Generation of a transgenic animal model of hyperthyroid Graves' disease
Abstract
Graves' disease (GD) is an organ-specific autoimmune disease characterized by hyperthyroidism. Agonistic anti-thyrotropin receptor antibodies (thyroid-stimulating antibodies, TSAb), whichmimic the thyrotropin (TSH) action, are thought to cause GD. The precise immunological mechanism of TSAb production, however, remains elusive. Previous immunization approaches using TSH receptor led to transient hyperthyroidism, but did not seem sufficient for comprehensive understanding of the development of autoimmune responses. To create GD-related autoimmunity in mice, we here generated TSAb-transgenic mice in which a patient-derived TSAb is expressed in B cells. Expression of the human TSAb in mice resulted in various manifestations of hyperthyroidism including increased free thyroxine levels with concomitantly decreased TSH levels, increased thyroid uptake of technetium pertechnetate, hyperthermia and thyroid hyperplasia. We found a correlation between the serum levels of human TSAb immunoglobulin and free thyroxine. In addition, conventional B cells expressing the TSAb were partially deleted in the periphery while B1 cells expressing the TSAb persisted and accumulated in the peritoneal cavity, a finding consistent with previous demonstrations that the maintenance of B1 cells plays an important role in the development of autoimmune diseases. Thus, our transgenic mouse mayprovide a novel and useful animal model for elucidating the pathogenesis and pathophysiology of GD.
Abbreviations:
-
- GD:
-
Graves' disease
-
- TSH:
-
Thyrotropin
-
- TSAb:
-
Thyroid-stimulating antibody
-
- FT4:
-
Free thyroxine
-
- Tg:
-
Transgenic
-
- hIgM:
-
Human IgM
-
- SP:
-
Spleen
-
- PerC:
-
Peritoneal cavity
-
- TPT:
-
Technetium pertechnetate
-
- BBT:
-
Basal body temperature
1 Introduction
Graves' disease (GD) is a common autoimmune disease characterized by the production of antibodies (Ab) against thyrotropin (TSH) receptor (TSHR) 1–4. Agonistic anti-TSHR Ab, or thyroid-stimulating Ab (TSAb), mimic TSH function and induce the overproduction of thyroid hormones. GD patients manifest various symptoms of hyperthyroidism including increased heart rate, excess perspiration and weight loss. TSAb can be detected in the serum of more than 90% of newly diagnosed GD patients with hyperthyroidism 3. The measurement ofTSAb in patients with GD is a useful predictor of relapse and remission 5.
A GD animal model will clarify the mechanisms underlying the development of GD including that of TSAb production. Recently, two attempts were made by immunizing mice using human TSHR: one by the injection of TSHR-transfected cells simultaneously expressing MHC class II 6; the other by genetic immunization using TSHR cDNA 7. Although these immunized mice showed some typical phenotypes associated with hyperthyroidism, the responses were temporary and TSAb were produced against non-self TSHR. The process of autoantibody production in these mice presumably lacks the persistent abrogation of self tolerance.
To circumvent the lack of an appropriate animal model for the study of GD, we undertook a different approach. In previous studies, we established a B cell clone producing a TSAb from a GD patient 8, 9. We subsequently demonstrated that this TSAb bound to TSHR and stimulated the thyroid in vitro 8, 10 and in vivo 11. These observations prompted us to generate a transgenic (Tg) mouse constitutively expressing the human TSAb. Since B cells expressing the TSAb are subjected to the host immune system, we postulated that these TSAb-Tg mice might develop immunological responses associated with autoimmunity, such as clonal deletion, anergy or the breakage of self tolerance. Indeed, we demonstrate in this report that these TSAb-Tg mice manifest many of the clinical features of hyperthyroidism and develop immunological responses against B cells that produce autoantibodies. These TSAb-Tg mice provide a novel and useful model for the study of endocrinological and immunological aspects of GD. In particular, studies concerning the disruption of self tolerance, a process which is responsible for the TSAb production, will be greatly facilitated.
2 Results
2.1 Establishment of transgenic mice expressing a patient TSAb
To express an agonistic TSAb in mice, we designed a transgene construct (Fig. 1A). A 5′ flanking region of the original human Ig (TSAb) gene 8 was used as a provisional promoter and a human Cμ segment was used for the constant region of H chain. We established two founder lines; one carried approximately ten copies of the transgene on chromosome (Ch.) 10 while the other carried ∼20 copies on a Ch. 7–Ch. 9 translocation (data not shown). As no phenotypic differences between these two lines were observed, we show combined results from both lines in the following studies.
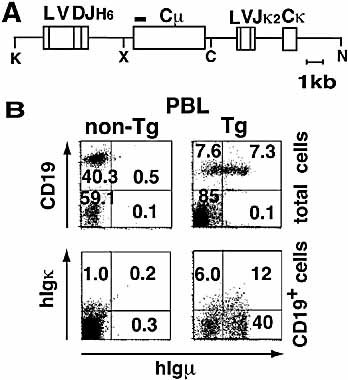
Schematic design of the transgene and the expression of hIgM on B cells in TSAb-Tg mice. (A) The transgene. Closed box indicates the probe used for Southern blot analysis. Relevant restriction sites are shown: K, KpnI; X, XhoI; C, ClaI; N, NotI. (B) HIgM and Igκ expression on PBL from Tg mice. Three-color flow cytometry using anti-CD19, anti-human IgM and anti-Igκ Ab was performed. Numbers represent the percentage of a particular subset.
2.2 B cell surface expression of human TSAb in TSAb-Tg mice
To determine whether the TSAb was expressed in the TSAb-Tg mice, we quantified serum human IgM (hIgM) by ELISA. Average levels of serum hIgM in TSAb-Tg mice were 47±38 μg/ml (mean ± SD, n=74), while transgene-negative littermates expressed undetectable levels of hIgM, thus confirming that the transgene was successfully expressed in the Tg mouse. Next we examined TSAb-Tg B cells for expression of surface hIgM. As shown in Fig. 1B, 12 % of B cells from the Tg mouse were hIgM+/hIgκ+. Moreover, total B cell numbers in the TSAb-Tg mice were much lower than expected, suggesting that B cells can be autoreactive, and clonal deletion, one of the key mechanisms for self tolerance, may have occurred (see below). Expression of the transgene was not observed in T cells (Fig. 1B) or other tissues (data not shown).
2.3 Development of hyperthyroidism in TSAb mice
Thyroid function in TSAb-Tg mice was assessed by measuring serum free thyroxine (FT4) and TSH levels. The FT4 levels of TSAb-Tg mice were significantly higher than those of non-Tg littermates (mean ± SD: Tg: 27.5±8.0 pmol/l, n=74 vs. non-Tg: 14.9±3.9 pmol/l, n=38, p<0.001) (Fig. 2A), while the TSH levels of Tg mice (mean ± SD: 1.3±1.2 ng/ml) were lower than those of non-Tg littermates (mean ± SD: 4.4±2.2 ng/ml) (Fig. 2B). Based on the thyroid function of non-Tg mice, we arbitrarily defined the hyperthyroid status as follows: FT4 >22.7 pmol/l (mean + 2 SD) and TSH <1.4 ng/ml (mean – 2 SD, using normalized logarithmic transformation). Fifty of 74 TSAb-Tg mice (68%) met this criterion (Fig. 2C). Of them, 24 mice had undetectable levels of TSH (<0.1 ng/ml). Thirteen mice that displayed increased FT4 but normal TSH levels. In these mice, the duration or degree of the increased FT4 levels might not be sufficient to suppress TSH levels. In the majority of TSAb-Tg mice examined, we observed positive correlations between serum hIgM and FT4 levels (Fig. 2D). These results indicate that the hIgM (TSAb) is indeed pathogenic, and that its level determines the severity of hyperthyroidism in the Tg mice.
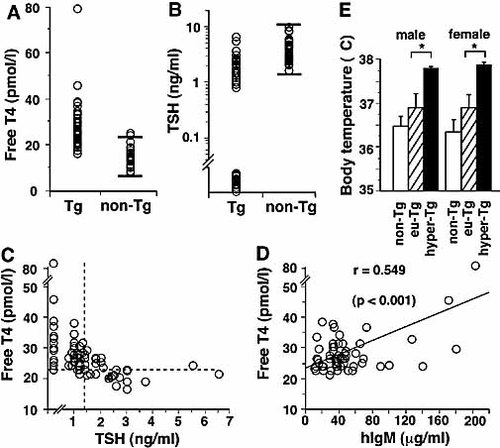
The human TSAb-Tg mice manifested hyperthyroidism. (A) Serum FT4 levels of Tg (n=74) and non-Tg (n=38) mice are shown. Horizontal bars in the non-Tg column represent the mean ± 2 SD boundaries. (B) TSH levels of the same mice as in (A). To calculate the deviation range (mean ± 2 SD) for non-Tg mice, values were normalized following logarithmic transformation. (C) Correlation of FT4 and TSH levels in each individual Tg mouse as in (A) and (B). Horizontal broken line corresponds to the upper bar level (mean + 2 SD) shown in (A), and vertical broken line to the lower bar level (mean – 2 SD) shown in (B). Mice in the upper left quadrant (n=50) satisfy the criteria for hyperthyroidism. (D) Correlation of FT4 and hIgM levels in individual Tg mice with low TSH levels (n=60). r, correlation coefficient; using Person's correlation coefficient test. (E) BBT measurements. Body temperatures of non-Tg (open box; male, n=6; female: n=7), euthyroid Tg (hatched box; male, n=7; female, n=4) and hyperthyroid Tg (closed box; male, n=6; female, n=3) mice are shown. The mean ± SE values are shown for each set of data; *p<0.05.
2.4 Clinical manifestations of hyperthyroidism in TSAb-Tg mice
Thyroid hormones stimulate various metabolic processes, including energy expenditure and the metabolism of various nutrients, and thus induce increased body temperature as one of the clinical manifestations of thyrotoxicosis 12. We observed higher basal body temperature (BBT) in both male and female hyperthyroid Tg mice compared to euthyroid Tg mice. There was no significant difference in the BBT between euthyroid Tg and non-Tg mice (Fig. 2E). These findings strongly suggest that thyroid function influences BBT in TSAb-Tg mice. In addition, hyperthyroid TSAb-Tg mice showed higher activity than euthyroid Tg mice as measured by distance moved in a given time scale (data not shown).
2.5 Increased thyroid technetium uptake by hyperthyroid Tg mice
Technetium pertechnetate (TPT) scintigraphy was performed to confirm that the hyperthyroidism in the TSAb-Tg mouse was caused by hyperactivity of the thyroid gland, not by destructive thyroiditis. The pertechnetate ion is transported into thyroid tissue by an iodide-concentrating mechanism but has a shorter half-life (6 h) than other radioiodines (131I: 8 days; 123I: 13 h). We injected mice with TPT via the tail vein and assessed the time course of TPT transport into the thyroid. In non-Tg mice, TPT was predominantly found in the stomach though trace amounts were present in the thyroid (Fig. 3A). Thyroidal TPT accumulation slightly increased up to 9% (mean ± SD: 7.8±0.64%) and saturated at 90–120 min (Fig. 3B) post-injection. In contrast, hyperthyroid TSAb-Tg mice accumulated TPT up to significantly higher levels (mean ± SD: 16.5±3.1%) and sustained this for at least 150 min. These results demonstrate that iodide transport is substantially increased in the hyperthyroid TSAb-Tg mice.
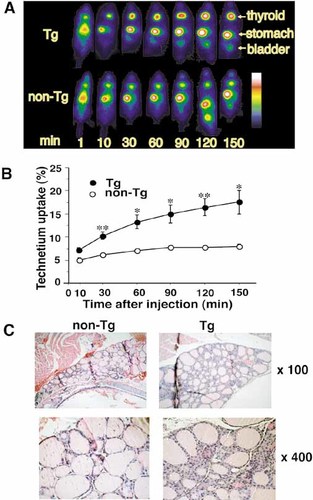
High technetium uptake and hyperplasia in the thyroid of the human TSAb-Tg mouse. (A) Technetium uptake planar images comparing the Tg and non-Tg mice. Images were taken at 1, 10, 30, 60, 120 and 150 min after injection of 2 mCi TPT into the tail vein. Striped color bar indicates arbitral intensity of the accumulated radioactivity. The white-red end indicates higher accumulation. (B) Time course of technetium uptake in the Tg (closed circle; n=3) and non-Tg (open circle; n=3) mice. The mean ± SE values are shown for individual time points; *p<0.05, **p<0.01, Student's t-test. (C) Thyroid gland histology. Magnification factors: top, ×100; bottom, ×400. Compared to the normal tissue, the thyroid gland from the Tg mouse displays diffuse hypercellularity and follicles with irregular sizes. Note that small cells are surrounded by square epithelial cells and large ones are surrounded by taller rounded cells.
2.6 Hyperplastic thyroid in hyperthyroid mice
Thyroid hyperplasia is a typical characteristic of GD. Histological examinations of the thyroids from hyperthyroid TSAb-Tg mice revealed irregular-sized follicles with diffuse hypercellularity (Fig. 3C). In large follicles with large nuclei, vacuoles due to hypersecretion were seen in the boundary of epithelial cells; richness of interstitial arterioles was also seen. These results are consistent with hyperthyroxinemia and high thyroid technetium uptake. Several accompanied features of GD were not detectable in our hyperthyroid TSAb-Tg mice, such as lymphocyte aggregates in the thyroid or evidence of muscle destruction associated with ophthalmopathy.
2.7 Clonal deletion of TSAb-positive B cells in peripheral lymphoid organs
As described earlier, B cell numbers in the TSAb-Tg mice were much lower than expected, suggesting that autoreactive TSAb-positive B cells might have been partially eliminated by clonal deletion. To test this hypothesis, B cells from bone marrow (BM), spleen (SP) and peritoneal cavity (PerC) of TSAb-Tg mice were analyzed. The percentages of hIgM+/hIgκ+ B cells in the whole lymphoid cells from the SP and BM of Tg mice were similar to those in the peripheral blood lymphocytes (PBL) (Fig. 4A). Total B cell numbers decreased in the SP and BM as well as in PBL (Fig. 4B). Statistical analysis confirmed that the total B cell numbers in TSAb-Tg PBL were significantly decreased compared with that of non-Tg littermates. However, percentages of hIgM+ B cells in the PBL and PerC of TSAb-Tg mice varied among mice. These results suggest that clonal deletion of TSAb-bearing B cells may be occurring in the TSAb-Tg mice.
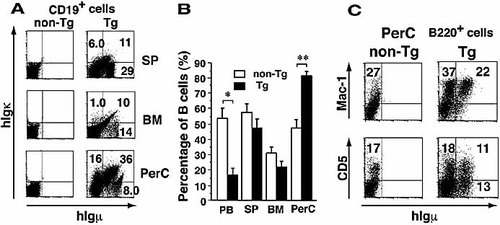
Clonal deletion and survival of TSAb-bearing B cells in the Tg mouse. (A) Flow cytometry of hIgM expression on the B cells of SP, BM and PerC. Cells were stained as described in Fig. 1B. (B) The B cell compartment size in lymphoid organs (open box: non-Tg, n=3; closed box: Tg, n=3). Data are means ± SE; (*p<0.05, **p<0.01, Student's t-test for unpaired data. (C) Flow cytometry of B1 cells in PerC.
2.8 B cells that survive clonal deletion accumulate in the PerC
If clonal deletion regulates the numbers of TSAb-bearing B cells in peripheral lymphoid organs, such cells may survive deletion in places where the immunological surveillance mechanism does not operate efficiently. Thus we investigated the number and composition of B cells in the PerC. We observed an increase of B cells in the PerC of the TSAb-Tg mice compared with that of non-Tg littermates (Fig. 4B); and the percentages of B cells expressing hIgM and Igκ in the PerC were much greater than those in the PBL (Fig. 4A). Further analysis of B cells in PerC using anti-Mac-1 Ab revealed that the substantial number of Mac-1+ B1 cells was present and that approximately 40% of B1 cells expressed hIgM (Fig. 4C). Furthermore, most of hIgM+ B cells were Mac-1+. Of hIgM+ B cells, the percentages of B1a (CD5+) and B1b (CD5–) cells were almost equal. These results are consistent with the previous ones that B cells in the PerC play an important role in autoimmunity 13.
2.9 Oral LPS administration induces hyperthyroidism in the TSAb-Tg mice
We examined whether administration of LPS induce the hyperthyroid state in the euthyroid TSAb-Tg mice. Before LPS administration, mean ± SD of serum FT4 and TSH levels of euthyroid TSAb-Tg mice were 11.4±1.15 pmol/ml and 3.8±0.64 ng/ml (non-Tg mice: 10.9±0.07 pmol/ml and 3.91±0.39 ng/ml), respectively. Seven days after oral administration of 10 μg LPS, their levels changed to 24.6±1.03 pmol/ml (n=5, p<0.0001) and 0.07±0.23 ng/ml (n=5, p<0.01) (non-Tg mice: 9.4±0.09 pmol/ml and 5.1±0.12 ng/ml), respectively (Table 1). These data suggest that the LPS administration activated TSAb-producing B or B1 cells in the Tg mice.
|
|
FT4 (pmol/l) |
TSH (ng/ml) |
|---|---|---|
|
TSAb-mice |
|
|
|
before LPS |
11.4 ± 1.15 |
3.80 ± 0.64 |
|
after LPS |
24.6 ± 1.03* |
0.77 ± 0.23* |
|
non-Tg mice |
|
|
|
before LPS |
10.9 ± 0.07 |
3.91 ± 0.39 |
|
after LPS |
9.4 ± 0.09 |
5.1 ± 0.12 |
- a) Values are expressed as mean ± SE (*p<0.01, n=5, vs. "before LPS").
3 Discussion
In this study, we generated hyperthyroidism in mice by expressing a patient-derived anti-TSHR Ab as a transgene. We were particularly interested in determining whether this strategy would evoke in mice similar autoimmune responses to those that are commonly seen in humans with GD. The human TSAb was successfully expressed in our Tg mice, and stimulated thyroid cells to produce excessive amounts of thyroid hormones (FT4). Consequently, these TSAb-Tg mice developed a series of hyperthyroidism symptoms typically seen in GD, which include increased serum FT4 levels concomitant with suppressed or decreased TSH levels, hyperthermia, hyperactivity, and an increase in body temperature. In addition, thyroid cells in these mice showed increased technetium uptake and displayed hyperplastic changes, all of which are typically seen in GD.
We took a new approach in generating a GD animal model. In the previous models, mice were immunized with human TSHR, which resulted in a transient autoimmune response. The non-persistent nature of the autoimmune response in the immunized mice is, supposedly, a consequence of the transient expression of the nominal Ag. However, the continuous presence of the Ag could induce tolerance, rather than leading to a persistent autoimmune response. To circumvent this problem, we persistently expressed the autoantibody, derived from a GD patient, in our Tg mouse. Indeed, many of the pathological and clinical changes associated with GD appeared in our TSAb-Tg mice. This is the first mouse model in which the constitutive expression of a human autoantibody has resulted in the successful development of an autoimmune disease.
Using this mouse model, we investigated the immunological factors which influence the development of hyperthyroidism in mice. The serum FT4 levels, a direct indicator of hyperthyroidism, strongly correlated with the serum hIgM levels. Nevertheless, the numbers of total B cells in PBL, SP and BM were significantly lower in the Tg mice compared to those of non-Tg littermates. Moreover, the number of Tg B cells variably decreased in the PBL, BM and SP in Tg mice and did not show correlation with serum hIgM or FT4 levels. These pieces of evidence suggest that a deletion process against the self-reactive B cells took place but the deletion of Tg B cells in the periphery did not abrogate the production of autoreactive TSAb in the Tg mice. Interestingly, we observed that the number of total B cells dramatically increased in the PerC of the Tg mice and that many of those B cells expressed hIgM (TSAb). Collectively, these findings suggest that B cells in PerC, rather than those in the periphery, are implicated in the development of GD in the Tg mice.
Similar observation was obtained from studies using a Tg model of autoimmune hemolytic anemia (AIHA), in which the mouse anti-erythrocyte autoantibody was expressed as a transgene 13, 14. Using this model, Honjo and co-workers demonstrated the importance of a subset of B cells, namely B1 cells 15, 16, in the development of AIHA. These investigators further demonstrated that B1 cells which had survived clonal deletion accumulated in the PerC, and that these B1 cells could produce autoantibodies following LPS, IL-5 or IL-10 administration 17, 18. In contrast, conventional B cells, even in the PerC as well as in the periphery, were deleted in this model. These findings, combined with our own observation, lead us to speculate that B cells in PerC can produce the TSAb, causing the development of hyperthyroidism in our Tg mice.
Administration of LPS into the Tg mice resulted in the elevated serum FT4 levels. Consistently, clinical studies demonstrated that the increase of CD5+ B cells, though in the PBL compartment, correlates well with the disease activity in GD patients 19, 20. Interestingly, this fact correlates with the data that the number of CD5+ B cells in the PerC tends to decrease; on the other hand, those in PB tend to increase when hyperthyroidism was induced by LPS administration. However, we did not determine the number of plasma cells, a population presumably secreting the TSAb, in these compartments (i.e. periphery and PerC). Moreover, a role of T cells has not yet been studied in the present model. In addition to B cell dysregulation, intrinsic T cell dysfunction may be involved in chronic autoimmunity 21, 22. Therefore, it is not clear how the present model reflects T cell help and dependency of the chronic Ab production on innate stimuli in the autoimmune response. For example, T cell-driven B2 differentiation into memory plasma cells has not been ruled out as a possible pathological mechanism in GD. Thus future investigation is needed to address these issues.
We noticed one difference between the AIHA and TSAb-Tg mice in addition to the similar accumulation of B1 cells in PerC and the deletion of peripheral B cells. Both B1 and B2 cells in TSAb-Tg mice increased in number in the PerC (data not shown), whereas only B1 cells but not B2 cells increased in AIHA mice. This difference may be associated with the distribution of the nominal Ag in each system. TSHR is widely expressed by the adipose tissue, thymus, kidney, heart and brain, in addition to the thyroid 23, 24, while erythrocytes are seen only in the blood. Such difference in Ag distribution could supposedly lead to different consequences in the development of autoimmunity in each animal model because autoreactive B cells might encounter their Ag at different locations and/or stages in their development. However, both animal models manifested the development of autoimmune responses, suggesting that the distribution of Ag did not meaningfully affect the onset of the immune responses in these animal models.
Most TSAb detected in patients with GD belong to the IgG class 1, 25. In fact, clone B6B7, which was isolated from PBL of a GD patient, is originally the IgG clone and has a significant number of somatic mutations in V region genes of H and L chains, indicating the involvement of somatic mutations for the TSAb specificity 8, 9. Although the isotype was changed from IgG to IgM in this TSAb-Tg mouse, this would not influence the binding affinity to TSHR or TSAb activity per se. Furthermore, we found IgM TSAb from PBL of GD patients and indicated the affinity maturation of TSAb driven by Ag in IgM-producing lymphocytes 8, 26. The isotype alteration, however, mightaffect on effector functions, i.e. complement fixation. This issue should be further investigated in the future study.
Clinical studies implicated the involvement of environmental factors in the development of GD in humans. We observed, as noted above, that an oral administration of LPS into the Tg mice causedan elevation of serum FT4 levels suggesting that the activation of B cells in PerC may augment the TSAb production in the Tg mice. Furthermore, this observation implies that certain bacterial infection in humans, through the activation of B1 cells in PerC, may episodically trigger or augment autoantibody production in human GD patients.
In summary, we developed a Tg mouse model of GD. These mice should provide us with unique opportunities not only to study the pathogenesis of GD but also to develop a new treatment strategy for this autoimmune disease.
4 Materials and methods
4.1 DNA construction
We isolated the V region cDNA of both H and L chains of the TSAb from an EBV-transformed B cell clone, B6B7, obtained from PBL of a patient with GD 8, 9. The characteristics of B6B7 were described previously 8–11, 27. Next, three PCR-amplified DNA fragments were tandemly ligated: the 5.7-kb L chain, the 3.7-kb VDJ fragment containing 5′ non-coding region and the 4.7-kb constant fragment of H chain (Fig. 1A). A 500-bp fragment upstream of the ATG initiation site was cloned as a putative promoter. An intron enhancer derived from the H chain and an intron enhancer/matrix attachment region of κ chain were also included. Cμ segment was used for the constant region of H chain, because the isotype change from IgG to IgM will not influence the activity.
4.2 Generation of transgenic mice
A 14.1-kb transgene was microinjected into fertilized eggs of C57BL/6J mice. We detected the transgene integration by Southern blot analyses with the probe (Fig. 1A). Mice were maintained in conventional but not pathogen-free conditions and analyzed at 12–20 weeks of age. All subsequent animal studies were conducted based on a guideline approved by the Institute of Laboratory Animals, Graduate School of Medicine, Kyoto University.
4.3 Flow cytometry
Lymphocytes from peripheral blood, BM, SP and PerC were analyzed by flow cytometry on a FACSCaliburTM (Becton Dickinson); anti-mouse CD19-biotin, anti-mouse B220-APC, anti-mouse CD5-PE and anti-mouse Mac-1-PE Ab were purchased from PharMingen. Goat anti-human μ-FITC and anti-human κL chain-PE were from Biosource Int. Streptavidin-PE-Cy5 was from DAKO.
4.4 ELISA system
The levels of secreted hIgM and hIgκ were measured by ELISA using goat anti-hIgM or anti-hIgκ Ab as the coating Ab, and alkaline phosphatase-conjugated anti-hIgM or anti-hIgκAb as the secondary Ab. All Ab were from Biosource.
4.5 Thyroid function
Serum FT4 and TSH concentrations were measured using a commercial kit (ENZAPLATE FT4, Bayer Medical; rat TSH 125I assay, Amersham Pharmacia Biotech).
4.6 Scintigraphy
Imaging was conducted using a scintigraphic gamma camera with a parallel collimator. TPT (99mTcO4–; Mediphysics; 2 mCi/0.1 ml) was injected into the tail vein of anesthetized mice and the radioactivity accumulated in the thyroid was measured. The uptake ratio was determined by measuring the accumulated radioactivity in the thyroid shaped by region of interest subtracting the radioactivity found in other parts of the body as background, and dividing by the total injected radioactivity. TPT decrement radioactivity was corrected as follows: A (corrected activity) = A′ (counted activity) × power (1/2, min/6.02 × 60 min).
4.7 Body temperature
We measured the anal temperature of mice at 7:00 p.m. for four consecutive days to minimize the body temperature fluctuation related to the estrous cycle.
4.8 Histology of thyroid and retro-orbital tissue
Thyroid glands and orbital tissue with the eyeball were placed in formalin. Five-micrometer-thick paraffin-embedded sections from each tissue were stained with hematoxylin-eosin.
4.9 Lipopolysaccharide administration
One-hundred micrograms LPS (Sigma Chemical Co., St. Louis, MO) dissolved in 500 μl was orally administered to the mice through a polyethylene tube of 1 mm diameter.
Acknowledgements
We thank Drs. T. Honjo, U. Storb, A. Shimizu, T. Tsubata, K. Shiota, K. Ishimura, A. Yasoda, K. Moriyama and Y. Hattori for valuable advices, Dr. T. Mori for warmhearted encouragement, and Miss M. Kouchi for excellent secretarial assistance. This work was partly supported by grants-in-aid from the Ministry of Education, Science, Culture, Sports and Technology of Japan, and the Ministry of Health, Labor and Welfare, Japan to T.A.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




