NK T cells stimulated with a ligand for TLR2 at least partly contribute to liver injury caused by Escherichia coli infection in mice
Abstract
Fas ligand (Fas L) expression was induced on intrahepatic NK1.1+ T cells in vivo after an intraperitoneal inoculation of Escherichia coli. Liver injury after E. coli infection, as assessed by serum GPT level and histological examination, was significantly reduced in Jα281–/– mice lacking NK1.1+ T cells or in gld/gld mice bearing mutated Fas L, indicating that NK T cells at least partly contribute to E. coli-induced liver injury in a Fas/Fas L-dependent manner. Bacterial numbers in organs and cytokine levelsin serum of Jα281–/– mice did not differ from those of Jα281+/+ mice following E. coli infection. Intrahepatic NK1.1+ T cells, which preferentially expressed Toll-like receptor 2 (TLR2) mRNA, responded in vitro to synthetic lipoprotein, a ligand for TLR2, by inducing Fas L expression on their surface. In a manner analogous to E. coli infection, lipoprotein and LPS could additively induce Fas L expression on NK1.1+ T cells, leading to liver injury in vivo in normal mice but not in gld/gld mice. In conclusion, it is suggested that induction of Fas L on NK T cells in response to bacterial components such as lipoproteins plays an important role in pathogenesis of E. coli-induced liver injuryin mice.
Abbreviations:
-
- Fas L:
-
Fas ligand
-
- TLR:
-
Toll-like receptor
-
- LP:
-
Lipoprotein
-
- GPT:
-
Glutamic-pyruvictransaminase
-
- -/-:
-
Knockout
-
- mRNA:
-
Messenger RNA
1 Introduction
The incidence of infection with Escherichia coli has increased in recent years among the patients undergoing abdominal surgery. These infections frequently result in liver injury and fatal shock, which are caused by endotoxin/ LPS derived from gram-negative bacteria 1. Massive TNF-α and IL-1β released from macrophages play a central role in LPS-inducedliver injury 2, 3. IL-12 and IL-18 are also known to play important roles in LPS-induced liver injury 4, 5.
Mouse liver contains unique α β T cells expressing NK1.1 Ag, a large fraction of which express an invariant TCR encoded by Vα14 and Jα281 gene segments 6, 7 and are specialized to recognize the processed glycolipids from enteric bacteria such as E. coli 8. Intrahepatic NK1.1+ T cells are found to be a target of IL-12 for NK-like effector mechanisms such as IFN-γ production and Fas ligand (Fas L)-mediated cytotoxicity 4, 9–12. There are several lines of evidence to suggest that NK T cells are involved in the formation of liver damage in animal models 13, 14. However, it remains to be elucidated whether NK T cells in the liver contribute to liver damage induced by infection with extracellular bacteria such as E. coli.
Toll was first identified as a protein controlling dorsoventral pattern formation in the early development of Drosophila 15 and was shown to participate in anti-microbial immune responses 16. Several mammalian Toll homologues have been identified and shown to play important roles in the recognition of various bacterial components. Toll-like receptor (TLR) 2 and TLR4 have been shown to be involved in signaling from lipoproteins (LP) and lipid A, respectively, whereas TLR9 has been shown to play a crucial role in recognition of bacterialDNA (CpG-DNA) 17–19. TLR3 and TLR5 have been reported to recognize double-stranded RNA (dsRNA) and bacterial flagellin, respectively 20, 21. TLR2 is expressed not only on phagocytes but also on some subsets of T cells including γ δ T cells and NK1.1+ T cells 22, 23. We have recently reported that TLR2 can at least partly contribute to liver injury caused by infection with an intracellular gram-negative bacteria, Salmonella choleraesuis via Fas L induction on liver NK T cells 13, 23.
In the present study, we focused on the roles of NK T cells and TLR2 on their surface in pathogenesis of liver injury induced by Escherichia coli infection. We found that induction of Fas L expression on NK T cells by a TLR2 ligand, lipoprotein, plays an important role in liver injury caused by E. coli infection.
2 Results
2.1 Contribution of Fas L on NK T cells to liver injury after E. coli infection
We have previously reported that Fas L expression was selectively induced on NK T cells in liver 7 days after Salmonella infection 23. To determine whether Fas L expression was induced on NK T cells in vivo by E. coli infection, we analyzed the expression of Fas L on intrahepatic lymphocytes of C57BL/6 mice 12 h after intraperitoneal inoculation with E. coli at a dose of 1.0×109 CFU/mouse by flow cytometry. As shown in Fig. 1, the level of Fas L expression on NK T cells was apparently increased after E. coli infection, although the relative number of NK T cells in whole liver lymphocytes was decreased (Table 1). On the other hand, the expression level on NK cells or T cells did not significantly increase after infection. Thus, Fas L was preferentially up-regulated on NK T cells after E. coli infection.
To investigate the involvement of Fas L on liver NK T cells in liver injury following E. coli infection, we next compared the serum glutamic-pyruvic transaminase (GPT) levels in Jα281–/– and gld/gld mice with non-functional Fas L expression and those in B6 control mice after infection of E. coli. The serum GPT levels in Jα281–/– and gld/gld mice were significantly lower than those in control B6 mice at 12 h after inoculation with 1.0×109 CFU of E. coli (Fig. 2, p<0.05).
The tissues of liver of E. coli-infected Jα281–/–, gld/gld, and control B6 mice were obtained at 12 h after infection. Histological examination stained with hematoxylin and eosin confirmed that necroinflammatory foci, cell death of hepatocytes characterized by cell shrinkage and chromatin condensation, and lymphocyte infiltration were obviously less in Jα281–/– and gld/gld mice than in control mice after infection (Fig. 3A). Apoptotic cells were next identified using the TUNEL technique. After E. coli infection, higher numbers of cells undergoing apoptosis could be observed in liver of control mice. The apoptotic cells showed characteristic shrinking, chromatin clumping, and nuclear fragmentation. Most of the events in the liver were located in the cells lining the sinusoids. In contrast, the amount of apoptotic cells remained low in Jα281–/– and gld/gld mice (Fig. 3B). These results suggest that the Fas/Fas L system mediated by liver NK T cells could contribute to liver injury following E. coli infection.
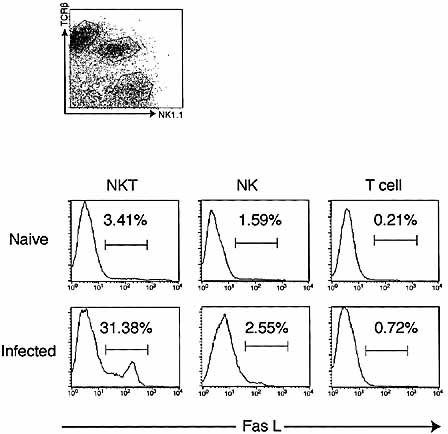
Expression of Fas L on intrahepatic lymphocytes after E. coli infection. Intrahepatic lymphocytes were isolated from the livers of naive and E. coli-infected C57BL/6 mice (1.0×109 CFU). Intrahepatic lymphocytes were stained with anti-TCRβ, anti-NK1.1 and anti-Fas L mAb and analyzed with a flow cytometer. Data are representative of three separate experiments.
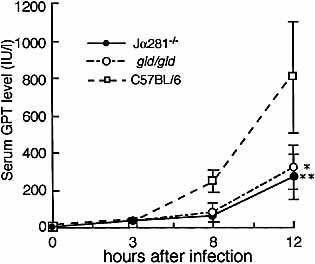
Kinetics of serum GPT levels in Jα281-knockout, gld/gld (gld), and C57BL/6 mice after Escherichia coli infection. Jα281-knockout, gld, and C57BL/6 mice were inoculated IP with 1.0×109 CFU of E. coli. Sera were collected 3, 8, and 12 h after E. coli challenge, and GPT activities in the sera were measured. Data are representative of three separate experiments and are expressed as the mean ± SD for ten mice in an experiment. *, **Significantly different from the value for B6 mice; p<0.05.
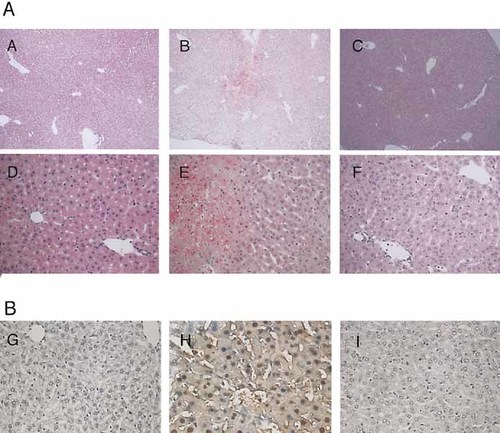
Comparison of histological findings in liver specimens from Jα281-knockout, C57BL/6, and gld/gld (gld) mice 12 h after infection with E. coli. (A) Formalin-fixed, paraffin-embedded thin specimens were stained with hematoxylin and eosin, and microscopical analyses were performed. Necroinflammatory foci and apoptotic hepatocytes, characterized by cell shrinkage and chromatin condensation, were clearly seen in specimens from B6 mice. (B) Apoptotic cells in situ in the liver after E. coli infection. Detection of DNA fragmentation in liver of E. coli-infected mice was performed using in situ terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method as described in Sect. 4. TUNEL-positive cells are dark brown. TUNEL staining revealed increased numbers of apoptotic cells in the liver of control mice compared with Jα281–/– or gld/gld mice. Data shown are representative of three independent experiments. (A), (D), and (G), liver specimens from Jα281-knockout mice; (B), (E), and (H), liver specimens from B6 mice; (C), (F), and (I), liver specimens from gld mice. Original magnification (A, B, C) ×100; (D, E, F, G, H, I) ×400.
|
|
NKT |
NK |
T cell |
|---|---|---|---|
|
Naive |
20.07 ± 2.02 |
12.41 ± 0.41 |
32.83 ± 6.28 |
|
Infected |
11.94 ± 2.54b) |
18.03 ± 5.84b) |
36.34 ± 7.98 |
- a) Values are given as % (mean ± SD) of five individual experiments.
- b) p <0.01 by Student's t-test compared with the values for each naive group, respectively.
2.2 Similar levels of bacterial burden in livers and cytokines in serum of Jα281–/– mice and control mice after E. coli infection
It is possible that the difference in susceptibilities of the liver to E. coli-induced injury is due to a difference in bacterial burdens in Jα281–/–, gld/gld, and +/+ mice. To test this possibility, we examined the numbers of bacteria in organs after infection with a sublethal dose (1×108 CFU: 1/5LD50) of E. coli. The bacteria in organs were eliminated in the similar kinetics in the liver, spleen and peritoneal cavity of Jα281–/– and gld/gld mice after infection with 1×108 CFU of E. coli. The numbers of bacteria in organs were similar in each group of mice 12 h after E. coli infection (Fig. 4). These results suggest that the degree of liver injury may not be due to a difference in bacterial loads in Jα281–/–, gld/gld, and +/+ mice.
Inflammatory cytokines are known to be involved in liver injury induced by LPS or Gram-negative bacterial infection 3, 5. Thus, we next examined kinetics of serum cytokines (TNF-α, IL-10, IL-12, and IL-1β) after E. coli infection. Jα281–/– and C57BL/6 mice were inoculated with E. coli at a dose of 1.0×108 CFU/mouse. Cytokine levels in the serum were determined by ELISA. As shown in Fig. 5, there were no significant differences in kinetics and peak levels of serum TNF-α, IL-10, and IL-1β concentrations between Jα281–/– mice and those in C57BL/6 mice, but the serum level of IL-12 in Jα281–/– mice was significantly lower than that in B6 mice at 3 h after E. coli infection (p<0.05). Serum IL-4 or IFN-γ was not detected in either Jα281–/– or B6 mice at any stage after E. coli infection (data not shown).
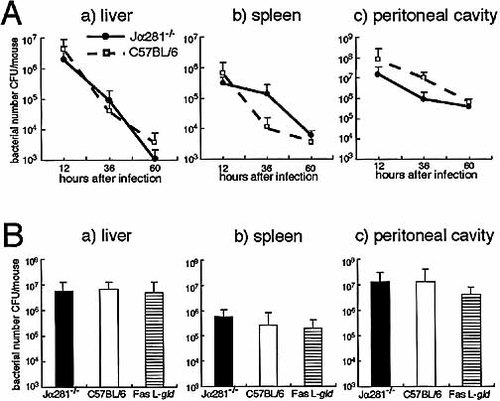
Bacterial growth in organs. (A) Kinetics of bacterial counts in the livers, spleens, and peritoneal cavities of Jα281-knockout and control mice infected IP with 1.0×108CFU/mouse of E. coli. Bacterial counts were measured at indicated times after infection. (B) Bacterial counts in organs of Jα281-knockout, C57BL/6, and gld/gld (gld) mice infected IP with 1.0×108CFU/mouse of E. coli. Bacterial counts were measured 12 h after infection. Data are representative of three separate experiments and are expressed as the mean ± SD for ten mice in each experiment.

Kinetics of cytokine production in serum of Jα281-knockout and control mice after E. coli infection at a dose of 1.0×108 CFU/mouse. Sera were collected at indicated times after E. coli infection and TNF-α, IL-12p40, IL-10, and IL-1β levels in the serum were determined by ELISA. Data are representative of three separate experiments and are expressed as the mean±SD for five mice in each experiment. IL-4 and IFN-γ were not detected in the serum (data not shown). *p<0.05 vs. the control group.
2.3 Expression of functional TLR2 by NK T cells in the liver
We previously reported that intrahepatic NK T cells freshly isolated from naive mice expressed significant levels of TLR2 messenger RNA (mRNA) and TLR2 signaling is suggested to contribute to liver injury induced by Salmonella infection via Fas L induction on NK T cells 23. To determine whether NK T cells express functional TLR on their surface, we studied the in vitro responses of intrahepatic lymphocytes to a ligand for TLR2 (lipoprotein) or for TLR4 (lipid A) by assessing induction of Fas L on the cell surface. Intrahepatic lymphocytes of naive C57BL/6 mice were treated with 10 μg/ml of synthetic lipoprotein or lipid A for 12 h, and the expression of Fas L on intrahepatic lymphocytes was analyzed using a flow cytometer. As shown in Fig. 6, the Fas L expression on NK T cells apparently increased after stimulation with a ligand for TLR2 (lipoprotein), whereas the levels of Fas L on NK T cells increased to a lesser extent after lipid A stimulation. In contrast, Fas L expression on NK and NK1.1– T cells did not increase in response to either lipid A or lipoprotein. Thus, TLR2 expressed on NK T cells may function to induce Fas L expression on the cell surface in response to its ligand.
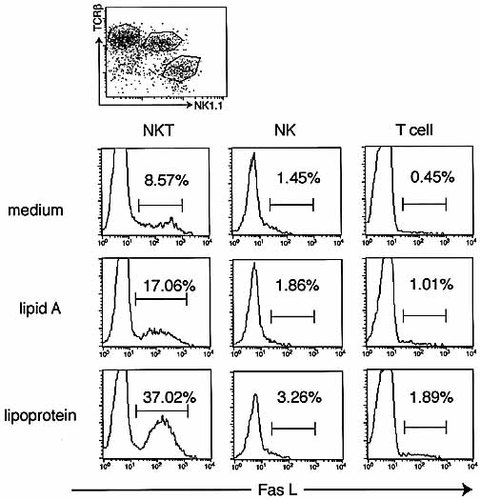
Expression of Fas L on intrahepatic lymphocytes after stimulation with lipoprotein or lipid A. Intrahepatic lymphocytes were isolated from the livers of naive C57BL/6 mice and were treated either with medium, lipoprotein (10 μg/ml), or lipid A (10 μg/ml). After incubation for 12 h, the cells were collected and stained with anti-TCRβ, anti-NK1.1 and anti-Fas L mAb and analyzed with a flow cytometer. Analysis gates were set on lymphocytes using forward and side scatter and on NKT, NK, or T cells. Data are representative of three separate experiments.
2.4 Lipoprotein and LPS act additively in inducing liver injury
To investigate whether in vivo administration of LP could induce liver injury via a Fas/Fas L-dependent manner in mice, C57BL/6 and gld/gld mice were given an i.p. injection of saline, 10 μg of LP, 10 μg of LPS, or 10 μg of LP plus 10 μg of LPS in 200 μl PBS. Twelve hours after injection, sera were collected and serum GPT activities were determined (Fig. 7A). The serum GPT levels were moderately increased at 12 h after inoculation of LP or LPS alone. Notably, LP in combination with LPS synergistically elevated serum GPT levels. This effect of LP and LPS on induction of liver injury was not seen in gld/gld mice. Interestingly, the synergistic effects of LP and LPS on the serum TNF-α level was not seen at any stage after injection (data not shown) These results indicate that LP and LPS act additively in inducing liver injury in vivo in a Fas/Fas L-dependent manner.
We next investigated whether administration of LP and/or LPS act in Fas L expression on liver NK T cells. The expression of Fas L on intrahepatic lymphocytes was analyzed using a flow cytometer 12 h after stimulation with 10 μg/ml of synthetic lipoprotein, LPS, or LP plus LPS. Analysis gates were set on NK T cells. As shown in Fig. 7B, the Fas L expression on NK T cells apparently increased after stimulation with LP and further increased after LP plus LPS. In contrast, Fas L expression on NK and NK1.1– T cells did not increase in response to any stimulation (data not shown).
We have previously reported that TLR2 gene transcription was up-regulated by TLR4 signaling via NFκB activation 24, 25. To address the mechanisms for additive effect of LP and LPS on induction of liver injury in Fas/Fas L-dependent manner, we examined the expression of TLR2 mRNA in intrahepatic lymphocyte subsets, including NK, NK T, and T cells. Intrahepatic lymphocyte subsets were purified from these mice 12 h after inoculation with LP, or LP plus LPS by cell sorting. Total RNA was extracted from each population, and the expression of TLR2 mRNA was analyzed by semiquantitative RT-PCR. As shown in Fig. 7C, TLR2 mRNA was expressed more abundantly in NK T cells of mice challenged with combination of LP and LPS than in NK T cells with LP, whereas TLR4 mRNA level remained constant in each subset (data not shown). These results indicate that LP and LPS additively induce enhanced liver injury with increased Fas L expression on NK T cells via up-regulation of TLR2 genes.
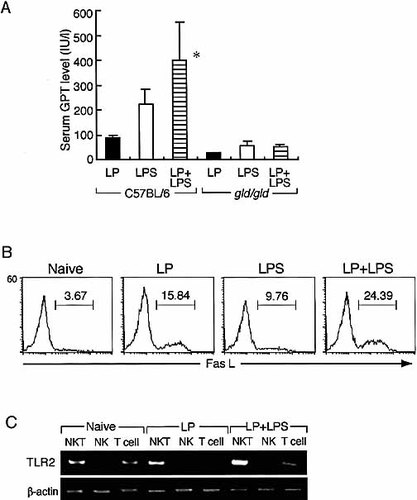
The serum GPT activities, expression of Fas L, and expression profiles of TLR genes on intrahepatic lymphocytes. (A) C57BL/6 and gld mice were inoculated intraperitoneally with LP (10 μg), LPS (10 μg), or LP (10 μg) plus LPS (10 μg) in 200 μl PBS. Sera and livers were collected at 12 h after inoculation, and GPT activities in the sera were measured. Each point and vertical bar indicate the mean ± SD of ten mice for each group. Data are representative of three separate experiments. *Significantly different from the value for other groups; p<0.05. (B) Expression of Fas L on intrahepatic NK T cells after stimulation with lipoprotein and LPS. Intrahepatic lymphocytes were isolated from the livers of naive C57BL/6 mice and were treated either with medium, lipoprotein (10 μg/ml), LPS (10 μg/ml), or lipoprotein (10 μg/ml) and LPS (10 μg/ml). After incubation for 12 h, the cells were collected and stained similarly and analyzed with a flow cytometer. Analysis gates were set on NK T cells. Data are representative of three separate experiments. (C) Expression of TLR2 in intrahepatic lymphocyte subsets of mice inoculated with LP, LPS, or LP plus LPS. NK, NKT, and T cells were purified by cell sorting (1×105–5×105 cells; purity >97%) 12 h after inoculation. Total RNA was extracted, and TLR2, or β-actin gene expression was examined by semiquantitative RT-PCR. The numbers of PCR cycles were 36 for TLR2 and 26 for β-actin. Data are representative of three separate experiments.
3 Discussion
In this study, we showed that Fas L expression on NK T cells was markedly elevated in vivo after E. coli infection and after in vitro stimulation with lipoprotein, aligand for TLR2. Jα281–/– and Fas L-deficient gld/gld mice were resistant to liver damage caused by E. coli infection or by lipoprotein in combination of a suboptimaldose of LPS. These results suggest that Fas L expression on NK T cells induced by TLR2 signaling is responsible for liver injury caused by E. coli infection. This is consistent with our recent finding that NK T cells contribute to liver injury induced by intracellular bacteria, Salmonella choleraesuis 13, 23. In case of Salmonellainfection, liver injury was detected 7 days after infection at which Th1 type cell-mediated acquired immunity was simultaneously induced 23. On the other hand, liver injury occurred at innate immunity phase shortly after infection with E. coli, an extracellular bacterium. Therefore, NK T cells could contribute to liver injury by the same mechanisms at innate or acquired immunity phase during the courses of infection with extracellular or intracellular gram-negative bacteria. Therefore, NK T cells could contribute to liver injury by the same mechanisms during the courses of infection with intracellular or extracellular gram-negative bacteria.
We have recently found that freshly isolated NK T cells from the livers of naive mice expressed relatively high levels of TLR2 and TLR4 mRNA 23 and that Fas L expression on liver NK T cells was up-regulated by Salmonella infection in TLR4-mutated mice but not in TLR2-deficient mice. These finding suggest that TLR2 but not TLR4 is involved in the enhanced Fas Lexpression on NK T cells after Salmonella infection. In the present study, we showed that freshly isolated NK T cells respond to synthetic lipoprotein, a ligand for TLR2, but not to synthetic lipid A, a ligand for TLR4, by inducing Fas L on their surface, confirming our earlier hypothesis that TLR2 on NK T cells can function to induce Fas L expression on NK T cells.
It should be noted that ligands for TLR2 and TLR4 act additively in inducing liver injury in a manner analogous to E. coli. Zhang et al. 26 reported that LP and LPScan act synergistically in the induction of lethal shock and in vivo cytokine production in LPS-responsive and LPS-nonresponsive mice, but how LPS functions in vivo to enhance LP activity is not clear yet. In this study, we showed that LP in synergy with LPS induced liver injury in B6 mice but not in gld/gld mice. Furthermore, Fas L expression on NK T cells was apparently increased after stimulation with LP plus LPS. Moreover, TLR2 mRNA level was highly up-regulated in response to combination with LP plus LPS. We have previously reported that TLR2 gene expression is rapidly induced by LPS or inflammatory cytokines in macrophages, and by TCR engagement or IL-2/IL-15 stimulation in T cells 22, 24, 25. Taken together, these findings led us to hypothesize that TLR4 signaling by LPS up-regulated TLR2 expression and lead to enhancement of Fas L expression on NK T cells, resulting in liver injury. Besides TNF-α and IL-1β, IL-12 and IL-18 are also known to play important roles in LPS-induced liver injury via stimulation of liver lymphocytes to produce IFN-γ 4, 5, which potentiates the Fas/Fas-L-mediated apoptosis in hepatocytes 4, 5. We have previously reported that a dominant Th2-like response of liver NK T cellsinduced by prostaglandin E series protects against liver injury induced by Escherichia coli infection 1. Thus, Th1/Th2 balance of NK1.1+α βT (NK T) cells is important in modulation of liver injury caused by E. coli infection. Therefore, it is alternatively possible that LPS may induce IL-12 and IL-18, leading to IFN-γ production by liver NK T cells and accelerate NK T cell-mediated liver injury in Fas/Fas L-dependent manner. Although IFN-γ was not detected in serum after E. coli infection, IFN-γ may be secreted in a verylow amount, thus acting in a narrow intracellular range in the infected sites. Leite-de-Moraes et al. 27 reported that IL-12 and IL-18 induced directly both Fas L and Fas expressions on NK T cells, which consequently elicited activation–induced cell death in NK T cells. Therefore, it can not be excluded that LPS may induce IL-12 and IL-18, leading to expression of Fas L on liver NK T cells and accelerate NK T cell-mediated liver injury in Fas/Fas L-dependent manner. Further experiments are needed to clarify these possibilities.
With respect to protection against E. coli infection, Jα281–/– and gld mice equally cleared bacteria after E. coli infection as compared with control mice, suggesting that protection against E. coli infection may be compensated in these knockout mice. We previously reported that γ δ T cells contribute to protection against E. coli infection 28. It is conceivable that liver injury may be mainly mediated by NK1.1+ T cells expressing Vα14/Jα281, whereas protection against E. coli infection may be compensated by various cells, including NK and γ δ T cells, in mice lacking NK1.1+ T cells via a Fas/Fas L-independent pathway.In conclusion,NK T cells directly respond to bacterial products by expressing Fas L and induce liver injury following E. coli infection through a Fas/Fas L-dependent pathway. Both ligands for TLR2 and TLR4 are important for the NK T cell-mediated liver injury in a Fas/Fas L-dependent pathway. Our results should be useful for the development of a prophylactic approach to control liver injury associated with infection with gram-negative bacteria such as E. coli.
4 Materials and methods
4.1 Animals and microorganisms
Jα281–/– mice with the C57BL/6 (B6) background were kindly provided by Dr. Taniguchi (Chiba University). Age- and sex-matched B6 wild-type mice (as normal controls) and gld/gld (gld) mice with non-functional Fas L expression with the B6 background were purchased from Japan SLC (Hamamatsu, Japan). These mice were bred in our institute under specific pathogen-free conditions. Six-to-eight-week-old female mice were used for the experiments. All experiments were carried out according to the criteria in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science. E. coli (American Type Culture Collection No.26; Manassas, VA) was inoculated intraperitoneally in all experiments.
4.2 Antibodies and reagents
PE-conjugated anti-NK1.1 mAb, biotin-conjugated anti-TCRα β mAb and Cy-chrome-conjugated streptavidin were purchased from PharMingen (San Diego, CA). Anti-Fas L hamster IgG mAb was purchased from Medical & Biological Laboratories (Nagoya, Japan). FITC-conjugated mAb to hamster IgG was purchased from ICN Pharmaceuticals (Aurora, Ohio). Synthetic E. coli-type lipid A, ONO4007, was kindly provided by Ono Pharmaceutical (Tokyo, Japan) and has been described 29. Synthetic lipoprotein, Palmitoyl-Cys((RS)2,3-di(palmitoyloxy)-propyl)-Ala-Gly-OH, was purchased from Bachem (Bubendorf, Switzerland). LPS (purified from E. coli 055:B5) was purchased from Sigma (St. Louis, MO).
4.3 Preparation of liver lymphocytes
Fresh liver was immediately perfused with sterile HBSS through the portal vein to wash out all remaining peripheral blood and then passed through stainless-steel mesh. After the coarse pieces had been removed by centrifugation at 50 g for 1 min, the cell suspensions were again centrifuged, resuspended in 8 ml of 45% Percoll (Sigma Chemical Co., St. Louis MO), and layered on 5 ml of 67.5%Percoll. The gradients were centrifuged at 600 g for 20 min at 20°C. Lymphocytes at the interface were harvested and washed twice with HBSS.
4.4 Flow cytometry analysis
For Fas L-staining, liver mononuclear cells isolated from C57BL/6 mice were first stained with hamster IgG anti-Fas L mAb and successively stained with FITC-conjugated mAb to detect hamster IgG. The cells that had been stained with anti-Fas L mAb were stained with PE-conjugated anti-NK1.1 mAb and biotin-conjugated anti-TCRβ mAb. To detect biotin-conjugated mAb, cells were stained with Cy-Chrome-conjugated streptavidin. To block Fc receptor (FcR)-mediated binding of the mAb, 2.4G2 (anti-FcR mAb) was added. All incubation steps were performed at 4°C for 30 min. The stained cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The cells were carefully gated by forward and side light scattering for the live lymphocytes. The data were analyzedusing FACSCalibur research software (Becton Dickinson).
4.5 Assay for GPT activity
Blood samples were collected at the time of sacrifice. Serum GPT activity was determined using a serum transaminase C II test kit (Wako Pure Chemical Industries, Osaka, Japan) according to themanufacturer's instructions. GPT activities (international units per liter) were calculated from the standard curve.
4.6 Histological studies
Liver specimens were removed and fixed with 10% buffered formalin, paraffin-embedded, and stained with hematoxylin and eosin for light microscopic examination. In situ terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed using an in situ apoptosis detection kit (Apoptag, Intergen, Purchase, NY). All steps were performed according to the instructions of the manufacturer. Briefly, paraffin-embedded sections were deparaffinized and rehydrated and then permeabilized by incubation with 20 mg/ml proteinase K (Sigma, St. Louis, MO) for 15 min at 37oC. Inactivation of endogenous peroxidases was accomplished by immersing the tissue sections in 3% hydrogen peroxide diluted in methanol for 10 min at room temperature. Then, the sections were incubated in equilibration buffer for 5 min. The sections were then incubated with the labeling solution containing terminal deoxynucleotidyl transferase in a humidified chamber for 1 h at 37oC. The reactions were terminated by rinsing the sections in a stop/wash buffer. The incorporated digitonigen-dUTP was detected by incubation with anti-digoxigenin peroxidase at room temperature for 30 min and positive reactions were revealed using 3,3′ diaminobenzidine. Methyl green was used for counterstaining for nuclei.
4.7 Bacterial growth in organs
Peritoneal exudates were obtained from the peritoneal cavity by lavage with 5 ml of HBSS at various times after E. coli infection. The liver was then perfused with 8 ml of sterile HBSS to wash out bacteria in the blood vessels. The bacterial counts in the homogenates of the liver and spleen or peritoneal exudates were established by plating serial 10-fold dilutions in sterile distilled water on tryptic soy agar (Nissui Laboratories, Detroit. MI). Colonies were counted 24 h later, after incubation at 37°C.
4.8 Cytokine assays
Serum was collected at various times after E. coli infection and TNF-α, IL-10, IL-12, IL-1β, IFN-γ, and IL-4 levels in serum were determined by enzyme-linked immunosorbentassay (ELISA) using Genzyme mAb according to the manufacturer's instructions (Genzyme, Cambridge, MA).
4.9 Sorting of intrahepatic lymphocyte subsets
The intermediate intensities of TCR (TCRint) NK1.1+ T cells, TCR– NK1.1+ cells and TCR+ NK1.1– T cells were purified as NK T cells, NK cells and T cells, respectively, in the B6 strain from isolated intrahepatic lymphocytes by cell sorting using a FACSVantage (Becton Dickinson) electric cell sorter. The purity of sorted cells was morethan 97% in all experiments.
4.10 Expression of TLR genes
Messenger RNA of liver tissue, purified NK cells, NK1.1+ T cells, NK1.1– T cells, and J774A.1 cells (a macrophage cell line as a positive control for TLR2) was extracted by using TRIZOL reagent (Gibco BRL, Rockville, MD), and cDNA synthesis was performed as described 22. Synthesized cDNA was amplified by polymerase chain reaction (PCR) using 20 pmol of each of the primers specific for TLR2 or β-actin. The primers were as follows: TLR2 sense, 5′-AAAGTCTAAAGTCGATCCGC-3′; antisense, 5′-ATATGCAACCTCCGGATAGT-3′; β-actin sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; antisense, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. The PCR cycles were run for 1 min at 94°C followed by 1 min at 54°C and 1 min at 72°C. Adenaturing step for 5 min at 94°C was included before the first cycle. The PCR product was subjected to electrophoresis on a 1.8% agarose gel (Life Technologies Laboratories, Grand Island, NY) and visualized by ethidium bromide staining.
4.11 Statistical analysis
Data were analyzed by Student's t-test, and a Bonferroni correction was applied for multiple comparison. The value of p<0.05 was considered statistically significant.
Acknowledgements
We thank Ono Pharmaceutical for providing synthetic lipid A, Dr. M. Taniguchi (Chiba University) for providing Jα281 KO mice, and Mrs. K. Itano and Miss. A. Nishikawa for providing excellent technical assistance. This work was supported in part by Grant-in-Aid from Scientific Research on Priority Areas Japan Society for the Promotion of Science, grant from the Japanese Ministry of Education, Science and Culture, and Yakult Bioscience Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




