The HIV protease inhibitor Indinavir reduces immature dendritic cell transendothelial migration
Abstract
Indinavir (IDV) is a protease inhibitor that successfully suppresses HIV-1 replication as part of anti-retroviral therapy. There is evidence to suggest that IDV may also act non-specifically upon host proteases. In this study we investigated whether IDV could modulate protease-dependent molecules involved in dendritic cell (DC) migration — a pivotal process in immunoregulation. Human monocyte-derived DC were exposed to IDV (IDV-DC) and transendothelial migration (TEM) to inflammatory chemokines was determined. TEM of IDV-DC was significantly impaired compared to non-treated DC (p<0.01). Phenotypic analysis revealed that IDV-DC had reduced DC-SIGN expression, correlating with reduced adhesion to immobilized ICAM-2. Nevertheless, the reduction in migration following exposure to IDV could not be fully attributable to DC-SIGN interactions alone. Investigation of IDV-DC interactions with the underlying matrix protein, fibronectin, demonstrated that IDV significantly impaired DC binding to immobilized fibronectin (p<0.01). IDV appeared to act upon VLA-4 and VLA-5 since addition of antagonist monoclonal antibodies (mAb) similarly reduced adhesion ofnon-treated DC to fibronectin. Combined blockade of DC using anti-VLA-4, VLA-5 and anti-DC-SIGN mAb inhibited TEM to a similar extent as IDV. Our results strongly suggest that IDV inhibits host proteases necessary for DC migration and may, therefore, affect DC immunoregulation in HIV-1-infected patients.
Abbreviations:
-
- DC-SIGN:
-
DC-specific intercellular adhesion molecule-3-grabbing nonintegrin
-
- HAART:
-
Highly active anti-retroviral therapy
-
- HUVEC:
-
Human umbilical vein endothelial cells
-
- IDV:
-
Indinavir
-
- IDV-DC:
-
DC treated with IDV for 16 h
-
- NT-DC:
-
Non-treated DC
-
- R5-HIV:
-
R5 strain of HIV
-
- TEM:
-
Transendothelial migration
1 Introduction
HIV-1 infection generally results in a poor prognosis with a gradual loss in normal immune functions. A key feature of HIV-1 pathogenesis is the infection and depletion of CD4 T cells that arecritically involved in regulating immune responses 1, 2. Dendritic cells (DC) are professional antigen-presenting cells that have the unique capacity to provide high levels of costimulation necessary for activation of both naive and antigen-experienced T cells 3. Central in co-ordinating the immune system are DC. Immature DC are able to migrate to an inflammatory site, capture antigen, undergo maturation and migrate to lymphoid organs where they can then activate and drive T cell proliferation.
Immature DC predominantly express CCR5 enabling migration to inflammatory sites, whereas mature DC express high levels of CXCR4, a receptor that facilitates migration to lymphoid organs 4. These chemokine receptors, together with CD4, are expressed by DC and function as coreceptors necessary for HIV-1 infection 5. In addition, DC express DC-SIGN, a C-type lectin that binds HIV-1 6, 7, ICAM-3 on T cells 7, and ICAM-2 on endothelial cells 8, permitting transport of infectious HIV-1 to CD4 T cell-rich areas, thus contributing to the rapid depletion in CD4 T cell numbers. CD4 T cell numbers may recover in HIV-1-infected patients if highly active anti-retroviral therapy (HAART) is administered 9. Inhibitors of HIV-1 aspartic protease such as Indinavir (IDV) are given to arrest HIV-1 replication, and have played a major part in the success of HAART regimens where they are generally used with inhibitors of HIV-1 reverse transcriptase. Protease inhibitors are designed to act specifically upon HIV-1 aspartic protease. However, a number of clinical side-effects have been attributed to the nonspecific action of IDV upon host proteases that are involved in important homeostatic functions (renin, pepsin, gastrin, lysosomal cathepsin D and cathepsin E, and proteases involved in lipid metabolism) 10, 11. There is evidence to suggest that proteases necessary for key immunological responses such as antigen processing, presentation and cell proliferation may also be impaired 12, 13. Central to the immune response is the ability of DC to perform these functions, facilitated by their capacity to migrate to localized chemokine gradients present at a site of infection. Chemokine engagement of DC chemokine receptors leads to a multistep, sequential process facilitating transendothelial migration (TEM), where protease-dependent conformational changes occur in integrins to increase their binding avidity 14, 15.
The aim of this study was to investigate whether one of the most common protease inhibitors used in clinical practice, IDV, affects proteases involved in DC migration — a process pivotal to immune surveillance and regulation. For this we utilized chemotaxis assays to evaluate DC migration across transwell membranes that were either untreated ("bare" transwell assays), coated with a fibronectin matrix alone, or coated with fibronectin on which a monolayer of endothelial cells had been established (TEM assays). Here we demonstrate that IDV was able to reduce TEM of DC through what appears to be an inhibition of the interaction of DC with both endothelial cells and the underlying or exposed fibronectin matrix, in a capacity distinct from its antiviral effects.
2 Results
2.1 TEM of DC is inhibited by exposure to IDV
To determine whether exposure to IDV at therapeutic concentrations affected immature DC TEM, their chemotactic response was assessed. A predominant chemokine receptor expressed on immature DC is CCR5; therefore, migration to the CCR5-binding chemokine, RANTES, was determined. DC TEM was significantly inhibited by treatment with 10 μM and 5 μM IDV for 16 h (Fig. 1a). Treatment with lower doses of IDV (0.1–1.0 μM) did not inhibit DC TEM.
DC chemotaxis across bare transwells was not inhibited by treatment of DC with 10 μM or 5 μM IDV for 16 h (Fig. 1b). This suggested that IDV affected specific interactions between DC and endothelial cells. Treatment of endothelial cells with 5 μM IDV (2–72 h) demonstrated that IDV had no effect on the expression of endothelial cell adhesion molecules involved in TEM or other molecules studied (Table 1). Furthermore treatment of endothelial cells with 5 μM IDV for 16 h did not inhibit DC TEM (Fig. 1c). In contrast, exposure of DC to 5 μM IDV for 16 h significantly inhibited TEM (Fig. 1c). TEM of DC to other CCR5-binding inflammatory chemokines (MIP-1α and MIP-1β) was also inhibited by IDV treatment (data not shown). Our data suggest that the protease inhibitor activity of IDV affects DC interactions with endothelial cells. Experiments using an aspartic protease inhibitor, pepstatin A, also demonstrated similar inhibition of DC TEM (data not shown).
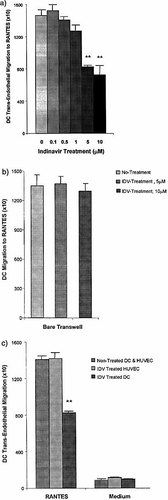
IDV inhibits TEM of DC. (a) TEM of DC and DC treated with IDV for 16 h within a therapeutic concentration range was performed and the number of migrated DC was determined. (b) Chemotaxis of NT-DC and IDV (5 μM and 10 μM for 16 h)-treated DC were tested in a bare transwell system. (c) TEM was performed using (i) NT-DC and non-treated HUVEC, and compared with (ii) treatment of HUVEC for 16 h IDV (5 μM), and (iii) treatment of DC for 16 h with 5 μM IDV (IDV-DC) prior to TEM. A significant reduction in TEM was observed in DC treated with IDV (5 μM for 16 h) (**p<0.01). Values represent mean ± SD of triplicate samples in four experiments.
|
HUVEC Antigen |
NT |
IDVa) 2 h |
IDV 6 h |
IDV 24 h |
IDV 72 h |
TNF-α 6 h |
IFN-γ 72 h |
|---|---|---|---|---|---|---|---|
|
HLA class I |
1 |
–b) |
– |
– |
– |
– |
↑3×c) |
|
HLA class II |
1 |
– |
– |
– |
– |
– |
↑5× |
|
PECAM-1 (CD31) |
1 |
– |
– |
– |
– |
– |
– |
|
ICAM-1 (CD54) |
1 |
– |
– |
– |
– |
↑2× |
↑2× |
|
ICAM-2 (CD102) |
1 |
– |
– |
– |
– |
– |
– |
|
VCAM-1 (CD106) |
1 |
– |
– |
– |
– |
↑9× |
– |
|
E-Selectin (CD62E) |
1 |
– |
– |
– |
– |
↑20× |
– |
- a) HUVEC were stimulated with 5 mm IDV (IDV) for 2–72 h.
- b) No change in mean fluorescence index (intensity x%), relative to the levels detected on non-treated, resting endothelium (NT).
- c) Expression was assessed as the x-fold increase in mean fluorescence index relative to the levels detected on non-treated, resting endothelium (values are the mean of three separate experiments, rounded up to the nearest integer).
2.2 IDV induces phenotypic changes of DC
Exposure of DC to 5 μM IDV over a 48-h period did not reduce expression of CCR5 or increase CXCR4 as might otherwise be expected following a reduction in chemotaxis to RANTES (Fig. 2a). Furthermore, IDV did not increase the expression of HLA class I, HLA class II, CD25 and costimulatory molecules (CD80, CD86, CD40), associated with maturation of DC over the 48-h period (Fig. 2a, b). However, expression of CD83, a typical DC maturation marker, was increased following exposure of DC to IDV. Ordinarily, soluble CD83 can be detected in the supernatant of NT-DC. However, exposure of DC to 5 μM IDV reduced the levels of soluble CD83 detected (data not shown). Elevated levels of CD83 on IDV-treated DC were detected at 12 h with maximal expression at 16 h and a return to basal levels after 24 h (Fig. 2b).
The expression of adhesion molecules ICAM-1, the β2 integrins (LFA-1, Mac-1, p150/95) and the β1 integrins (VLA-4 and VLA-5) was unaffected by exposure to IDV (Fig. 2a). However, a significant reduction in DC-SIGN expression was detected following 8-h exposure to IDV, with a maximal reduction observed after 16 h (Fig. 2b). DC-SIGN expression returned to basal, constitutive levels after 20-h exposure to IDV. Interestingly, maximal changes in DC-SIGN and CD83 expression, affected by IDV treatment, were sustained if DC were supplemented with further doses of IDV every 16 h to maintain optimal levels of anti-protease activity (Fig. 2b).
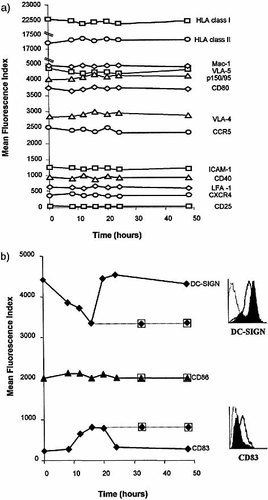
Phenotypic analysis of DC following exposure to IDV. (a) DC were treated with 5 μM IDV over time and phenotypic analysis was expressed as mean fluorescence index. Exposure of DC to IDV did not alter the expression of any antigens tested. (b) A reduction in DC-SIGN expression and an increase in CD83 expression were observed and found to be maximal at 16 h; this was sustained following further doses of IDV every 16 h (32 h and 48 h; dotted line). The FACS plots represent the change in expression of DC-SIGN and CD83 on IDV-DC (black line) compared with NT-DC (filled-black profile). Values are representative of four experiments.
2.3 IDV reduces DC-SIGN interactions with ICAM-2 to inhibit TEM
To determine whether reduced DC-SIGN expression on DC treated with 5 μM IDV for 16 h (IDV-DC) could directly affect DC interactions with ICAM-2 on endothelial cells, (a) adhesion to immobilized ICAM-2, and (b) TEM were assessed. Exposure of DC to IDV inhibited adhesion to ICAM-2, to the same level as mAb blockade of DC-SIGN on NT-DC. Furthermore, there was no additional inhibitory effect by anti-DC-SIGN mAb on IDV-DC binding to ICAM-2 (Fig. 3a). The reduction in DC-SIGN expression caused by IDV was sufficient to inhibit DC interactions with ICAM-2, to a level that excludes the possible involvement of LFA-1, the only other DC ligand for ICAM-2. In fact, mAb blockade of LFA-1/ ICAM-2 interactions further reduced TEM of IDV-DC, indicating that IDV does not inhibit this interaction (data not shown). Our data suggest that IDV reduced DC-SIGN expression to a level that could no longer support the interaction of DC with ICAM-2.
TEM studies demonstrated that blockade of DC-SIGN on NT-DC significantly reduced TEM (p<0.02) (Fig. 3b). Interestingly, IDV inhibited DC TEM to a greater extent than blockade with anti-DC-SIGN mAb (Fig. 3b). Blockade of DC-SIGN on IDV-DC did not cause an additional inhibition of migration. These results strongly suggested that IDV not only affected DC-SIGN, but also other molecules involved in TEM. Because the endothelial cell monolayers were grown on fibronectin-coated transwells, we next turned our attention to the interactions of DC with this matrix support.
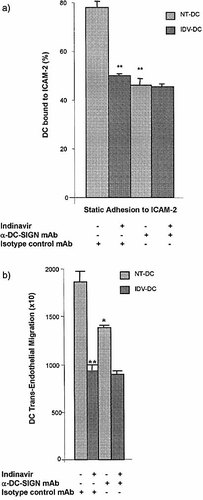
IDV inhibits TEM by reducing DC-SIGN interactions with ICAM-2. (a) Static adhesion to ICAM-2 was performed using DC treated with IDV, and/or anti-DC-SIGN mAb and the percentage of adherent DC calculated. IDV-DC showed significantly reduced binding to ICAM-2 compared to NT-DC (p<0.01). Enhanced antagonism by blockade of IDV-DC with anti-DC-SIGN mAb did not result in further reduced binding to ICAM-2. (b) TEM of NT-DC and IDV-DC was assessed following exposure to anti-DC-SIGN and the number of migrated DC was determined. Migration of NT-DC was significantly inhibited by blockade with anti-DC-SIGN mAb (*p<0.02). A greater reduction in migration was observed for IDV-DC compared to NT-DC incubated with anti-DC-SIGN mAb (**p<0.01). DC-SIGN blockade of IDV-DC did not provide additional reduction in migration. Values represent mean ± SD of triplicates samples in four experiments.
2.4 Exposure to IDV reduces DC migration across fibronectin
Trans-fibronectin migration of IDV-DC was significantly reduced compared to migration of NT-DC (p<0.02) (Fig. 4). Importantly, migration of IDV-DC across bare transwells was unaffected, suggesting that the migration of DC involves specific interactions with fibronectin (Fig. 4). These results suggested that the reduction in TEM caused by exposure to IDV (Fig. 1) was, in part, due to an alteration of DC interactions with fibronectin.
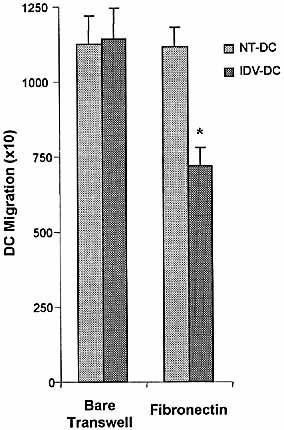
IDV inhibits DC migration across fibronectin. Migration of NT-DC and IDV-DC were assessed across bare and fibronectin-coated transwells. The number of migrated DC was determined. IDV-DC showed significantly inhibited migration across fibronectin-coated transwells without affecting transmigration across bare transwell (*p<0.02). Each value represents mean ± SD performed in triplicate and repeated in four experiments.
2.5 Blockade of VLA-4 and VLA-5 reduces DC migration across fibronectin to a similar level as IDV-DC
Blockade of fibronectin ligands with anti-VLA-4 and anti-VLA-5 mAb on NT-DC significantly reduced migration across fibronectin (p<0.01) (Fig. 5a). A similar level of reduction was observed for IDV-DC (p<0.01) (Fig. 5a). No additional antagonistic effect of anti-VLA-4 and anti-VLA-5 blocking mAb was observed on the trans-fibronectin migration of IDV-DC. This suggested that IDV inhibited the capacity of VLA-4 and VLA-5 on DC to interact with fibronectin.
As expected, surface levels of VLA-4 and VLA-5 were not altered by IDV treatment as these molecules are expressed constitutively, irrespective of whether they are in active or inactive forms (Fig. 2a). However, the active conformational forms of VLA-4 and VLA-5 were reduced on IDV-DC compared with NT-DC following detection with a specific mAb (data not shown). Therefore, we assessed whether IDV altered the binding of DC to fibronectin. Blockade of VLA-4 and VLA-5 on NT-DC significantly reduced binding to immobilized fibronectin (Fig. 5b). IDV-DC also showed reduced binding to fibronectin to a similar level as blockade with anti-VLA-4 or VLA-5 mAb. An additional reduction in adhesion of IDV-DC to fibronectin was not observed following treatment with anti-VLA-4 or VLA-5 mAb, suggesting that IDV maximally inhibits the interaction of VLA-4 and VLA-5 interactions with fibronectin (Fig. 5b).
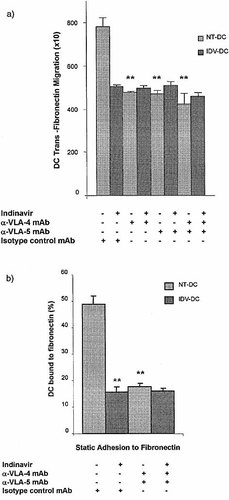
IDV inhibits VLA-4 and VLA-5 interactions with fibronectin. (a) NT-DC and IDV-DC were incubated with blocking anti-VLA-4 or anti-VLA-5 mAb and migration across fibronectin determined. Migration of NT-DC was significantly inhibited by blockade with anti-VLA-4 and VLA-5 mAb (**p<0.01). IDV-DC incubated with anti-VLA-4 and VLA-5 mAb did not show a further reduction in trans-fibronectin migration compared to IDV. (b) Static adhesion to fibronectin was performed using DC treated with IDV, and/or anti-VLA4 and VLA-5 mAb. Nonadherent DC were counted and the percentage of adherent DC calculated. IDV-DC showed significantly reduced binding to fibronectin and this was to a similar level as blockade of NT-DC with anti-VLA-4 and VLA-5 mAb (**p<0.01) (Fig. 5b). Blockade of fibronectin ligands on IDV-DC did not result in a further reduction in adhesion to fibronectin. Values represent mean ± SD in experiments performed in triplicate in four experiments.
2.6 Exposure to IDV reduces DC TEM through combined effects on DC-SIGN, VLA-4 and VLA-5
To confirm that IDV had combined effects on DC-SIGN, VLA-4 and VLA-5, TEM assays were used. IDV-DC and NT-DC were treated with a combined blockade of DC-SIGN, VLA-4 and VLA-5. Combined mAb blockade of NT-DC significantly inhibited TEM to the same extent as IDV treatment of DC (p<0.01) (Fig. 6). No additional reduction in TEM was observed for the combined blockade of DC-SIGN, VLA-4 and VLA-5 on IDV-DC. Our overall interpretation is that IDV (i) reduces the expression of DC-SIGN and, therefore, inhibits the binding of DC to ICAM-2 on endothelial cells, and (ii) inhibits the binding of DC via VLA-4 and VLA-5 to the fibronectin matrix support. TEM of DC in these assays involves both sets of interactions. Our results suggest that IDV maximally inhibits these interactions during TEM, as no further inhibition is achieved by blockade of DC-SIGN, VLA-4 and VLA-5 on IDV-DC.
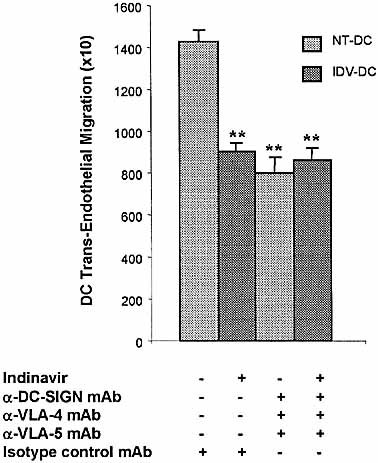
IDV modulates VLA-4, VLA-5 and DC-SIGN to inhibit TEM of DC. TEM of IDV-DC was compared to migration of NT-DC blocked with a combination of antibodies against VLA-4, VLA-5 and DC-SIGN. Blockade of VLA-4, VLA-5 and DC-SIGN in combination, significantly reduced TEM of NT-DC to a similar level as IDV-DC (**p<0.01). IDV-DC when blocked with the combination of mAb above did not show further reduced TEM. Values represent mean ± SD of triplicate samples performed in four experiments.
2.7 Expression of DC-SIGN and CD83 on DC prepared from HIV-1-infected subjects receiving IDV
DC from HIV-1-infected subjects were examined to determine whether similar phenotypic changes could be induced by IDV. Analysis of DC-SIGN and CD83 expression on DC from HIV-1-infected subjects that have been previously exposed to IDV demonstrated similar changes in expression as observed in IDV-DC from healthy individuals (Fig. 7). An increase in CD83 and decrease in DC-SIGN expression was observed following 16-h treatment with IDV. These results demonstrate that DC from HIV-1-infected subjects previously exposed to IDV have similar changes in DC-SIGN and CD83 expression as DC from healthy subjects following re-exposure to IDV.
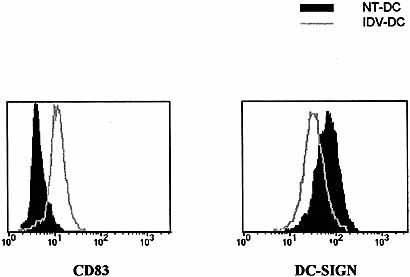
Altered DC-SIGN and CD83 expression on DC from HIV-1-seropositive individuals. DC prepared from HIV-1-positive individuals receiving IDV were treated with 5 μM IDV for 16 h (IDV-DC) and expression of CD83 and DC-SIGN was assessed. Exposure of DC from HIV-1 infected subjects to therapeutic concentrations of IDV (5 μM), resulted in a reduction in DC-SIGN and increase in CD83 expression. Values are representative of samples performed in duplicate for five patients.
3 Discussion
IDV is a widely used and generally well-tolerated protease inhibitor that targets HIV aspartic protease, preventing the cleavage of viral proteins necessary for generating viable virions 16. However, HIV protease inhibitors may act on host proteases and cause a number of clinical side-effects 10, 11. Proteases play a crucial role in the expression and function of molecules involved in cellular homeostasis and immune regulation. DC orchestrate the immune system by capturing and presenting antigen to naive T cells and providing high levels of costimulation. This is critically dependent upon the capacity of DC to migrate between peripheral and lymphoid tissues where multistep navigational control is essential for chemotaxis 17. Inflammatory chemokines induce immature DC chemotaxis, whereas homing chemokines attract mature DC 4, 18. Proteases have been shown to play a role in the activation of molecules involved in leukocyte migration 15, 19, 20. Therefore, we wanted to determine whether IDV affected migration and traffic of DC.
Exposure of DC to therapeutic levels of IDV significantly inhibited their migration across endothelial cells and the underlying fibronectin matrix. IDV treatment of endothelial cells did not affect the expression of adhesion molecules nor did it inhibit TEM of NT-DC. In marked contrast, IDV-DC showed reduced transendothelial and transfibronectin migration. Phenotypic analysis revealed a reduction in DC-SIGN expression on IDV-DC. Similar phenotypic changes were observed in DC from HIV-1-infected individuals receiving IDV treatment. Interestingly, we observed maximally reduced DC-SIGN expression and increased CD83 expression when IDV was present for 16 h in culture.
DC-SIGN is a DC-specific adhesion molecule that has been shown to bind to ICAM-2 (expressed constitutively by endothelial cells, and not up-regulated upon activation) to support DC trafficking 8, 21. As expected, expression of endothelial ICAM-2 was unaffected by exposure to IDV, whereas DC-SIGN expression was reduced on IDV-DC.
The reduction in DC-SIGN expression on IDV-DC may limit interactions with ICAM-2, thus reducing migration across an endothelial monolayer. In support of this, we have shown that exposure of DC to IDV significantly reduced specific adhesion to immobilized ICAM-2. Importantly, antibody blockade of DC-SIGN on IDV-DC did not have an additive inhibitory effect upon binding to immobilized ICAM-2. These results indicate that IDV reduces DC-SIGN expression to a level that cannot support DC-SIGN mediated TEM. Interestingly, the decrease in the level of DC-SIGN was sustained if DC were supplemented with further doses of IDV every 16 h. This suggests that the reduction in DC-SIGN expression can be maintained if protease inhibitor is administered at periodic intervals, reflecting the clinical situation where patients receive regular doses of IDV. Our results suggest that proteases play an important role in the surface expression of DC-SIGN on DC that has not been previously reported.
Intriguingly, the level of inhibition of TEM caused by blockade of DC-SIGN was not as great as that induced by IDV, indicating that other molecules were also involved. Fibronectin is an extracellular matrix protein that forms part of the basement membrane underlying endothelial cells in vivo. Leukocytes that have migrated through the endothelium bind to fibronectin and other extracellular matrix proteins via the β1-integrins 22. DC express the major ligands for fibronectin, the β1-integrins VLA-4 and VLA-5 23. Surface levels of VLA-4 and VLA-5 remain constant after chemokine receptor activation, but they undergo a protease-dependent conformational change that increases binding affinity for fibronectin 20. Chemokine binding to its receptor initiates a cascade of signaling events that activates proteases such as calpain, that ultimately enables the conformational change of integrins 15, 24. This enables high affinity binding to its ligand and facilitates the multistep process involved in leukocyte TEM 25.
IDV-DC showed significantly reduced migration across fibronectin but not across bare transwells. This suggested that IDV inhibited specific interactions between DC and fibronectin. As expected, surface levels of VLA-4 and VLA-5 expressed upon DC were not reduced by IDV. However, the capacity of IDV-DC to bind to fibronectin was reduced. This may be attributable to inhibition of the protease-dependent, high-affinity conformational change of VLA-4 and VLA-5 by IDV. In addition, we were able to determine that VLA-4 and VLA-5 were affected by IDV since blockade of IDV-DC with mAb did not further inhibit static binding to fibronectin or trans-fibronectin migration. It has been shown that the co-ordination of β1-integrin interactions with fibronectin is a sequential process where either VLA-4 or VLA-5 are engaged at any particular point 15, 25. These studies may explain why no additional reduction in migration or adhesion to fibronectinwas observed using combined blockade of VLA-4 and VLA-5. The reduction in interactions resulting from blockade of VLA-4 and VLA-5 was greater in static adhesion assays to fibronectin than observed in trans-fibronectin migration. The static adhesion assay represents a snapshot of the direct interaction between VLA-4 and VLA-5 with fibronectin, whereas the trans-fibronectin migration assay is a dynamic process.
The results of this study demonstrate that IDV inhibits DC migration across endothelial monolayers and fibronectin-coated transwells toward the inflammatory CCR5-binding chemokine, RANTES. Phenotypic analysis demonstrated that CCR5 expression was not down-regulated on IDV-DC. Furthermore, the chemotactic response to RANTES was similar for both IDV-DC and NT-DC across bare transwells, suggesting that IDV did not inhibit CCR5-mediated chemotaxis. Therefore, the migration of DC appears to be inhibited following exposure to IDV through reduced DC-SIGN expression and disruption of VLA-4and VLA-5 interactions with fibronectin. Thus, DC in HIV-infected individuals undergoing HAART may have altered migratory function.
We have previously demonstrated that immature DC migrate in response to R5-HIV, suggesting that DC may be directed to a site of HIV infection along virion gradients 26. HIV virions are able to infect cells expressing CD4 and the chemokine co-receptors CCR5 or CXCR4, and can attach to DC-SIGN to extend virion lifespan 6. Therefore, DC are candidates for HIV-1 infection and dissemination as they express these receptors throughout their lifespan. DC that are infected or carrying HIV-1 upon their surface may infect CD4 T cells, ultimately leading to lower CD4 T cell numbers. The results from our study suggest that the IDV-induced down-regulation of DC-SIGN expression may inhibit DC TEM, impairing dissemination of HIV-1 by a cell that can uniquely activate naive CD4 T cells.
An increase in CD83 expression was detected in IDV-DC without the corresponding increases in costimulatory molecule expression, loss of CCR5, and up-regulation of CXCR4 that would be expected on matured DC 4. Gruber et al. 27 reported that IDV did not increase CD83 expression on monocyte-derived DC. A reason for this difference compared with our results, may be that IDV was added to culture medium at 15 μg/ml 7 days prior to the experiments. We have clearly demonstrated that IDV activity is diminished after 24 h and needs to be readministrated at least every 16 h to maintain increased expression of CD83. Elevated levels of CD83 may be of biological importance in response to viral infection since its expression has been reported to bedown-regulated by herpes simplex and pox viruses 28. However, the precise function of CD83 has not been determined.
CD83 may be an adhesion molecule that binds monocytes, T cells 29 and DC 30. Expressed on the surface of thymic DC, it may be crucial for the development and generation of CD4 T cells 31. It has also been proposed that an increase in CD83 expression may enhance CD4 T cell production 32. Interestingly, recent studies suggest that membrane-bound CD83 may be proteolytically cleaved to give soluble CD83 33. Exposure of DC to soluble CD83 may inhibit their ability to stimulate T cells 30. The results in our study suggest that the increase in surface CD83 expression may result from IDV inhibiting its proteolytic cleavage, leading to accumulation of CD83 on the cell surface. These studies suggest that IDV may enhance CD4 T cell production not only by inhibiting viral replication, but also by inhibiting the relevant DC proteases and increasing surface expression of CD83, e.g. on thymic DC.
In summary, HAART regimens play a major role in successfully controlling HIV-1 replication, although it is evident that protease inhibitors may have nonspecific effects on host protease activity. The data suggest that DC from individuals on a HAART regime containing IDV may express lower levels of DC-SIGN and have impaired VLA-4 and VLA-5 activity. Thus, the action of IDV on host proteases that may be critically involved in regulating DC TEM and may alter the capacity of DC to perform their normal immune functions.
4 Materials and methods
4.1 Preparation of DC
Human monocyte-derived DC were prepared using an established method 34 from healthy individuals or from for HIV-1-infected subjects undergoing IDV anti-retroviral therapy (Churchill Hospital, Oxford). Briefly, monocytes isolated from peripheral blood mononuclear cells were cultured with IL-4 and GM-CSF and immature DC were used between days 5–8.
IDV sulfate (IDV, Crixivan, Merck Sharpe & Dohme Ltd., GB) was dissolved in PBS and Pepstatin A (Sigma, GB) dissolved in ethanol (0.0003% v/v). The therapeutic concentration of IDV (7.7 μg/ml, 12.5 μM) was based upon Cmax values (steady state concentrations present in the blood from standard administered doses) from patients receiving 200 mg IDV four times a day.
4.2 Endothelial and DC phenotype
DC and endothelial cells were washed twice with 0.5% BSA/PBS. For surface staining, 105 cells were incubated with primary antibody for 40 min at 5°C washed three times and secondary FITC-conjugated antibody (Dako Ltd., Denmark) was added, and incubated as above. Expression was detected by flow cytometry and analyzed using FACSort (Becton Dickinson Immunocytometry System, GB). DCwere phenotyped with antibodies against CD83, CD25, CD40, LFA-1, Mac-1, p150/95 (Dako), CD80, CD86, (Ancell, Switzerland), VLA-4 (R&D Systems, Abingdon, GB), VLA-5, conformation sensitive CD29 (Chemicon International, Inc., CA), DC-SIGN (a kind gift from Dr. Marco Colonna, Basel Institute, Switzerland), CCR5, CXCR4 (PharMingen, CA) and ICAM-1 (British Biotech. Ltd., Oxford, GB). Isotype controls (IgG1, IgG2a, IgG2b) were obtained from (Dako). Soluble CD83 was detected in cultured DC supernatant using anti-CD83 recognizing the external N terminus (Novo Castra, GB) and biotinylated CD83 mAb recognizing the external C terminus (Biosciences, GB), in a quantitative, sandwich, asymmetrical ELISA.
Endothelial cells were phenotyped for HLA class I (Dako), HLA-DR (Coulter Immunology, FL), ICAM-1, CD31 and VCAM-1 (British Biotech). Endothelial cells were activated using 200 U/ml TNF-α (R&D Systems) for 6 h and 10 ng/ml IFN-γ for 72 h (R&D Systems).
4.3 Migration of DC across ‘bare’ transwells to chemokines
DC migration assays were performed under the optimized conditions previously described 4. Briefly, 5×104 DC were added in the upper transwell chamber (Transwell System, 5 μm pore, Costar, Cambridge, MA), and migration to chemokine across the polycarbonate membrane was assessed following 2-h incubation at 37°C. The number of migrated cells was quantified by FACSort flow cytometry (Becton Dickinson Immunocytometry System, CA). The rate of acquisition was standardized using 5×104 fluorescent beads as internal controls (7.6 μm CC3F/PE/PE-CY5 beads, Flow Cytometry Standards Corp., San Juan).
4.4 Transendothelial and trans-fibronectin migration
DC migration across fibronectin and endothelial monolayers was performed under optimized conditions previously described using the transwell method 4. Fibronectin-coated transwells were prepared by incubating bare transwells with 50 μg/ml fibronectin, (Boehringer Mannheim, Lewes, GB) for 45 min at room temperature. These transwells were used for trans-fibronectin migration. TEM assays were set up by seeding 5×104 human umbilical vein endothelial cells (HUVEC; Bio-Whittaker, MA) grown in supplemented EBM-2 medium (Bio-Whittaker) onto fibronectin-coated transwells and incubated for approximately 3 days to enable confluency. The integrity of endothelial cell monolayers was determined using [14C]mannitol (Amersham, GB) equilibration as previously described 35. For migration across HUVEC, 2×105 DC were added to the transwell; for migration across fibronectin 5×104 DC were used. Blocking mAb against β1 integrin (25 μg/ml; Chemicon International), β2 integrin (25 μg/ml; Ancell) and DC-SIGN (20 μg/ml) were used at room temperature in the treatment of DC.
4.5 Static adhesion assays
DC adhesion to both immobilized fibronectin and ICAM-2 was performed using an established method with minor modifications 36. Briefly, 96-well flat-bottom plates (Greiner, GB) were coated with 50 μl fibronectin (50 μg/ml; Boehringer Mannheim, GB) overnight at 5°C, and then incubated with 1% Tris/BSA buffer (pH 8.0) for 30 min at 37°C prior to the assay. For immobilization of ICAM-2, 50 μl goat anti-human Fc-specific F(ab′)2 (4 mg/ml; Serotec, GB) was incubated for 1 h at 37°C and blocked with 1% Tris/BSA buffer for a further 30 min. TheICAM-2 plate was coated overnight at 5°C with 500 ng/ml recombinant human ICAM-2/Fc chimera and then incubated with 1% Tris/BSA for 30 min before the assay was performed.
DC were pre-treated with optimal blocking concentrations of α-VLA-4 (2 μg/ml), α-VLA-5 (20 μg/ml) or α-DC-SIGN (20 μg/ml), for 15 min at room temperature. DC (4x104) were then aliquoted into each well with 6 nM RANTES and allowed to adhere for 30 min at 37°C. Nonadherent cells were removed by six washes with warm Tris/BSA buffer, quantitated by flow cytometry and the percentage adherent cells calculated.
4.6 Statistics
Two-tailed Student's t-tests were performed to determine significant differences (p<0.05) between experimental groups.
Acknowledgements
This work was supported by the Medical Research Council (G9608278), UK and the Elizabeth Glaser Pediatric AIDS Foundation (PAF), USA. S. R. J. is an Elizabeth Glaser Scientist of the PAF.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




