Maintenance of B lymphocyte-related clones in the cerebrospinal fluid of multiple sclerosis patients
Abstract
A longitudinal study of Ig V gene segments utilized by B cells from the cerebrospinal fluid (CSF) of two patients with multiple sclerosis (MS) was carried out using RT-PCR methodologies. One patient with a relapsing-remitting (RR)-MS was investigated at onset and at relapse, 1 year later. A patient with secondary-progressive (SP)-MS was tested 9 and 13 years after disease onset. Sequenceanalyses of VHDJH segments bearing VH3 and VH4 that were obtained from Cγ cDNA genes demonstrated a substantial proportion of shared clones in the samples taken at different times; these clones were identical or closely related, i.e. had the same third complementary determining region (CDR) of the H chain variable region gene (HCDR3) with different mutations in the VH segment. Collectively, these data demonstrate that in MS patients there is a strong selective pressure, which could be exerted by antigen (or autoantigen) stimulation, for the maintenance and partial diversification of certain VHDJH Cγ sequences.
Abbreviations:
-
- CSF:
-
Cerebrospinal fluid
-
- MS:
-
Multiple sclerosis
-
- RR-MS:
-
Relapsing-remitting MS
-
- SP-MS:
-
Secondary-progressive MS
-
- CNS:
-
Central nervous system
-
- CDR:
-
Complementarity determining region
1 Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of the central nervous system (CNS). Although T lymphocytes, B lymphocytes and macrophages are implicated in the pathogenesis of the disease, the exact mechanisms involved in these lesions are poorly understood 1, 2. Autoimmunity may play an important role since autoantibodies against myelin antigens have been described and found to cause demyelination 3–5.
The pathologic process characteristic of MS is confined primarily to the CNS. Therefore, micro-methods are required to study this process. Several groups recently demonstrated the presence of oligoclonal B cells in the cerebrospinal fluid (CSF) of MS patients or in brain tissue obtained at autopsy using PCR methodologies to detect the VHDJH gene segments encoding the Igvariable regions 6–9. In certain studies, these oligoclonal accumulations were found to be comprised of several clonally related VHDJH sequences, i.e. sequences that shared the same third complementary determining region (CDR) of the H chain variable region gene (HCDR3) and V region genes but differed in the number and type of point mutations in the V gene segments 6, 8. These accumulations of oligoclonal B cells were detected mostly only in the CSF and were virtually absent from the peripheral blood 6. Collectively, these data suggested an ongoing process of B cell stimulation/selection occurring in vivo.
In previous studies, accumulations of oligoclonal B cells were demonstrated at only one time point in the life history of the disease due to obvious constraints in the design of experiments involving humans. In this investigation, we had the opportunity to test γ cDNA from the CSF of two MS patients at two different time points. Clones marked by the same VHDJH gene segments could be detected at these intervals, suggesting that an antigen or group of antigens provided sustained stimulation throughout the course of the disease.
2 Results and discussion
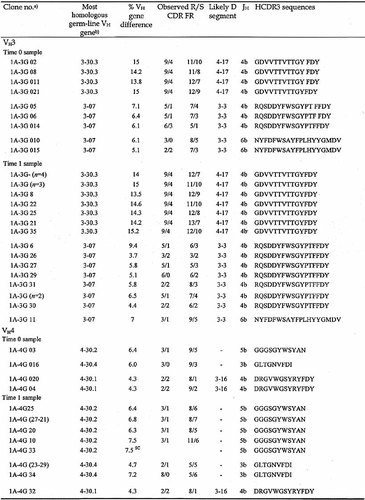
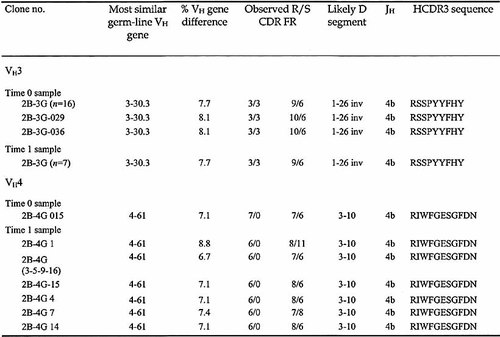
The HCDR3 segments in cells from the CSF of two MS patients, one with the relapsing-remitting (RR) and one with the secondary-progressive (SP) form of the disease, were analyzed from samples taken at intervals of 1 year and 4 years, respectively. In the RR-MS patient, the first sample was obtained at the onset of the disease, whereas in the SP-MS patient the first sample was taken 9 years after the initial diagnosis. For the sake of simplicity, the first CSF sample from each patient is defined as the time 0 sample and the second as the time 1 sample. We observed a typical oligoclonal pattern (discrete and dominant HCDR3 lengths 6) in the two samples from each patient. The presence of VHDJH bands of identical length in the samples taken at different times suggested a persistence of the same clonal progeny (data not shown). In order to explore this issue further, the VH3- and VH4-containing γ cDNAs from the two time points were amplified and cloned. A summary of the results is reported in Table 1 and 2; only those molecular clones found in the CSF samples at both time points are presented. All of the analyzed sequences have been deposited (EMBL AJ245201-AJ245220 and AJ556635-556779).
In patient 1, three groups of VH3-expressing clones that were clearly related to each other, as demonstrated by the presence of the same HCDR3, were detected at both time points. These represented 50% (9/18) and 81% (22/27) of the VH3-expressing clones analyzed at time 0 and time 1, respectively. The time 0 sample from patient 1 also contained three groups of VH4-expressing clones related to clones found at time 1, representing 21% (4/19) and 47% (9/19) of the VH4 clones analyzed at times 0 and 1, respectively (Table 1).
In patient 2, the time 0 sample contained a predominant group of VH3-expressing clones (66%, 18/27). This group was still abundantly represented in the time 1 sample taken 4 years later (35%, 7/20). In addition, a molecular clone expressing a VH4 gene family and the HCDR3 sequence RIWFGESGFDN was present as a single copy at time 0 (0.5%, 1/19) but was abundant among the clones analyzed at time 1 (11/18, 61%).
In both patients, the shared clones exhibited intraclonal diversification. For example, in patient 1, all clones within the three groups of shared clones had identical HCDR3 but expressed different mutations in the VH3 genes at time 0, suggesting that diversification was taking place (Table 1). In the time 1 sample, two out of three of the groups of shared clones were identical (Table 1, brackets) or related (i.e. they had the same HCDR3 with different new mutations in the VH gene). Analysis of clones sharing the HCDR3 sequence RQSDDYFWSGYPTFFDY revealed some interesting mutations in the time 1 sample (Fig. 1). Clones 1A-3G 26 and 1A-3G 29 presented an identical point mutation at codon 35 (AGC→ACC; Ser→Thr) that did not alter the amino acid charge of CDR1. In addition, clone 1A-3G26 shared an identical mutation at codon 53 (CAA→CCA; Gln→Pro) wit clones 1A-3G 30 and 1A-3G 31, a change to a more basic amino acid. Finally, clones 1A-3G 29 and 1A-3G 33 contained identical mutations at codons 75 (GCC→TCC; Ala→Ser) and 79 (CTG→GTG; Leu→Val), respectively. Some of the these shared mutations probably occurred at different times since they were observed in related, but not identical clones, suggesting a special selective pressure by (auto)antigen(s) (Fig. 1). The fact that mutations resulting in amino acid substitutions were found primarily in the CDR is also consistent with this antigen selection hypothesis (Fig. 1 and data not shown).
Collectively, these findings suggest a persistent antigenic stimulation/selection of certain B cell clones rather than a process of clonal expansion promoted by non-specific stimuli via Toll-like or cytokine receptors 10. Diversification/selection of these clones could have occurred in the germinal center (GC) of lymph nodes from which the B cells migrated to the CSF and CNS. Alternatively, diversification could have occurred in the CNS, outside of classical GC structures. Such extra-GC diversification has been reported for certain autoimmune conditions 11. Whatever the mechanisms may be, the persistence of some clones and their progeny in the patients suggests a pathogenic role for B cells in this disease.
In patient 2, the group of clones expressing the HCDR3 sequence RSSPYYFHY at time 0 was comprised of 88% (16/18) identical clones and two related clones (2B-3G 029 and 2B-3G 036). No further diversification had occurred among these clones at time 1 (Table 2). In contrast, in the same sample there was clonal diversification within the group of seven clones using the VH4-61 gene. This group emerged from a clone that existed in a single copy at time 0. In these seven related clones, the new mutations (often of the same sense type) were all located outside the CDR, and none involved the same nucleotide position. Therefore, the process of diversification may have been less marked in the SP-MS patient than the RR-MS patient. These findings suggest a more active diversification pattern in the initial stages of the disease, followed by a persistence of "memory" clones that were selected for their antigenic specificities. These clones may include some long-lived plasma cells 12.
In evaluating this study, it should be stressed that collection of longitudinal CSF samples from MS patients is somewhat exceptional. CSF is usually taken at disease onset in some, but not all patients, and disease course is monitored thereafter by other means, including nuclear magnetic resonance. Repeated spinal taps are only allowed when particular clinical conditions justify an invasive procedure. Although extension of these studies to a larger cohort of patients would be desirable, data from two patients with different forms of MS are already of significant value, particularly since they point out a persisting B cell response that may contribute to the onset and maintenance of the pathological condition.
- a) Clone number denotes the patient (e.g. 1A) followed by number of the VH family analyzed (e.g. 3). The capital letter (G) indicates the isotype of cDNA and is followed by the corresponding clone number. The clones of time 0 samples are indicated by "0" before the clone numbers.
- b) Genes identified by two-number code, with the first number indicating the family and the second the relative position in the locus from VH to JH – indicates that a Dsegment is not assignable; SC, stop codon; R, replacement mutations; S, silent mutations; n, number of identical clones.
- a) See legend to Table 1
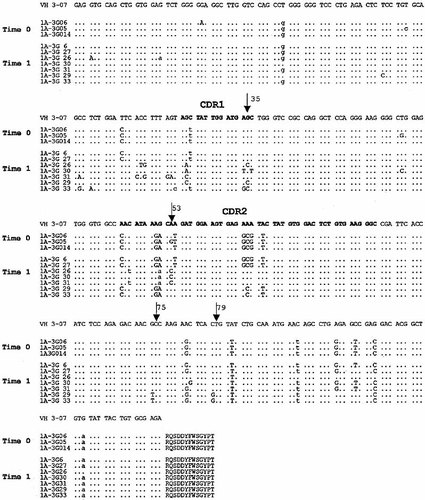
Intraclonal diversification of the γ VH3 transcripts in the CSF from a RR-MS patient (patient 1). Clonally related sequences isolated from time 0 (disease onset) and time 1 (relapse, 1 year) CSF samples were aligned and compared with the most homologous germline VH3 sequences (VH3-07). The dots denote germ-line identity. Capital and lower case letters represent replacement and silent mutations, respectively. The arrows indicate identical mutations that occurred at different times in related clones.
3 Materials and methods
3.1 Patients
CSF samples from two patients with the diagnosis of MS supported by clinical and laboratory criteria were collected over two different time intervals. The patients were categorized according to clinical course as relapsing-remitting (RR-MS, patient 1) or secondary progressive (SP-MS, patient 2). At the time of analysis, both patients were free of therapy.
Patient 1 was studied at the onset of the disease (time 0) and after 1 year (time 1), when initial symptoms recurred. The patient underwent a completed clinical laboratory workup before interferon therapy was started. Patient 2 was studied at 9 years (time 0 in our analysis) and 13 years (time 1) after the diagnosis, following an exacerbation of the symptoms. Each patient gave informed consent for CSF examination, and the study was approved by the Institutional Review Board.
3.2 PCR procedures
Total RNA was extracted from CSF cells and PBMC using the Rneasy Extraction Minikit (Qiagen-S.P.A., Milan, Italy) and reverse transcribed into cDNA with Oligo-(dT) primers in a final volume of 40 μl. The techniques for PCR amplification and cloning of rearranged IgV genes have been described previously 13. Briefly, first-strand cDNA (1 μl) was amplified using sense Ig VH3 and Ig VH4 gene family-specific primers and antisense γ constant region primers 6. To analyze the length HCDR3, the first PCR products were re-amplified using two nested consensus primers, a sense FR3 and an antisense JH primer 14, and the products were electrophoresed through a 7.5% acrylamide gel. Bands were visualized by silver staining (Promega, Madison, WI.)
First PCR products were purified with the Qiaex II Gel extraction kit (Qiagen) and cloned into the TOPO-TA vector (InVitrogen, Carlsbad, CA), and sequencing was performed as previously described 6.
Acknowledgements
This work was supported by grants from the Italian Multiple Sclerosis Federation (FISM) and MURST. Monica Colombo was supported by a fellowship from Italian Multiple Sclerosis Federation (FISM).
- WILEY-VCH