) between experimental groups were determined using the Mann-Whitney U test.
Acknowledgements
We would like to thank Prof. Nancy Rothwell, Prof. Shizuo Akira and Dr Kiyoshi Takeda for kindly providing animals. This work was supported by the Wellcome Trust.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




