International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short-term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial
Abstract
Aims
The implications of geographical variation are unknown following adjustment for hospital length of stay (LOS) in heart failure (HF) trials that included patients whether or not they had systolic dysfunction. We investigated regional differences in an international acute HF trial.
Methods and results
The PROTECT trial investigated 2033 patients with acute HF and renal dysfunction hospitalized at 173 sites in 17 countries with randomization to rolofylline or placebo. We grouped enrolling countries into six regions. Baseline characteristics, in-hospital management, and outcomes were explored by region. The primary study outcome was 60-day mortality or cardiovascular/renal hospitalization. Secondary outcomes included 180-day mortality. Of 2033 patients, 33% were from Eastern Europe, 19% from Western Europe, 16% from Israel, 15% from North America, 14% from Russia, and 3% from Argentina. Marked differences in baseline characteristics, HF phenotype, in-hospital diuretic and vasodilator strategies, and LOS were observed by region. LOS was shortest in North America and Israel (median 5 days) and longest in Russia (median 15 days). Regional event rates varied significantly. Following multivariable adjustment, region was an independent predictor of the risk of mortality/hospitalization at 60 days, with the lowest risk in Russia (hazard ratio 0.39, 95% confidence interval 0.23–0.64 vs. Western Europe) due to lower rehospitalization; mortality differences were attenuated by 180 days.
Conclusions
In an international HF trial, there were differences in baseline characteristics, treatments, LOS, and rehospitalization amongst regions, but little difference in longer term mortality. Rehospitalization differences exist independent of LOS. This analysis may help inform future trial design and should be externally validated.
Introduction
Clinical trials are often conducted globally to reduce timelines and costs, and satisfy regulatory authorities.1 However, concerns exist regarding the impact of regional differences in patient characteristics, medical practice patterns, trial conduct, and outcomes.2, 3 The generalizability of study results to patient populations without significant representation in clinical trials has also been questioned.4 Even though randomized clinical trials have rigorous entry criteria, geographic variation in patient characteristics and outcomes has been demonstrated in several heart failure (HF) trials.5-8 Many of these studies did not include adjustment for length of stay (LOS). LOS for acute HF hospitalization differs by global region and has been associated with patient outcomes.9, 10 Furthermore, most of the worldwide trials have been performed in patients with decreased LVEF, but much less is known about the geographical variation in trials of acute HF that included patients whether or not they had systolic dysfunction. The PROTECT trial was a placebo-controlled, randomized study of the A1-adenosine receptor antagonist rolofylline in patients admitted with worsening HF that was conducted in 17 countries.11 We explored the international differences among patients participating in PROTECT, and whether these differences influenced clinical outcomes.
Methods
Patient population
The international PROTECT trial enrolled 2033 patients admitted to hospital with acute HF and mild or moderate renal impairment. The design and results of PROTECT have been published.11, 12 Briefly, patients were randomly assigned to the i.v. administration of the A1-receptor antagonist rolofylline or placebo. Death from any cause or rehospitalization for cardiovascular or renal causes through day 60 was a pre-specified secondary endpoint. Vital status was also assessed at 180 days. The investigation conforms with the principles outlined in the Declaration of Helsinki. The PROTECT study was approved by the appropriate regulatory authorities and Ethics Committees prior to patient enrolment, and written informed consent was obtained from each patient before entry (ClinicalTrials.gov numbers, NCT00328692 and NCT00354458).
Data collection and definitions
Patients were enrolled from 2007 to 2009. For the purpose of this analysis, we grouped enrolling countries into six global regions: North America (Canada and the USA), Israel, Russia, Western Europe (Belgium, France, Germany, Italy, The Netherlands, Sweden, and the UK), Eastern Europe (Czech Republic, Hungary, Poland, Romania, and Ukraine), and Argentina. These classifications were determined based on previous studies of the association between world region and outcome.2 For natriuretic peptide levels, a point-of-care device for measuring the level of NT-BNP was provided to study sites if needed, but measurements of >3000 pg/mL were not quantified in these circumstances. Worsening HF was defined as physician-determined assessment of worsening symptoms or signs of HF occurring >24 h after the start of study drug to day 7 or discharge, whichever occurred first, that required institution or an increase in dose of intravenous or mechanical therapy for HF. Quality of life was evaluated at the day 14 visit using the EQ5D questionnaire. The EQ5D consists of a 5-item patient assessment of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.13 A utility score was calculated from the EQ5D responses using weights estimated in a UK population using the time trade-off method,14 with a score of 1.0 representing perfect health and 0 representing death (negative utilities are also possible, representing states perceived to be worse than death). An independent Clinical Events Committee adjudicated the primary reason for rehospitalization and cause of death through day 60.
Statistical methods
Baseline characteristics of the study population were summarized by region as frequencies and percentages for categorical variables and by the medians and 25th and 75th percentiles for continuous variables. The primary outcome for the present analysis was time to death or cardiovascular/renal hospitalization from randomization to 60 days by global region. As additional endpoints, index hospital LOS and events rates for worsening HF to 7 days, time to death or hospitalization to 60 days, and death through 180 days were investigated. Kaplan–Meier estimates of the event rates were calculated over the relevant follow-up period. For the mortality and hospitalization endpoints, hazard ratios (HRs) and corresponding confidence intervals (CIs) for each region were calculated using Cox regression analysis with and without adjustment for baseline covariates. Western Europe was chosen as the reference group for presenting HRs. Adjustment covariates were identified in a previous analysis and included clinically relevant demographic (age), clinical [NYHA class, past hospitalization for HF, body mass index (BMI), systolic blood pressure (SBP), respiratory rate, heart rate, ischaemic heart disease (IHD), oedema, and rales], and laboratory values [blood urea nitrogen (BUN), sodium, albumin, creatinine, glucose, alanine aminotransferase (ALT), and white blood cell count]. The cause-specific hazard was modelled to determine the association between region and the non-fatal components of the composite endpoints. Since PROTECT outcomes were evaluated from the time of randomization in the hospital, we did not feel that it was appropriate to adjust for LOS for the 60- and 180-day endpoints because the LOS is not known at the time of randomization. Given that differences in LOS may influence event rates, we performed an additional analysis of death or rehospitalization from discharge to 30 days post-discharge and for mortality at 150 days post-discharge that also included adjustment for LOS. A P-value <0.05 was used to indicate statistical significance for all comparisons, with no adjustment for multiple comparison. All statistical analyses were performed at Duke Clinical Research Institute (Durham, NC, USA) using SAS version 9.22 (SAS Institute, Cary, NC, USA).
Results
Of 2033 patients enrolled in PROTECT, 33% were from Eastern Europe, 19% Western Europe, 16% Israel, 15% North America, 14% Russia, and 3% Argentina. The specific contributions by country are included in the Supplementary material online, Table S1. Baseline characteristics are presented in Table 1.
| Argentina (n = 55) | Israel (n = 318) | Russia (n = 283) | Eastern Europe (n = 676) | Western Europe (n = 388) | North America (n = 313) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 72 (62–78) | 76 (70–81) | 69 (60–76) | 71 (61–77) | 75 (66–82) | 67 (57 – 77) | <0.0001 |
| Male sex | 39 | 62 | 58 | 68 | 70 | 74 | 0.0005 |
| Caucasian race | 100 | 100 | 100 | 100 | 98 | 71 | <0.0001 |
| Hispanic ethnicity | 96 | 0 | 1 | 0 | 5 | 12 | <0.0001 |
| Ischaemic heart disease | 53 | 82 | 86 | 67 | 60 | 63 | <0.0001 |
| Medical history | |||||||
| Myocardial infarction | 35 | 62 | 60 | 47 | 44 | 41 | <0.0001 |
| Hypertension | 86 | 86 | 88 | 76 | 71 | 82 | <0.0001 |
| Stroke or PVD | 26 | 15 | 15 | 21 | 19 | 18 | 0.09 |
| Diabetes mellitus | 44 | 54 | 29 | 43 | 49 | 53 | <0.0001 |
| Asthma or COPD | 15 | 16 | 25 | 14 | 21 | 30 | <0.0001 |
| Atrial fibrillation/flutter | 50 | 47 | 59 | 56 | 61 | 49 | 0.0004 |
| Moderate-severe MR | 11 | 25 | 17 | 45 | 32 | 40 | <0.0001 |
| Prior PCI | 18 | 54 | 1 | 22 | 29 | 26 | <0.0001 |
| Prior CABG | 22 | 35 | 2 | 13 | 27 | 38 | <0.0001 |
| Biventricular pacing | 0 | 12 | 0 | 6 | 13 | 24 | <0.0001 |
| AICD | 2 | 15 | 0 | 9 | 21 | 44 | <0.0001 |
| LVEF, % (n = 975) | 30 (25–35.5) | 30 (23–43) | 34 (26–42) | 30 (25–40) | 30 (25–40) | 25 (20–35) | <0.0001 |
| LVEF <40% of those with a measured EF (n = 975) | 85 | 70 | 69 | 70 | 70 | 76 | 0.35 |
| NYHA class 1 month prior to admission | <0.0001 | ||||||
| 0–II | 49 | 20 | 0 | 20 | 46 | 16 | |
| III | 38 | 60 | 15 | 54 | 46 | 61 | |
| IV | 13 | 20 | 85 | 27 | 9 | 23 | |
| Hospitalization for HF previous year | 51 | 41 | 48 | 51 | 46 | 59 | 0.0006 |
| Orthopnoea at day 1 | <0.0001 | ||||||
| None | 0 | 3 | 1 | 3 | 9 | 5 | |
| One pillow | 15 | 10 | 9 | 11 | 16 | 14 | |
| Two pillows | 40 | 45 | 48 | 38 | 37 | 35 | |
| >30° | 45 | 42 | 42 | 47 | 39 | 47 | |
| Dyspnoea on exertion at day 1 | <0.0001 | ||||||
| None | 0 | 0 | 0 | 1 | 0 | 1 | |
| Mild | 2 | 2 | 0 | 1 | 6 | 8 | |
| Moderate | 42 | 48 | 1 | 40 | 44 | 55 | |
| Severe | 56 | 50 | 99 | 59 | 50 | 37 | |
| Physical examination | |||||||
| Weight, kg | 78 (65–85) | 76 (67–87) | 81 (71–94) | 78 (68–89) | 78 (69–92) | 87 (74–103) | <0.0001 |
| BMI, kg/m2 | 27 (24–30) | 28 (25–32) | 29 (25–34) | 27 (24–31) | 27 (24–31) | 29 (25–35) | <0.0001 |
| Systolic blood pressure, mmHg | 120 (110–140) | 128 (114–140) | 130 (115–140) | 125 (110–140) | 120 (110–140) | 114 (103–131) | <0.0001 |
| Pulse, b.p.m | 80 (70–91) | 72 (66–81) | 88 (76–99) | 80 (70–92) | 76 (68–87) | 76 (67–87) | <0.0001 |
| Respiratory rate, resp/min | 21 (19–23) | 22 (20–24) | 24 (22–26) | 20 (17–22) | 20 (18–24) | 20 (18–22) | <0.0001 |
| Oedema at day 1 | <0.0001 | ||||||
| 0 | 9 | 19 | 3 | 17 | 12 | 16 | |
| 1+ | 11 | 16 | 12 | 16 | 28 | 20 | |
| ≥2+ | 80 | 65 | 86 | 67 | 60 | 64 | |
| Rales at day 1 | <0.0001 | ||||||
| None | 4 | 3 | 11 | 7 | 8 | 25 | |
| <1/3 | 29 | 29 | 30 | 21 | 35 | 42 | |
| 1/3–2/3 | 56 | 51 | 55 | 60 | 49 | 31 | |
| >2/3 | 11 | 18 | 4 | 12 | 8 | 3 | |
| JVP at day 1 | <0.0001 | ||||||
| <6 cm | 0 | 13 | 9 | 15 | 13 | 7 | |
| 6–10 cm | 28 | 63 | 54 | 45 | 48 | 34 | |
| >10 cm | 71 | 23 | 38 | 40 | 39 | 59 | |
| Laboratory values on day 1 | |||||||
| Sodium, mEq/L | 138 (135–140) | 139 (137–142) | 141 (138–143) | 141 (138–143) | 139 (137–142) | 138 (135–140) | <0.0001 |
| Potassium, mEq/L | 4.0 (3.7–4.5) | 4.3 (4.0–4.7) | 4.4 (4.0–4.8) | 4.3 (3.9–4.7) | 4.2 (3.8–4.6) | 4.0 (3.6–4.4) | <0.0001 |
| Potassium >5.0 mEq/L | 9 | 10 | 16 | 10 | 7 | 5 | 0.0003 |
| BUN, mg/dL | 31 (22–38) | 32 (23–45) | 24 (19–34) | 27 (21–38) | 34 (25–47) | 33 (23–48) | <0.0001 |
| Creatinine, mg/dL | 1.5 (1.2–2.0) | 1.4 (1.1–1.9) | 1.2 (1.0–1.5) | 1.3 (1.1–1.7) | 1.5 (1.3–1.9) | 1.5 (1.2–2.0) | <0.0001 |
| eGFR, mL/min/1.73 m2a | 41 (34–56) | 42 (31–58) | 54 (43–66) | 48 (37–62) | 41 (31–54) | 43 (31–56) | <0.0001 |
| eGFR <30 mL/min/1.73 m2 | 21 | 23 | 8 | 12 | 22 | 22 | <0.0001 |
| Glucose, mg/dL | 134 (103–158) | 135 (110–178) | 119 (97–158) | 131 (105–164) | 126 (101–157) | 116 (97–154) | <0.0001 |
| ALT, U/L | 22 (16–33) | 18 (13–24) | 21 (14–32) | 21 (16–33) | 21 (15–31) | 23 (15–34) | <0.0001 |
| Albumin, g/dL | 3.8 (3.5–4.1) | 3.9 (3.6–4.2) | 3.8 (3.5–4.1) | 3.9 (3.6–4.2) | 3.9 (3.6–4.2) | 3.7 (3.5–4) | <0.0001 |
| Haemoglobin, g/dL | 12.9 (11.1–14.7) | 11.8 (10.8–13.0) | 13.5 (12.2–14.7) | 13.1 (11.9–14.4) | 12.3 (11.2–13.5) | 11.7 (10.6–13.3) | <0.0001 |
| Anaemia at baseline (Hb <12 g/dL in women and <13 g/dL in men) | 52 | 65 | 31 | 38 | 58 | 64 | <0.0001 |
| Total cholesterol, mg/dL | 148 (114–180) | 146 (121–174) | 158 (131–191) | 143 (120–173) | 137 (114–170) | 122 (100–148) | <0.0001 |
| Uric acid, mg/dL | 8.05 (6.6–10.5) | 8.3 (6.7–9.85) | 8.6 (7.1–10.3) | 8.6 (7.1–10.4) | 9.3 (7.2–11.1) | 9.5 (7.8–11.4) | <0.0001 |
| Screening BNP, pg/mL | n = 1 | n = 7 | n = 10 | n = 162 | n = 124 | n = 233 | 0.60 |
| 3000 (3000–3000) | 2382 (741–3000) | 1605 (978–2280) | 1211 (800–2432) | 1262 (729–2197) | 1288 (863–2120) | ||
| NT-proBNP, pg/mL, n (%) | 0.0003 | ||||||
| ≤2500 | 6 (11.1) | 56 (18.1) | 21 (7.7) | 54 (10.0) | 27 (10.3) | 7 (9.0) | |
| 2501–2999 | 3 (5.6) | 40 (12.9) | 21 (7.7) | 41 (7.6) | 25 (9.5) | 4 (5.1) | |
| ≥3000 | 45 (83.3) | 213 (68.9) | 231 (84.6) | 443 (82.3) | 211 (80.2) | 67 (85.9) |
- Values are presented as percentage or median (interquartile range) unless noted.
- AICD, automated implantable cardioverter defibrillator; ALT, alanine aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; Hb, haemoblobin; HF, heart failure; JVP, jugular venous pressure; MR, mitral regurgitation; PVD, peripheral vascular disease.
- aeGFR was calculated in the central laboratory using the Modification of Diet in Renal Disease (MDRD) formula.
Patients from North America tended to be younger, were more often men, and had a lower EF. North Americans had lower blood pressure and greater elevation in jugular venous pressure (JVP). Implantable cardioverter/defibrillator (ICD) and CRT as well as prior coronary artery bypass grafting (CABG) surgery were higher in North America. In North America, a higher percentage of patients had an NT-proBNP value ≥3000 pg/mL. In comparison, patients from Israel tended to be older, with more prior PCI, higher EF and blood pressure, and lower weight and device implantation.
Compared with Eastern Europeans, those from Western Europe tended to have more prior CABG and device therapy. Western Europeans also tended to have lower blood pressure and higher creatinine. Western European patients demonstrated differences from North Americans, including older age, less device use, and lower NYHA class symptoms 1 month previously.
Russian patients were more likely to have IHD, but little prior revascularization. Most patients from Russia had NYHA class IV symptoms with severe dyspnoea on exertion at baseline. They had the highest EF and tended to have the most oedema. The patients from Argentina included more women with non-IHD compared with other regions.
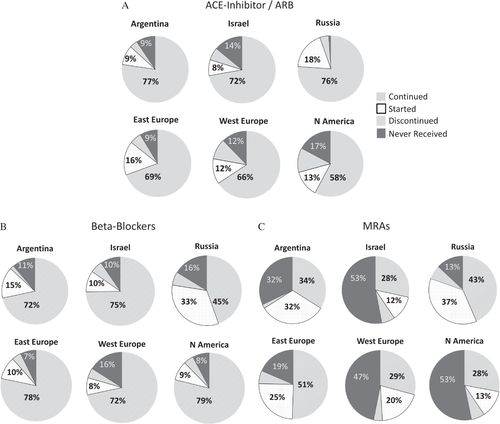
Differences in medication use by region are presented in Table 2. Two weeks prior to admission, North American patients were the most likely to be prescribed a beta-blocker, but had the lowest use of ACE inhibitor/ARB and modest mineralocorticoid receptor antagonist (MRA) use. Russian patients had the lowest beta-blocker use, but high MRA and digoxin use. Patients from North America were the most likely to receive in-hospital inotropes and had the highest total diuretic dose. Figure 2 presents medication changes from 2 weeks prior to admission to discharge/day 7 based on region for ACE inhibitors/ARBs, beta-blockers, and MRAs. Despite high baseline use of ACE inhibitors/ARBs, Russian patients had robust initiation of these therapies during hospitalization. In terms of beta-blocker use, Russia had the lowest baseline use, but a substantial proportion of the patients were initiated on the therapy during admission. For MRAs, Russia, Eastern Europe, and Argentina had the largest percentage of patients initiated on the therapy during hospitalization. Approximately 50% of patients in Israel, Western Europe, and North America did not receive MRAs at discharge/day 7. Table S2 in the Supplementary material online includes the percentage of patients receiving these therapies based on eligibility by clinical characteristics including LVEF. Increasing inpatient i.v. diuretic dose was independently associated with reduced ACE inhibitor/ARB use (odds ratio 0.95, 95% CI 0.93–0.97 for each 100 mg increase in i.v. diuretic).
| Medication | Argentina (n = 55) | Israel (N = 318) | Russia (n = 283) | Eastern Europe (n = 676) | Western Europe (n = 388) | North America (n = 313) | P-value |
|---|---|---|---|---|---|---|---|
| Medications 2 weeks prior to admission | |||||||
| ACE inhibitor/ARB | 82 | 79 | 81 | 75 | 75 | 69 | 0.02 |
| Beta-blocker | 73 | 80 | 50 | 82 | 76 | 84 | <0.0001 |
| MRA | 36 | 35 | 49 | 57 | 33 | 34 | <0.0001 |
| Nitrates | 13 | 25 | 39 | 22 | 24 | 29 | <0.0001 |
| Digoxin | 25 | 13 | 43 | 30 | 24 | 30 | <0.0001 |
| Medications between randomization and discharge or day 7 if earlier | |||||||
| Inotropic agents | 4 | 6 | 3 | 7 | 6 | 10 | 0.014 |
| Vasodilator agents | 24 | 2 | 21 | 12 | 5 | 7 | <0.0001 |
| Total i.v. loop diuretic, mga | 260 (160–399) | 260 (120–480) | 200 (120–360) | 240 (120–500) | 380 (160–800) | 400 (180–900) | <0.0001 |
| Total oral loop diuretic, mga | 200 (160–360) | 350 (200–480) | 80 (0–200) | 200 (60–360) | 210 (60–480) | 300 (40–520) | <0.0001 |
| Medications at discharge or day 7 if earlier | |||||||
| ACE inhibitor/ARB | 87 | 80 | 95 | 86 | 78 | 71 | <0.0001 |
| Beta-blocker | 87 | 85 | 78 | 88 | 79 | 88 | <0.0001 |
| MRA | 66 | 41 | 80 | 75 | 49 | 41 | <0.0001 |
| Nitrates | 10 | 16 | 26 | 15 | 19 | 33 | <0.0001 |
| Digoxin | 26 | 13 | 52 | 37 | 28 | 32 | <0.0001 |
- Values are presented as percentage or median (interquartile range).
- MRA, mineralocorticoid receptor antagonist.
- a Total diuretic dose from day 1 to earliest of day 7/discharge.
Table 3 presents the quality of life, LOS, and event rates. Table S3 in the Supplementary material online presents cause-specific readmission and death information through 60 and 180 days, respectively. Approximately 50–60% of readmissions within 60 days were for HF across world regions, except for Russia where only 33% of readmissions were for HF, and other cardiovascular causes constituted half of the remaining hospitalizations. HF was the most common cause of death through 180 days across the different regions, followed by sudden cardiac death. Specifically, death was due to HF in 36% of patients in Israel, 38% in Russia, 46% in Western Europe, 52% in North America, and 71% in Argentina. Sudden cardiac death was the cause in 0% of patients in Argentina, 10% in North America, 11% in Western Europe, 14% in Israel, 17% in Eastern Europe, and 32% in Russia.
| Argentina (n = 55) | Israel (n = 318) | Russia (n = 283) | Eastern Europe (n = 676) | Western Europe (n = 388) | North America (n = 313) | P-valueb | |
|---|---|---|---|---|---|---|---|
| EQ5D utility score (day 14) | 0.81 (0.71–1.00) | 0.68 (0.52–0.81) | 0.64 (0.52–0.81) | 0.73 (0.59–0.88) | 0.71 (0.29–0.85) | 0.75 (0.59–0.85) | <0.0001 |
| Length of stay (including in-hospital deaths) | 4 (4–6) | 5 (4–7) | 15 (14–20) | 8 (7–13) | 10 (7–15) | 5 (4–8.5) | <0.0001 |
| Death, worsening HF, rehospitalization for HF (1–7 days) | 3 (5.5) | 49 (15.6) | 25 (8.8) | 99 (14.7) | 77 (20.3) | 41 (13.2) | 0.0006 |
| Death to 7 days | 1 (1.8) | 2 (0.6) | 9 (3.2) | 17 (2.5) | 8 (2.1) | 0 (0) | 0.022 |
| Worsening HF to 7 days | 2 (3.6) | 47 (15.0) | 17 (6.0) | 89 (13.3) | 70 (18.5) | 39 (12.5) | <0.0001 |
| Rehospitalization for HF to 7 days | 1 (1.8) | 1 (0.3) | 0 (0) | 0 (0) | 2 (0.5) | 5 (1.6) | 0.006 |
| Death or CV/renal rehospitalization (1–60 days) | 9 (16.4) | 117 (36.8) | 34 (12.0) | 171 (25.3) | 117 (30.2) | 112 (35.8) | <0.0001 |
| Death or rehospitalization (1–60 days) | 11 (20.0) | 139 (43.7) | 39 (13.8) | 184 (27.2) | 143 (36.9) | 136 (43.5) | <0.0001 |
| Death to 60 days | 1 (1.8) | 23 (7.2) | 23 (8.1) | 67 (9.9) | 39 (10.1) | 22 (7.0) | 0.18 |
| CV/renal rehospitalization to 60 days | 8 (14.5) | 105 (33.0) | 12 (4.2) | 118 (17.5) | 88 (22.7) | 98 (31.3) | <0.0001 |
| Rehospitalization to 60 days | 10 (18.2) | 128 (40.3) | 18 (6.4) | 131 (19.4) | 116 (29.9) | 123 (39.3) | <0.0001 |
| Death through day 180 | 7 (12.7) | 50 (15.7) | 34 (12.0) | 125 (18.5) | 79 (20.4) | 63 (20.1) | 0.042 |
- a Presented as median (Q1–Q3) for EQ5D and length of stay; number of events (%) for the remainder of the outcomes.
- b Kruskal–Wallis test for EQ5D and length of stay; χ2 test for binary variables.
- CV, cardiovascular; EQ5D, a measure of health status from the EuroQol Group; HF, heart failure.
On unadjusted analysis, enrolment in Russia and Argentina was associated with reduced death or cardiovascular/renal rehospitalization at 60 days (Table 4). For the endpoint of death or all-cause rehospitalization at 60 days, enrolment in Russia, Argentina, and Eastern Europe was associated with reduced risk (e.g. 13.8% event rate in Russia; HR 0.32, 95% CI 0.22–0.45 vs. Western Europe), whereas enrolment in Israel and in North America was associated with the highest event rates (43.7% and 43.5%, respectively). For the endpoint of death at 180 days, only enrolment in Russia was associated with reduced risk. Figure 1 presents the unadjusted time to event for the 60- and 180-day endpoints by region.
| Western Europe (n = 388) | Argentina (n = 55) | Israel (n = 318) | Russia (n = 283) | Eastern Europe (n = 676) | North America (n = 313) | P-valueb | |
|---|---|---|---|---|---|---|---|
| Unadjusted analysis, HR (95% CI) | |||||||
| Death or CV/renal hospitalization (60 days) | 1.0 | 0.49 (0.25–0.96)a | 1.27 (0.98–1.64) | 0.35 (0.24–0.51)a | 0.80 (0.63–1.01) | 1.22 (0.94–1.58) | <0.0001 |
| Death or rehospitalization (60 days) | 1.0 | 0.48 (0.26–0.88)a | 1.25 (0.99–1.58) | 0.32 (0.22–0.45)a | 0.68 (0.55–0.85)a | 1.22 (0.96–1.54) | <0.0001 |
| Death (60 days) | 1.0 | 0.17 (0.02–1.25) | 0.70 (0.42–1.17) | 0.79 (0.47–1.33) | 0.97 (0.66–1.45) | 0.68 (0.40–1.14) | 0.22 |
| CV/renal hospitalization (60 days) | 1.0 | 0.56 (0.27–1.16) | 1.51 (1.14–2.01)a | 0.16 (0.09–0.30)a | 0.73 (0.55–0.96)a | 1.42 (1.07–1.90)a | <0.0001 |
| Rehospitalization (60 days) | 1.0 | 0.52 (0.27–1.00)a | 1.42 (1.10–1.82)a | 0.18 (0.11–0.29)a | 0.59 (0.46–0.76)a | 1.36 (1.06–1.76)a | <0.0001 |
| Death (180 days) | 1.0 | 0.58 (0.27–1.25) | 0.74 (0.52–1.05) | 0.56 (0.38–0.84)a | 0.89 (0.67–1.18) | 0.96 (0.69–1.34) | 0.047 |
| Adjusted analysisc, HR (95% CI) | |||||||
| Death or CV/renal hospitalization (60 days) | 1.0 | 0.54 (0.26–1.11) | 1.10 (0.80–1.52) | 0.39 (0.23–0.64)a | 0.78 (0.58–1.04) | 0.91 (0.66–1.26) | 0.0010 |
| Death or rehospitalization (60 days) | 1.0 | 0.52 (0.27–1.01) | 1.12 (0.84–1.49) | 0.31 (0.19–0.50)a | 0.63 (0.48–0.83)a | 0.93 (0.69–1.25) | <0.0001 |
| Death (60 days) | 1.0 | 0.23 (0.03–1.72) | 0.40 (0.19–0.81)a | 0.97 (0.45–2.09) | 1.24 (0.74–2.09) | 0.42 (0.22–0.83)a | 0.0004 |
| CV/renal hospitalization (60 days) | 1.0 | 0.57 (0.26–1.25) | 1.36 (0.95–1.93) | 0.15 (0.06–0.34)a | 0.63 (0.45–0.89)a | 1.14 (0.80–1.64) | <0.0001 |
| Rehospitalization (60 days) | 1.0 | 0.53 (0.27–1.07) | 1.30 (0.96–1.78) | 0.14 (0.07–0.29)a | 0.51 (0.37–0.69)a | 1.11 (0.80–1.53) | <0.0001 |
| Death (180 days) | 1.0 | 0.62 (0.24–1.57) | 0.59 (0.37–0.94)a | 0.80 (0.46–1.41) | 1.23 (0.85–1.78) | 0.75 (0.49–1.16) | 0.007 |
- Hazard ratios use Western Europe as a reference group.
- CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
- a P < 0.05 for comparison of HR with Western Europe.
- b P-value represents global Wald χ2 test from a Cox model of regional differences on the outcome.
- c Adjusted for blood urea nitrogen, past hospitalization for heart failure, sodium, systolic blood pressure, ischaemic heart disease, oedema, albumin, creatinine, glucose, rales, body mass index, alanine aminotransferase, age, NYHA class, respiratory rate, heart rate, and white blood cell count.
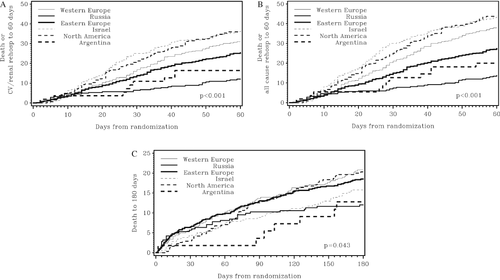
Following multivariable adjustment, region remained associated with risk of death or cardiovascular/renal rehospitalization at 60 days (P = 0.001), with enrolment in Russia being associated with a reduced risk compared with Western Europe, with no other region differing significantly from Western Europe (Table 4). In multivariable analysis of death or all-cause rehospitalization at 60 days, enrolment in Russia and Eastern Europe was associated with reduced risk, entirely due to reduced rehospitalization. Region was also associated with 180-day mortality (P = 0.007), although only Israel had a significantly lower risk compared with Western Europe. The risk of death or rehospitalization from discharge to 30 days post-discharge by region, including adjustment for LOS, was lowest in Russia and Eastern Europe (Table 5). Region was not independently associated with mortality following adjustment, including LOS, when events up until day 150 post-discharge were included.
| Western Europe (v = 388) | Argentina (n = 55) | Israel (n = 318) | Russia (n = 283) | Eastern Europe (n =676) | North America (n = 313) | P-valueb | |
|---|---|---|---|---|---|---|---|
| Unadjusted analysis, HR (95% CI) | |||||||
| Death or CV/renal rehospitalization (30 days post-discharge) | 1.0 | 0.42 (0.17–1.04) | 1.27 (0.92–1.74) | 0.26 (0.15–0.45)a | 0.64 (0.47–0.87)a | 1.19 (0.85–1.64) | <0.0001 |
| Death or rehospitalization (30 days post-discharge) | 1.0 | 0.39 (0.17–0.88)a | 1.20 (0.90–1.59) | 0.24 (0.14–0.39)a | 0.55 (0.41–0.72)a | 1.13 (0.84–1.51) | <0.0001 |
| Death (150 days post-discharge) | 1.0 | 0.80 (0.34–1.87) | 0.82 (0.53–1.26) | 0.47 (0.28–0.82)a | 0.88 (0.61–1.26) | 1.24 (0.83–1.84) | 0.0207 |
| Adjusted analysisc, HR (95% CI) | |||||||
| Death or CV/renal rehospitalization (30 days post-discharge) | 1.0 | 0.42 (0.15–1.17) | 1.20 (0.80–1.82) | 0.26 (0.12–0.56)a | 0.57 (0.39–0.84)a | 1.04 (0.68–1.59) | <0.0001 |
| Death or rehospitalization (30 days post-discharge) | 1.0 | 0.43 (0.17–1.08) | 1.17 (0.81–1.68) | 0.20 (0.10–0.41)a | 0.50 (0.35–0.71)a | 1.03 (0.71–1.51) | <0.0001 |
| Death (150 days post-discharge) | 1.0 | 0.93 (0.32–2.72) | 0.80 (0.44–1.46) | 0.63 (0.30–1.31) | 1.34 (0.82–2.20) | 1.18 (0.69–2.04) | 0.1338 |
- Hazard ratios use Western Europe as a reference group
- CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
- a P < 0.05 for comparison of HR with Western Europe.
- b P-value represents global Wald χ2 test from a Cox model of regional differences on the outcome.
- c Adjusted for blood urea nitrogen, past hospitalization for heart failure, sodium, systolic blood pressure, ischaemic heart disease, oedema, albumin, creatinine, glucose, rales, body mass index, alanine aminotransferase, age, NYHA class, respiratory rate, heart rate, white blood cell count and length of stay.
Discussion
The PROTECT trial is a unique data set in which to investigate the impact of the enrolling region on clinical characteristics, management, and outcomes of patients with acute HF and elevated natriuretic peptide levels irrespective of LVEF. We demonstrated marked differences in the co-morbidity burden, symptom complex, vital signs, laboratory test results, LOS, and outcome by region.
Regional heart failure phenotypes
In PROTECT, there appeared to be different phenotypes of patients enrolled from the various global regions. While these phenotypes oversimplify global heterogeneity, the generalizations are illustrative of regional variation. North American patients were younger with an increased burden of co-morbidities, lower blood pressure, and high usage of device therapies and surgical revascularization. Interestingly, despite higher device use and CABG rates, North American enrolment was not associated with reduced long-term mortality on adjusted analysis. In comparison, European patients were older with less surgical revascularization. The younger age of patients from North America was relatively unexpected as North Americans were older than Eastern European patients in other trials.7 The lower rate of Caucasian patients and of patients with previous myocardial infarction in North America, compared with the other areas, may have influenced these results. Western Europeans exhibited a haemodynamic and laboratory profile similar to that of North American patients, while Eastern European patients had less severe metabolic derangements. North American and Western European patients also received higher doses of loop diuretics, compared with the other areas. Those from Russia had IHD with low rates of revascularization, with prominent dyspnoea and oedema, but preserved renal function and higher EF. The severity of reported dyspnoea and NYHA symptom class in Russia could be related to regional differences in the symptom complex of patients, the patient experience, or documentation. Patients from Israel were the oldest and tended to have metabolic disease out of proportion to their weight. Argentina included more women, and the non-ischaemic aetiology is also relevant.
Clinical trial implications
These phenotypes have implications for the design and interpretation of HF trials. For instance, the global variation in the prevalence of COPD, whether related to factors such as smoking, genetic susceptibility, or case ascertainment, may affect beta-blocker use and outcomes.15, 16 COPD may influence symptoms such as dyspnoea. Differential dosage of diuretics may also influence dyspnoea, renal function, and other medication use with implications on trial endpoints. Other co-morbidities such as obesity and renal disease may impact study drug pharmacokinetics and background therapies. Additional aspects of the HF phenotype, including lower blood pressure and IHD, may affect drug tolerance or response.17 We demonstrated marked regional differences in the percentage of deaths due to sudden fatal cardiac events, ranging from 10% in North America to 32% in Russia. These findings suggest that the observed regional variations in medication use and device therapy may translate into marked differences in overall and cause-specific events. In particular, longer hospitalization may lead to improved initiation (and potentially also up-titration) of guideline-directed medical therapy. The background therapy and co-morbidity profile of the target population for a therapeutic agent should be considered when determining the potential enrolling regions for a trial.
Length of stay
The LOS varied across these regions, which might influence both rehospitalization and mortality rates. There was a three-fold difference in median LOS between North American and Russia. The impact of regional differences in LOS has recently been highlighted in patients with CAD18 and HF,10 where shorter LOS has been independently associated with increased 30-day readmission rates. The regional differences in LOS also demonstrate the complexity of analysing trial endpoints (e.g. rehospitalization) from randomization rather than discharge, since a trial participant cannot be rehospitalized if he/she remains hospitalized for the index event.
Medication differences and clinical implications
The observed differences in medication use by region inform clinical practice and trial design. Previous HF trials6, 7 and registries19, 20 have demonstrated regional variation in cardiovascular medication use even after accounting for indication. In PROTECT, North American patients had high beta-blocker usage, but low usage of ACE inhibitors and MRAs. These findings are particularly interesting in light of a recent meta-analysis demonstrating that beta-blocker use was associated with a lower magnitude of survival benefit in patients enrolled in the USA compared with other world regions.21 Western Europe demonstrated similar baseline medication use compared with North America, while Eastern Europe and Russia tended to have higher use of MRAs. Importantly, the evidence for these medications is restricted to those with reduced EF. While multiple factors, including blood pressure and renal disease, probably contributed to the underuse of these therapies in certain regions, these observations indicate that further opportunities remain to improve the medical care for HF patients. Regional differences in public policy and medication acquisition (e.g. public vs. private payment) may also play a role in these observations. Thus, information from national and regional registries may help to identify public health issues within different populations which can then be targeted with performance improvement initiatives in order to improve patient outcomes. For instance, preventing ventricular arrhythmias and improving LV synchrony with device therapy may represent key targets in developing countries.
In-hospital medication changes
Russia demonstrated the largest increases in the use of evidence-based HF therapies for reduced EF from admission to discharge, despite having high baseline use of MRAs and ACE inhibitors. The hospitalized setting may be an ideal time to initiate these therapies, since patients can be closely observed for adverse effects. In addition, the high usage of digoxin in Russia is notable given data suggesting that digoxin may improve outcomes in high-risk HF subgroups.22 These data suggest a different strategy of acute HF management in Russian patients characterized by longer hospitalization with more vasodilator use and less total diuretic use. Previous studies have suggested similar differences in treatment characteristics by world region.23 This lack of connection between vasodilator use to target acute (and potential rapidly reversible pathophysiology) and increased LOS demonstrates how multiple factors including both clinical and cultural characteristics determine admission duration. In an analysis of the association between inpatient HF medications (adjusting for regional differences), increasing diuretic dose was independently associated with reduced ACE inhibitor/ARB use. Thus, inpatient management strategies may have an association with discharge medication use.
Outcomes
The 60-day event rates varied across the regions. For the outcome of death or cardiovascular/renal hospitalization at 60 days, patients enrolled in Russia had a 61% reduction in risk compared with Western Europe, whereas no significant difference was found between the other geographical areas, at multivariable analysis. Similarly, a previous analysis of the EVEREST trial demonstrated that in Eastern Europe there was a lower risk of cardiovascular mortality or HF hospitalization relative to North America due to fewer rehospitalizations.7 These observations may have been due to less severe HF, on average, in these populations despite adjustment for major covariates. Alternatively, the various regional approaches to acute HF management characterized by differential use of diuretics and vasodilators over several days to weeks along with heterogeneity in the use of chronic HF medications may impact outcomes. In fact, after adjusting for LOS, the relative risk for short-term death or cardiovascular/renal hospitalization in Russia was reduced from 61% to 74% lower risk compared with Western Europe (at 60 days post-randomization and 30 days post-discharge, respectively). Thus, LOS may have an association with outcomes, but regional differences provide independent information related to outcomes.
For 180-day mortality, only patients enrolled in Israel had a lower risk, and adjustment for LOS attenuated this association. A prior propensity-matched analysis of the EPHESUS trial (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) investigating transatlantic variation in HF outcomes in post-myocardial infarction patients with EF ≤40% also demonstrated similar mortality risk between regions.8 Thus, following risk adjustment, rehospitalization rather than mortality appears to be the driving factor for geographical differences in composite outcomes. This is consistent with recent data showing a poor correlation between rehospitalizations and mortality.24-26
Limitations
The limitations of this analysis include its retrospective design and relatively small regional sample sizes. Since the selection of sites for trial enrolment reflects organizational feasibility rather than clinical epidemiology, not all world regions are represented in PROTECT. These observed differences in clinical characteristics, management strategies, and outcomes do not necessarily translate into real-world epidemiology. There is a lack of consensus about the grouping of different countries into world regions for analysis. For instance, the regional differences analysis from the EVEREST trial (n = 4133) grouped patients into four regions and had different overall regional contributions: 13.6% Western Europe, 16.9% South America, 30.3% North America, and 39.2% Eastern Europe.7 Since patients enrolled in a clinical trial meet specific inclusion/exclusion criteria, they represent only a subgroup of acute HF patients and may demonstrate differences from registry cohorts. These considerations may limit generalizability and highlight the need for validation in other data sets.
Conclusion
Differences in baseline characteristics, treatment, LOS, and outcomes were observed across six global regions in PROTECT. Regional differences in rehospitalization exist independently of the duration of index hospitalization. Regional approaches to HF management characterized by different strategies of diuretic and vasodilator use and LOS may affect outcomes. Mortality at 180 days is, in contrast, much less dependent on geographical areas. These findings have implications for the design and interpretation of future trials, particularly related to patient selection criteria and choice of clinical sites and trial endpoints.
Funding
The PROTECT study was supported by NovaCardia, a subsidiary of Merck, but no extramural funding was used to support the current analysis.
Conflict of interest: M.M. and B.M.M. have received honoraria and reimbursements from NovaCardia, sponsors of the study, and from Merck, which purchased the rights to rolofylline after completion of the PROTECT pilot study. B.A.D. and G.D. are employees of Momentum Research Inc., which was contracted to perform work on the project by Merck. J.G.F.C. was on the Steering Committee for the study, served on the advisory board for MSD, and received payments for both. J.R.T. has received research funds and consulting fees from Merck and has also received research funds and consulting fees from Abbott, Amgen, Biogen Idec, Corthera, Cytokinetics, Johnson & Johnson/Scios, Novartis, Relypsa, and Solvay for research in related areas. A.A.V. has received speaker and consultancy fees from Merck. D.M.B. is an employee of Merck. M.M.G. has received institutional research support and served on a scientific Advisory Board for Merck. P.P. has received honoraria from Merck, consulting fees from Vifor Pharma and Amgen, Inc., honoraria from Vifor Pharma, and travel/accommodation expenses covered by Vifor Pharma and Amgen, Inc. C.M.O. is a consultant to Merck and Amgen. All other authors have no conflicts of interest to declare.




