Cruzipain induces autoantibodies against cardiac muscarinic acetylcholine receptors. Functional and pathological implications
Abstract
The goal of this study was to investigate whether cruzipain, a Trypanosoma cruzi immunodominant antigen, was able to induce antibodies reactive to the cardiac M2 muscarinic acetylcholine receptor (M2 mAChR). Immunization with cruzipain that was devoid of enzyme activity triggered IgG antibodies against cardiac M2 mAChR. By radioligand competition assay we proved that the anti-cruzipain IgG fraction, purified from serum, inhibited binding of the specific M2 mAChR radioligand [3H]quinuclidinyl benzilate. We also demonstrated that anti-cruzipain IgG reacted against the second extracellular loop of the M2 mAChR. The corresponding affinity-purified serum anti-M2e2 IgG (reacting against a synthetic peptide corresponding to this loop in humans) displayed agonist-like activity associated with specific M2 mAChR activation — increase of cGMP, inositol phosphate accumulation and nitric oxide synthase activity — triggering a decrease in myocardial contractility. Moreover, the same IgG fraction decreased heart frequency, related to inhibition of adenylate cyclase activity. These results imply thatcruzipain plays a role in the production of antibodies against M2 mAChR, which have been related to the pathogenesis of dysautonomic syndrome described in Chagas' disease.
Abbreviations:
-
- [3H]MI::
-
[Myo-3H]inositol
-
- IP:
-
Inositol phosphate
-
- KRB:
-
Kreb's-Ringer's bicarbonate
-
- L-NMMA:
-
NG-monomethyl-L-arginine
-
- mAChR:
-
Muscarinic acetylcholine receptor
-
- NOS:
-
Nitric oxide synthase
-
- O.D.:
-
Optical density
-
- QNB:
-
Quinuclidinyl benzilate
1 Introduction
Chagas' disease is caused by the protozoan parasite Trypanosoma cruzi. This parasitosis is widespread in many Latin-American countries, affecting 16–18 million people. During chronic infection 25–30% of patients develop different degrees of cardiopathy. Autoimmune processes have been proposed to explain the pathology, since parasite intracellular forms are rarely found in chagasic myocarditis. Furthermore, an inflammatory mononuclear cell infiltrate is found in the heart, and automyocardial antibodies are present in blood 1, 2. The pathology is normally expressed 20–30 years after primary infection. The mechanisms underlying the pathology of Chagas' disease have been subject of active research. It has been suggested that humoral and cellular immune mechanisms are involved in the pathogenesis of the disease 3–5.
It is suspected that some chronic manifestations of Chagas' disease are mediated through the association of autoantibodies with proteins found on the surface of host cells. Studies have implicated members of the G-protein-coupled receptor superfamily as some of these host cells' surface antigen candidates. We have identified autoantibodies against neurotransmitter receptors in human and murine chagasic sera. These antibodies were not only able to bind to β-adrenergic and muscarinic acetylcholine receptors (mAChR) of myocardium, but they also displayed agonist-like activity, triggering neurotransmitter-receptor-mediated biological effects 6–8. Therefore, they may play a role in the development of myocarditis and the dysautonomic manifestations observed in Chagas' disease 8. Components of parasite membrane have also been shown to bind directly and activate mAChR of both host cardiac cells and immunocompetent cells 9, 10. At present, it is not known which T. cruzi surface proteins are responsible for these interactions between the host cell neuroreceptors and parasites.
Cruzipain, a major T. cruzi antigen 11, is found in every developmental form of the parasite 12 and it is highly immunogenic in human and murine infection 13. In an experimental model we demonstrated that immunization of BALB/c mice with cruzipain elicits specific humoral and cellular immune responses against myosin from skeletal and heart muscles. This immune response leads to structural and functional damage in these tissues 14, 15. The presence of autoantibodies was associated with an increase of B cell numbers and high levels of IL-4, IL-5 and IL-10 cytokines, suggesting an activation of these cells 16. Cruzipain-immunized mice presented alterations in the heart conduction system, such as bundle-branch block and sinus bradycardia 16. Similar manifestations were observed on normal heart tissue when using serum IgG from chronic chagasic humans and mice 7, 17. However, the precise mechanism that triggers production of these antibodies has not yet been elucidated.
In this paper we study whether anti-cruzipain IgG is a candidate autoantibody that can bind to cardiac mAChR on host cells and if it can trigger signal transduction to alter normal myocardial function.
2 Results
2.1 Detection of antibodies against myocardial mAChR in sera from cruzipain-immunized mice
Cruzipain-reactive antibodies that are also reactive to heart antigens have been reported 16. To test the ability of anti-cruzipain IgG to interact with myocardial mAChR, a competition-binding assay was performed on normal mouse cardiac membranes. Fig. 1 shows that anti-cruzipain IgG inhibited the specific mAChR antagonist, [3H]-QNB (quinuclidinyl benzilate), binding in a concentration-dependent manner, whereas normal IgG did not. Saturation studies and computer-assisted fitting curves showed that 1×10–7 M anti-cruzipain IgG interacted irreversibly, decreasing the number of binding sites (Bmax) without changes in the equilibrium dissociation constant (Kd). In contrast, control IgG affected neither Bmax nor Kd (Table 1).
In previous work, it has been shown that IgG from chagasic patients selectively interacts with the second extracellular loop of human M2 mAChR. Therefore, to evaluate the molecular interaction between anti-cruzipain IgG and this receptor, the 24-mer synthetic peptide, M2e2 (corresponding to the second extracellular loop), was used as coating antigen in ELISA. Fig. 2A shows an increase in optical density (O.D.) values in a concentration-dependent manner using anti-cruzipain sera. Values obtained with immune sera were always >3 standard deviations above those of control IgG. Reaction specificity was assessed by the ability of the anti-M2e2 IgG purified from the corresponding sera to increase the O.D. values (Fig. 2B). Affinity-purified anti-M2e2 IgG (7×10–8 M) maximally increased O.D. values; this concentration had an equivalent effect to 5×10–7 M of total anti-cruzipain IgG. The excluded fraction (i.e. the fraction excluded from M2e2 affinity-columns) showed O.D. values similar to control IgG. Incubating the antibodies with the synthetic peptide, M2e2, neutralized the specific reaction of the affinity-purified anti-M2e2 IgG derived from the anti-cruzipain serum.
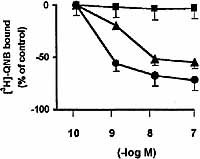
Inhibition of [3H]-QNB binding on normal cardiac membranes by anti-cruzipain IgG (triangles), control IgG (squares) and carbachol (circles). Cardiac membranes were incubated for 30 min at 30°C with different concentrations of IgG or carbachol and then with 0.3 nM [3H]-QNB. Binding of 0% represents that achieved when no competing extract was added. Values are expressed as mean±SEM of sera pools from 10 immune mice and 10 control mice performed in triplicate. Both anti-cruzipain and carbachol p < 0.001, vs. control IgG.
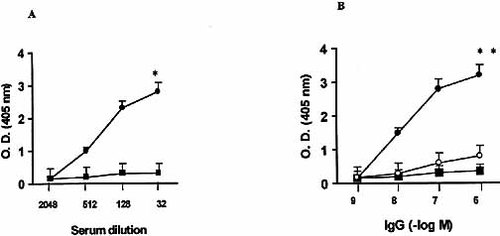
Immune reactivity, tested by ELISA, of affinity-purified antibodies from anti-cruzipain IgG that specifically recognize M2e2. (A) Effect of anti-cruzipain sera (circles) and control sera (squares) at different dilutions. *, p<0.001 compared with control sera. (B) Effect of increasing concentrations of affinity-purified anti-M2e2 IgG (black circles), the anti-cruzipain IgG fraction excluded from M2e2 affinity columns (squares) and anti-M2e2 IgG previously incubated for 30 min at 30°C with M2e2 peptide (white circles). Values are mean ± SEM of five experiments performed in triplicate. IgG was obtained from pooled sera. **, p<0.001 compared with the excluded fraction.
|
Treatment |
Kd (nM) |
Bmax (fmol/mg protein) |
|---|---|---|
|
None |
0.18 ± 0.04 |
462 ± 23 |
|
Anti-cruzipain IgG (1 × 10–7 M) |
0.15 ± 0.03 |
204 ± 13* |
|
Control IgG (1 × 10–7 M) |
0.20 ± 0.04 |
461 ± 26 |
- Cardiac membranes (3–5 mg/ml protein) were or were not incubated with IgG preparations from cruzipain-immunized mice (anti-cruzipain IgG) or with IgG from OVA+CFA-immunized mice (control IgG). *, p<0.001 between controls vs. anti-cruzipain IgG.
2.2 mAChR-mediated effect of anti-cruzipain IgG
To evaluate the effect of anti-cruzipain IgG on myocardial intracellular signals coupled to M2 mAChR, changes in cAMP levels, inositol phosphate (IP) accumulation, nitric oxide synthase (NOS) activity and cGMP production were measured.
Mice atria were exposed to 1×10–7M anti-cruzipain IgG for different times to accurately determine its kinetic behaviour and the time for their maximal effect. As shown in Table 2, there was a significant decrease in atrial cAMP exposed to anti-cruzipain IgG or the corresponding affinity-purified anti-M2e2 IgG. The maximal decrement was obtained at 2–3 min and persisted for 10 min (Fig. 3). Furthermore, it was blocked by AF-DX 116 (1×10–6 M). Effective neutralization was also observed when preincubating the anti-cruzipain IgG with M2e2. The effect of anti-cruzipain IgG and anti-M2e2 IgG resembled that of the authentic agonist, carbachol, at 5×10–8 M. Control IgG or the M2e2-excluded IgG fraction had no effect on intracellular cAMP levels.
To assess the action of anti-cruzipain IgG on phospholipase C (PLC)-induced intracellular second-messenger production in cardiac preparations, atria were incubated with [myo-3H]-inositol ([3H]MI). Thereafter, water-soluble radiolabeled IP levels formed in the presence or absence of anti-cruzipain IgG or affinity-purified anti-M2e2 were determined. Table 3 shows that total anti-cruzipain IgG and the corresponding anti-M2e2 IgG were able to produce a significant increase in phosphoinositide formation. The effect of the immune IgG was similar to carbachol action. The anti-M2e2 IgG effect could be abolished by pre-treating cardiac tissue with AF-DX 116 or U-73122, indicating that M2 mAChR and PLC-mediated hydrolysis of IP were involved in this effect. M2e2 also neutralized the IgG action, indicating the specificity of the reaction (Table 3).
Knowing that the endogenous nitric oxide signaling system is involved in activation of atrial M2 mAChR 18, we analyzed whether the immune IgG led to increased NOS activity and production of cGMP on murine myocardium. As can be seen in Fig. 4, the affinity-purified anti-M2e2 increased both NOS activity and cGMP production in a concentration-dependent manner, whereas the excluded IgG fraction (i.e. the fraction with no affinity for M2e2) showed no effect.
To verify the nature of the mechanism involved in increased cGMP synthesis and NOS activity, mice atria were incubated with several inhibitors. Table 4 shows that AF-DX 116 inhibited the stimulatory action of the affinity-purified anti-M2e2 IgG on both cGMP production and NOS activity. Furthermore, inhibition of PLC (by U-73122), calcium calmodulin (by TFP) and NOS (by NG-monomethyl-L-arginine [L-NMMA]) blunted M2 mAChR-dependent activation of NOS activity and cGMP accumulation. Additionally, the natural substrate L-arginine completely reversed the inhibition of L-NMMA. As control, the increment of cGMP was abolished by the inhibitor of soluble guanylate cyclase (ODQ). Moreover, carbachol (5×10–8 M) and anti-cruzipain IgG mimicked the effect of the specific anti-M2e2 IgG. Control IgG had no effect.
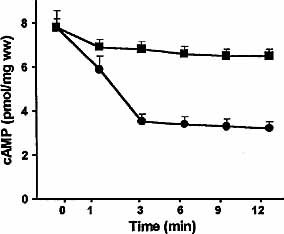
Time-course of cAMP decrement in murine myocardium exposed to 5×10–8 M anti-M2e2 IgG (circles) compared with control IgG (1×10–7 M) (squares). Values are mean ± SEM of five experiments performed in triplicate. p<0.001 between control and immune IgG.
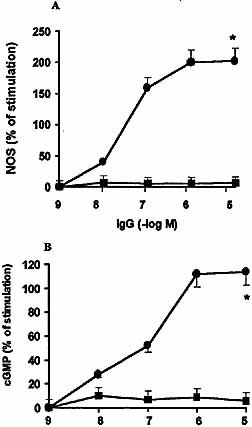
Increase of murine myocardial NOS activity (A) and cGMP production (B) by increasing concentrations of anti-M2e2 IgG (circles) or the anti-cruzipain IgG fraction excluded from M2e2 affinity columns (squares). Values are mean ± SEM of five experiments performed in triplicate. *, p<0.001 compared with the excluded fraction.
|
Treatment |
cAMP (pmol/mg tissue, wet weight) |
|---|---|
|
None |
7.80 ± 0.21 |
|
Anti-cruzipian IgG (1 × 10–7 M) |
3.60 ± 0.12* |
|
Anti-M2e2 IgG (5 × 10–8 M) |
3.28 ± 0.11* |
|
Anti-M2e2 IgG (5 × 10–8 M) + AF-DX 116 (1 × 10–6 M) |
7.98 ± 0.30 |
|
Anti-M2e2 IgG (5 × 10–8 M) + M2e2 (1 × 10–5 M) |
6.89 ± 0.32 |
|
M2e2 (1 × 10–5 M) |
7.86 ± 0.18 |
|
Control IgG (1 × 10–7 M) |
7.92 ± 0.25 |
|
M2e2-excluded IgG (1 × 10–7 M) |
7.75 ± 0.24 |
|
Carbachol (5 × 10–8 M) |
3.18 ± 0.15* |
- Values are mean ± SEM of five separate experiments performed in duplicate in each group. Cyclic AMP values were determined after 15 min of treatment. Initial levels of induced cAMP ("None") were performed using 1×10–7 M PGE2. Prior treatment of anti-cruzipain IgG or anti-M2e2 with the indicated M2e2 peptide was performed for 20 min at 37°C before addition to the atrial preparations. M2 mAChR antagonist (AF-DX 116) and M2e2 were added 20 min before IgG. *, p<0.001 between control vs. carbachol or IgG preparations.
|
Treatment |
% of IP formation |
n |
|---|---|---|
|
None |
14.2 ± 2.1 |
6 |
|
Anti-cruzipain IgG (1 × 10–7 M) |
48.3 ± 2.7* |
5 |
|
Anti-M2e2 IgG (5 × 10–8 M) |
52.5 ± 1.8* |
5 |
|
Anti-M2e2 IgG (5 × 10–8 M) + AF-DX 116 (1 × 10–6 M) |
15.5 ± 2.6 |
5 |
|
Anti-M2e2 IgG (5 × 10–8 M) + U-73122 (5 × 10–6 M) |
13.4 ± 2.2 |
5 |
|
Anti-M2e2 IgG (5 × 10–8 M) + M2e2 (1 × 10–5 M) |
12.2 ± 1.6 |
6 |
|
Control IgG (1 ± 10–7 M) |
12.4 ± 1.8 |
5 |
|
Carbachol (5 × 10–8 M) |
49.7 ± 3.0* |
5 |
- Values are expressed as a % of total radioactivity taken as a 100%. Results are mean ± SEM of six separate experiments performed in duplicate in each group. Values were determined after 60 min of treatment with [3H]MI and for an additional 30 min in the presence or absence of AF-DX 116 or U-73122. Tissues were then left for a further 15 min in the absence ("None") or presence of different IgG preparations or carbachol. *, p<0.0001 between control vs. carbachol or IgG.
|
Treatment |
cGMP |
NOS activity |
|---|---|---|
|
|
(pmol/g of tissue, wet weight) |
|
|
None |
25 ± 2 |
174 ± 12 |
|
Anti-cruzipain IgG (1 × 10–7 M) |
48 ± 4* |
318 ± 20* |
|
Anti-M2e2 IgG (5 × 10–8 M) |
52 ± 5* |
350 ± 28* |
|
Anti-M2e2 IgG (5 × 10–8 M) + AF-DX 116 (1 × 10–6 M) |
20 ± 2 |
162 ± 15 |
|
Anti-M2e2 IgG (5 × 10–8 M) + U-73122 (5 × 10–6 M) |
23 ± 2 |
177 ± 19 |
|
Anti-M2e2 IgG (5 × 10–8 M) + TFP (5 × 10–6 M) |
26 ± 2 |
181 ± 13 |
|
Anti-M2e2 IgG (5 × 10–8 M) + ODQ (5 × 10–6 M) |
22 ± 2 |
ND |
|
Anti-M2e2 IgG (5 × 10–8 M) + L-NMMA (5 × 10–4 M) |
21 ± 2 |
18 ± 2 |
|
Anti-M2e2 IgG (5 × 10–8 M) + L-NMMA (5 × 10–4 M) + L-arginine (5 × 10–5 M) |
50 ± 4* |
350 ± 20* |
|
Control IgG (1 × 10–7 M) |
28 ± 3 |
168 ± 11 |
|
Carbachol (5 × 10–8 M) |
54 ± 3* |
352 ± 12* |
- Results are mean±SEM of six separate experiments performed in duplicate in each group. Cyclic GMP and NOS were measured incubating atria with or without enzymatic inhibitors for 20 min and then for an additional 15 min with carbachol or IgG. ND, not detected. *, p<0.0001 between control vs. carbachol or IgG.
2.3 Functional effects of anti-cruzipain antibody
As seen in Figs. 5 and 6, addition of anti-cruzipain IgG and specific anti-M2e2 IgG results in a decrease of atrial contractility and frequency, the specific affinity-purified anti-M2e2 IgG being more potent than anti-cruzipain total IgG. Control IgG showed no effect in the studied system (Figs. 5 and 6, upper panels).
The functional effects of anti-cruzipain IgG were neutralized by the same factors (AF-DX116 and M2e2) that inhibited signal transduction coupled to M2 mAChR (Figs. 5 and 6, lower panels).
To determine if an endogenous nitric oxide signaling system participates in the negative inotropic action of anti-cruzipain IgG, isolated mouse atria were incubated with inhibitors of NOS activity and cGMP synthesis. Fig. 5, lower panel, shows that L-NMMA inhibition of NOS activity or inhibition of soluble guanylate cyclase by ODQ prevented the inotropic negative effect of anti-cruzipain IgG. On the other hand, db-cAMP (a cAMP analogue) was able to reverse the negative chronotropic effect of the anti-cruzipain IgG (Fig. 6, lower panel).
These results indicate that the negative inotropic effect is related to the NO-cGMP pathway, whereas the negative chronotropic action is related to intracellular cAMP reduction triggered by anti-cruzipain antibodies.
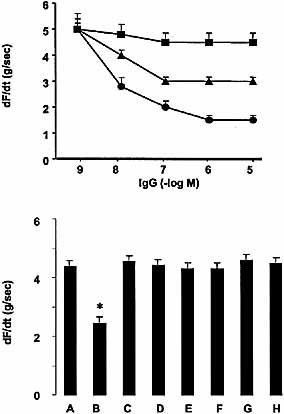
Upper panel: concentration-response curve of anti-cruzipain IgG (triangles), affinity-purified anti-M2e2 IgG (circles) and control IgG (squares) upon atrial contractility (dF/dt) of normal mice. Atria were equilibrated for 30 min before adding different concentrations of IgG preparations. Each concentration was allowed to react with atria for 5 min. Changes in dF/dt are expressed as (g/sec). Values are mean ± SEM of five experiments. Anti-cruzipain IgG and affinity-purified anti-M2e2 showed p<0.001 vs. control IgG. Lower panel: histograms show the atrial electrically stimulated dF/ dt (g/sec) values in the presence of control IgG (1×10–7 M) (A), anti-cruzipain IgG (1×10–7 M) (B), AF-DX 116 (1×10–6 M) + anti-cruzipain IgG (1×10–7 M) (C), anti-cruzipain IgG (1×10–7 M) pre-incubated with M2e2 peptide (1×10–5 M) (D), anti-cruzipain IgG (1×10–7 M) pre-incubated with cruzipain (1×10–5 M) (E), anti-cruzipain IgG (1×10–7 M) + L-NMMA (1×10–5 M) (F), anti-cruzipain IgG (1×10–7 M) + ODQ (1×10–5 M) (G) and the anti-cruzipain IgG fraction excluded from M2e2 affinity columns (1×10–7 M) (H). *, p<0.0001 compared with control IgG.
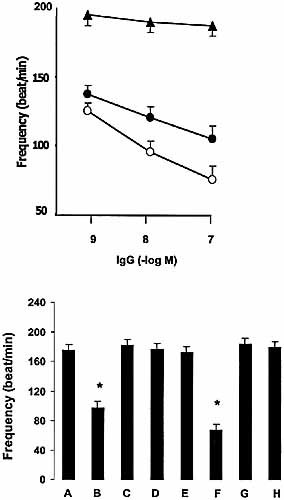
Upper panel: concentration-response curve of anti-cruzipain IgG (black circles), affinity-purified anti-M2e2 IgG (white circles) and control IgG (black triangles) upon normal mice atrial frequency (beats/minute). Spontaneously beating atria were equilibrated for 30 min before adding different concentrations of IgG. Each concentration was allowed to react with atria for 5 min. Values are mean ± SEM of five experiments. Anti-cruzipain IgG and affinity-purified anti-M2e2 showed p<0.001 vs. control IgG. Lower panel: histograms show the atrial rate (beats/minute) values in the presence of: control IgG (1×10–7 M) (A), anti-cruzipain IgG (1×10–7 M) (B), AF-DX 116 (1×10–6 M) + anti-cruzipain IgG (1×10–7 M) (C), anti-cruzipain IgG (1×10–7 M) pre-incubated with M2e2 peptide (1×10–5 M) (D), anti-cruzipain IgG (1×10–7 M) pre-incubated with cruzipain (1×10–5 M) (E), anti-cruzipain IgG (1×10–7 M) + ODQ (1×10–5 M) (F), anti-cruzipain IgG (1×10–7 M) + db cAMP (1×10–4 M) (G) and the anti-cruzipain IgG fraction excluded from M2e2 affinity columns (1×10–7 M) (H). *, p<0.0001 compared with control IgG.
3 Discussion
The set of heterogeneous lesions that typically occur in Chagas' disease following infection by T. cruzi ultimately results from the interactions between parasite and host tissues that allow the parasite to invade cells, replicate and evade attack by the immune system. The biochemical mechanisms that underlie these interactions, and how they then are manifested as symptoms during pathogenesis, are poorly understood. To date, few candidate parasite proteins and their receptors on host cells have been identified. Here we describe cruzipain, a T. cruzi antigen, as an antigen that triggers IgG which is able to interact with cardiac M2 mAChR on host cells, leading to activation of signaling pathways downstream of these receptors. Since the receptors become activated, normal cell signaling is also disrupted. Immunization with cruzipain elicits a strong immune response 15. Beside the inflammatory properties of cruzipain as a proteinase, the role of this antigen, when rendered devoid of enzymatic activity, has been demonstrated during heart damage in immunized mice 15. In our study, protease activity was previously inhibited. Therefore, a direct action of the enzyme on the M2 mAChR can be excluded.
Previous studies have demonstrated the existence of circulating IgG that reacts with M2 mAChR-rich tissues 19, 20, in sera from chagasic patients 5, 8 and mice 7. These antibodies have shown to be associated with development of cardiomyopathy 21. This has led to the hypothesis that digestive-tissue damage and heart-tissue damage which arise from the dysautonomic disorder — commonly seen in chronic Chagas' disease sufferers — might be a consequence of the production of such autoantibodies 21–23. Since we demonstrate that anti-cruzipain IgG binds and activates cardiac M2 mAChR in a similar way as anti-receptor antibodies do, it is tempting to speculate that this antigen is the candidate parasite protein responsible for the induction of antibodies that cross-react with heart M2 mAChR. Further evidence for cross-reactivity between cruzipain and M2 mAChR was provided, firstly, by the fact that anti-M2e2 IgG could be purified from anti-cruzipain serum and, secondly, because M2e2 peptide was able to suppress the biological effect of anti-cruzipain serum, indicating that the second extracellular loop of the receptor can be considered essential for the biological action of these anti-receptor antibodies.
When considering that mice immunized with cruzipain showed alterations in the heart conduction system and sinus bradycardia 15, and that anti-cruzipain antibodies from the same mice are able to decrease contractile tension and frequency on normal atria, one might speculate that these antibodies could mediate long-term interaction with cardiac M2 mAChR. Thus, by behaving as an M2 mAChR agonist, anti-cruzipain IgG could lead to prolonged receptor phosphorylation or desensitization phenomenon that could be responsible for the most common electrocardiographic finding in chagasic patients and murine infection 17, 21. Finally, cruzipain that is rendered devoid of enzymatic activity is also able to induce IgG antibodies against myosin 15. This antibody, directed against an intracellular protein, was described in mice suffering from autoimmune myocarditis 24. This affliction strongly resembles experimental chronic Chagas' heart disease on the basis of histological and immunopathological data 24. Thus both chagasic mice 7 and autoimmune-myocarditis mice 24 showed enlargement of the QRS wave, and antibodies that recognized antigens located intracellularly (myosin) and on the surface of the cells (M2 mAChR).
In summary, the IgG purified from sera of mice immunized with cruzipain interacts with the second extracellular loop of M2 mAChR. This interaction could be due to cross-reactivity between cruzipain and the cardiac cholinoceptor, followed by loss of self-tolerance. It might be of great interest to study if this cross-reactivity is also observed in the human in vivo T. cruzi infection to show if anti-cruzipain antibodies play a pathophysiological role in the disease. Chronic fixation of IgG and activation of M2 mAChR could induce myocardial dysfunction and injury, leading to intracellular myosin exposure with induction of anti-myosin antibodies. These events might perpetuate myocardial damage and lead to the symptoms commonly observed in chronic cardiac Chagas' disease.
4 Materials and methods
4.1 Cruzipain purification
Epimastigote forms of T. cruzi Tulahuen strain were grown at 28°C in brain heart infusion medium (Becton Dickinson) supplemented with 0.5% tryptose, 10% FCS, 200 mg/ml hemin and 100 U/ml streptomycin. Parasites were harvested at the exponential growth phase, centrifuged at 5,000×g for 10 min at 4°C, and washed with PBS. Parasites were resuspended in three volumes of 0.25 M sucrose and 5 mM KCl; next the irreversible protease inhibitors, 1 mM N-α-p-tosyl-L-lysine chloro methyl ketone and 1 mM PMSF (phenylmethylsulfonylfluoride) (Sigma) were added. Epimastigotes were disrupted by three cycles of freezing (–20°C) and thawing (4°C). The homogenates were centrifuged at 7,000×g for 15 min at 4°C. Saturated ammonium sulfate solution, adjusted to pH 7.1 with NH4OH, was added at 50% saturation to the supernatant, with stirring in an ice bath. The precipitate obtained after centrifugation of this suspension was carefully dissolved and dialyzed in 50 mM Tris-HCl, 150 mM NaCl, pH 7.4. CaCl2, MgCl2 and MnCl2 were added to a final concentration of 5 mM each. Subsequently, the samples were submitted to affinity chromatography. The absence of enzyme activity was checked by 10% SDS-PAGE containing 0.1% gelatin as substrate. It was important to eliminate cruzipain activity to avoid the inflammatory properties of cruzipain as a proteinase 4. After electrophoresis performed at 120 V, the gel was incubated with buffer — 50 mM sodium phosphate, pH 5.7, at 37°C overnight — and it was then stained with Coomassie brilliant blue R250. Samples were neither reduced nor boiled.
4.2 Immunization of BALB/c mice
Inbred female BALB/c mice, aged 6–8 weeks (purchased from Centro Nacional de Energía Atómica, Buenos Aires, Argentina), were immunized by intradermal injection, at the base of the tail and along the back, with cruzipain that had been irreversibly inactivated and was emulsified in complete Freund's adjuvant (CFA) (Sigma). The mice were injected on days 0, 14 and 28 with 10 μg of cruzipain in 0.1 ml of PBS, mixed with 0.1 ml of CFA 12. Control mice were injected with 0.2 ml of OVA (10 μg) + CFA following the same procedure. Mice were maintained according to National Research Council's guide for the care and use of laboratory animals.
4.3 Purification of mouse IgG
Sera pooled from 10 immunized mice and sera pooled from 10 control mice were obtained 14 days after the third immunization; immune and control pools were then dialyzed overnight against the elution buffer (PBS, pH 8.0). Immune and control IgG fractions were isolated by affinity chromatography using two protein-G–sepharose columns (Amersham Pharmacia) that were previously equilibrated with elution buffer. Immune and control IgG fractions were eluted with 0.1 M citric acid, pH 3.0, neutralized with 2 M Tris and finally they were dialyzed against PBS, pH 7.3. The degree of IgG purification was tested by SDS-PAGE and its concentration was determined.
4.4 M2 mAChR synthetic peptide
A 24-mer M2 peptide (VRTVEDGECYIQFFSNAAVTFGTA), corresponding to the sequence of the second extracellular loop of the human mAChR (residues 169–192), was synthesized by the F-moc-amino acids activated using HOBt/DCC strategy. An automatic peptide-synthesizer (Applied Biosystems Model 431A) was used throughout.
4.5 Purification of anti-peptide IgG by affinity chromatography
IgG fraction pools were independently subjected to affinity chromatography on the synthesized peptide covalently linked to AffiGel 15 gel (Bio-Rad, Richmond, CA, USA). The IgG fraction was loaded on the affinity column equilibrated with PBS and the fraction that did not bind peptide was first eluted with the same buffer. Specific anti-peptide autoantibodies were then eluted with 3 M KSCNand 1 M NaCl followed by immediate extensive dialysis against PBS. The IgG concentration of both antibodies that did not bind peptide and specific antibodies that bound the muscarinic-receptor peptide was determined by radial immunodifussion assay and their immunological reactivity against the M2 mAChR peptide was evaluated by enzyme immunoassay.
4.6 Preparation of membranes
Hearts were removed and placed on a glass plate containing a modified Kreb's-Ringer's bicarbonate (KRB) buffer, gassed with 5% CO2 in oxygen, pH 7.4; thus, fat, the large vessels, the connective tissue and blood were eliminated. Homogenization was accomplished at 4°C by homogenizing the tissue twice in four volumes of cold buffer containing 0.25 M sucrose, 50 mM Tris-HCl, pH 7.4, and 10 mM MgCl2 using a Polytron PT-20 at a setting of three for 15 sec. The homogenates were filtered through four layers of gauze and centrifuged at 700×g for 15 min, 10,000×g for 15 min and 40,000×g for 30 min. The pellet was resuspended in 2.5 ml of 50 mM Tris-HCl, pH 7.4, and 10 mM MgCl2.
4.7 Binding assay
IgG preparations were preincubated with cardiac membranes (3–5 mg/ml protein) for 3 min at 30°C in 5 mM Tris-HCl, pH 7.4, and 10 mM MgCl2. The cardiac membranes were washed twice by centrifugation and resuspended. For [3H]-QNB binding, 100 μl of membrane suspension (150 μg protein) was incubated with a fixed (0.3 nM) or increasing concentrations of [3H]-QNB (Dupont/New England Nuclear, Boston, MA, USA; specific activity 40 Ci/mmol) in a shaking bath at 30°C for 30 min in a total volume of 150 μl of 50 mM Tris-HCl, pH 8, and 10 mM MgCl2. In all cases, binding was stopped by the addition of ice-cold buffer and, after filtration under mild pressure, Whatman GF/c filters were rinsed and counted in a Beckman spectrometer. Nonspecific binding was determined by filtering aliquots incubated in the presence of 1 μM atropine, which did not exceed 15% of the specific binding.
4.8 ELISA
A 50-μl volume of the M2e2 peptide (20 μg/ml), in 0.1 M Na2CO3 buffer, pH 9.6, was used to coat microtiter plates (Costar) at 4°C overnight. After blocking the wells with 1% BSA in PBS for 1 h at 37°C, different dilutions of sera or purified IgG from non-infected or infected mice treated or non-treated with M2e2 (50 μl) were allowed to react with the peptide for 2 h at 37°C 21. The wells were then thoroughly washed with 0.05% Tween-20 in PBS. A 50-μl volume of goat anti-mouse-IgG conjugated to alkaline phosphatase (1:5,000, v/v; Sigma) was added and incubated for 1 h at 37°C. After several washing steps, p-nitrophenyl phosphate (1 mg/ml) was added as substrate and the reaction was stopped at 30 min. values of O.D. were measured with an ELISA reader (Uniskan Laboratory System).
4.9 cGMP and cAMP accumulation assays
Hearts were incubated in 1 ml of KRB for 30 min and different concentrations of IgG preparations or carbachol were added for the last 15 min. When blockers were used, they were added 20 min before the addition of reagents. For cAMP determination, mouse atria were incubated in the presence of 0.1 mM IBMX. To evaluate the inhibitory effects on AMP production, PGE2 (1×10–7 M) was added for the last 3 min as an adenylate cyclase stimulator. The residue was dissolved in different buffers for cGMP 6 or cAMP 5. Aliquots of 100 ml were taken for the nucleotide determination using a RIA procedure with [125I]cGMP or [125I]cAMP RIA kit from Dupont/New England Nuclear, USA.
4.10 Measurements of total labeled IP
Hearts were incubated for 120 min at 37°C in 0.5 ml of KRB (gassed with 5% CO2 in oxygen) with 1 mCi [3H]MI, specific activity 46.0 Ci/mmol, from Dupont/New England Nuclear; LiCl (10 mmol/l) was added for measurement of inositol monophosphate accumulation, as previously described 10. Carbachol and IgG preparations were added 15 min before the end of incubation period and blockers were added 30 min before addition of reagents. After incubation, atria were quickly washed with KRB and water-soluble IP were extracted. Briefly, tissues were homogenized in 0.3 ml of KRB with 10 mmol/l LiCl and 2 ml chloroform:methanol (1:2, v/v) to stop the reaction. Chloroform (0.62 ml) and water (1 ml) were then added. Samples were centrifuged at 2,000×g for 15 min and the aqueous phase of the supernatants (1–2 ml) was applied to a 0.7 ml column of Bio-Rad AG 1 × 8 anion exchange resin, which had been previously washed with 10 mmol/l Tris-formate, pH 7.4. The resin was then washed with 20 volumes of 5 mmol/l MI to get the first peak of [3H]MI incorporated in the tissue, followed by six volumes of water and, finally, IP were eluted with 1 mol/l ammonium formate in 0.1 mol/l formic acid (second peak). Fractions of 1 ml were recovered and radioactivity was determined by scintillation. Results corresponding to the second peak were expressed as the absolute values of the Simpson's equation. To determine the absence of [3H]MI in the second peak corresponding to IP, thin-layer chromatography on silica gel 60F 254 sheets (Merck, USA) was performed using appropriate MI and IP standards as described previously 6.
4.11 Determination of NOS activity
NOS activity was measured on mouse heart by production of [U-14C]citrulline from [U-14C]arginine according to the procedure described previously 6. Briefly, after a 20-min preincubation in KRB 18, tissues were transferred to 500 ml of prewarmed KRB equilibrated with 5% CO2 in oxygen in the presence of [U-14C]arginine (0.5 μCi). Appropriate concentrations of enzymatic inhibitors were added and tissues were incubated for 20 min, and for an additional 15 min with carbachol or IgG under carbogen at 37°C. Tissues were then homogenized with an Ultraturrax in 1 ml of medium containing 20 mmol/l Hepes, pH 7.4, 0.5 mmol/l EGTA, 0.5 mmol/l EDTA, 1 mmol/l dithiothreitol, 1 mmol/l leupeptin and 0.2 mmol/l PMSF at 4°C. After centrifugation at 2000×g for 10 min at 4°C, supernatants were applied to 2-ml columns of Dowex AG-50 WX-8 (sodium form); [U-14C]citrulline was eluted with 3 ml of water and quantified by liquid scintillation counting. Measurement of basal NOS activity by the above-mentioned procedure was inhibited to a 95% by 0.5 mmol/l L-NMMA. In order not to drastically modify basal NOS activity, we chose 10 μmol/l L-NMMA to inhibit NOS activation. The activity of the partially purified enzyme was 345+35 nmol/mg/min, as assessed by means of 2′,5′-ADP-Sepharose chromatography. The Ca2+-dependency of NOS was assayed in the presence of 1 mmol/l EDTA and free Ca2+ was controlled by the addition of CaCl2 solution to the reaction mixture. In the absence of free Ca2+ (1 mmol/l EDTA) there was no measurable formation of NO, and a 50% of maximal stimulation of enzyme activity was observed with 0.5 mmol/l of free Ca2+.
4.12 mAChR functional studies
Animals were decapitated and the atria were removed quickly and placed in a glass chamber containing KRB solution, pH 7.4, that was gassed with 5% CO2 in oxygen at 30°C. After a stabilization period of 30 min, spontaneous tension and frequency were recorded using a force transducer coupled to an ink-writing oscillograph, as previously described 6. Then, the preparations were paced by means of a bipolar electrode using a SK4 Grass stimulator, with stimuli duration of 2 msec and a voltage that was 10% above threshold. The constant resting tension applied to the atria (preload tension) was 350 mg. Inotropic effects (dF/dt) were assessed by recording the maximum rate of tension development during electrical stimulation at a frequency of 250 beats/min. To obtain the maximum serum or IgG effect, different concentrations of sera or IgG were added to normal mouse atria every 15 min.
4.13 Drugs
Carbachol, AF-X116, RG E2, L-NMMA and db-cAMP were from the Sigma Chemical Company (Saint Louis, MO), and U-73122 and ODQ were from Tocris Cookson Inc (Ellisville, MO).
4.14 Statistical analysis
Statistical significance was determined by the two-tailed t-test for independent populations. Analysis of variance and Student-Newman-Keuls test was employed when multiple comparisonswere necessary. Differences between means were considered significant if p was equal to or less than 0.05.
Acknowledgements
This work was supported by grants from Buenos Aires University (UBACyT) and The Argentine Research Council (CONICET, PIP). The authors also thank Mrs ElvitaVannucchi for her expert technical assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




