Cardio-adipose tissue cross-talk: relationship between adiponectin, plasma pro brain natriuretic peptide and incident heart failure
Abstract
Aims
There is increasing evidence of cross-talk between the heart, body metabolism, and adipose tissue, but the precise mechanisms are poorly understood. Natriuretic peptides (NPs) have recently emerged as the prime candidate for a mediator. In patients with heart failure (HF), infusion of NPs increases adiponectin secretion, indicating that NPs may improve adipose tissue function and in this way function as a cardio-protective agent in HF. Accordingly we investigated the interplay between plasma adiponectin, plasma proBNP, and development of HF.
Methods and results
We prospectively followed 5574 randomly selected men and women from the community without ischaemic heart disease or HF. Plasma adiponectin and proBNP were measured at study entry. Median follow-up time was 8.5 years (interquartile range 8.0–9.1 years). During follow-up 271 participants developed symptomatic HF. Plasma adiponectin and proBNP were strongly associated (P < 0.001). Participants with increasing adiponectin had increased risk of incident HF (P < 0.001). After adjustment for confounding risk factors (including age, gender, smoking status, body mass ratio, waist–hip ratio, glucose, glycated haemoglobin, systolic and diastolic blood pressure, lipid profile, high sensitivity C-reactive protein, estimated glomerular filtration rate, and physical activity) by Cox regression analysis, adiponectin remained an independent predictor of HF: the hazard ratio (HR) per 1 standard deviation (SD) increase in adiponectin was 1.20 [95% confidence interval (CI) 1.06–1.30; P = 0.003]. However, the association vanished when plasma proBNP was included in the analysis, HR 1.08 (95% CI 0.95–1.23; P = 0.26).
Conclusions
In conclusion, plasma adiponectin and proBNP are strongly associated. Increasing plasma adiponectin is associated with increased risk of HF. However, concomitantly elevated proBNP levels appear to explain the positive association between adiponectin and risk of HF.
Introduction
Cardiac natriuretic peptides (NPs) (such as BNP and its precursors NT-proBNP and plasma proBNP) are well established biomarkers in cardiovascular disease.1 NPs are produced by the myocardium and released in response to increased mechanical load and wall stress.1 Accordingly NPs are elevated in patients with heart failure (HF) and are strong predictors of both worsening HF and mortality.2, 3
There is increasing evidence of cross-talk between the heart, total body metabolism, and adipose tissue, but the precise mechanisms are poorly understood.4 Due to its anti-inflammatory, antiatherogenic, and insulin-sensitizing effects, adiponectin is one of the most intensively studied hormones produced by adipose tissue.5 Adiponectin levels are, however, paradoxically elevated in HF and also predict a poorer outcome in this population.6 This could possibly be explained by the relationship with NPs.
Recently, NP levels were demonstrated to be independently associated with a favourable adipose profile (reduced visceral and liver fat), suggesting NPs as a candidate mediator of cardiac–adipose tissue cross-talk.7 In experimental models, NPs stimulate lipolysis, promote adipocyte browning, and increase weight loss, but data in humans are sparse.8 In a recent study, i.v. infusion of NPs was shown to increase plasma adiponectin,9 and plasma levels of adiponectin and NPs are strongly associated in patients with HF.10 In conjunction, these studies suggest that NP may function as a regulator of adipose tissue function and adiponectin secretion.
To test this hypothesis, we investigated the relationship between plasma adiponectin and proBNP in the general population, and assessed the influence of plasma proBNP on the association between adiponectin and development of HF.
Methods
Study population
The present study included 5574 men and women (20–94 years of age) without ischaemic heart disease (IHD) or HF from the 4th Copenhagen City Heart Study, a longitudinal cohort study of cardiovascular diseaseand risk factors which have been described previously.11 In total, 6035 persons were examined (participation rate 49.5% of all invited participants). We excluded individuals with prevalent IHD or HF (n = 327) as well as participants in whom data on plasma adiponectin or proBNP were missing (n = 134), leaving a total of 5574 participants Patients were followed for a median of 8.5 years [interquartile range (IQR) 8.0–9.1 years]. Follow-up information on the occurrence of HF was obtained from the highly validated Danish National Board of Health's National Patient Registry which contains data on the entire cohort [using the International Classification of Disease 10th revision (ICD-10) codes I11.0, I42.0, and I50].12 Importantly, all events identified using these registries were carefully validated using medical records (e.g. medical chart, laboratory tests, hospital summaries, etc.), excluding possibly misclassified events.
Health examination
Ischaemic heart disease was defined as a history of hospital admission due to either acute myocardial infarction, PCI, or coronary artery bypass grafting. HF was defined as a history with a hospitalization due to HF. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication. Hypercholesterolaemia was defined as use of cholesterol-lowering medicine or a total cholesterol ≥7.0mmol/L. Physically active was defined as moderate lenient physical activity above 4 h a week and/or strenuous lenient physical activity above 2 h a week. Alcohol consumption was calculated as grams of ethanol per week using standard ethanol concentrations in beer, wine, liquor, and spirits.
All subjects gave informed consent to participate, and the study was performed in accordance with the second Declaration of Helsinki and approved by the regional ethics committee.
Blood samples for measurement of plasma adiponectin and proBNP were immediately centrifuged at 3000 r.p.m. for 10 min and plasma was stored at −80°C until subsequent analysis. Total plasma adiponectin was determined by a validated in-house time-resolved immunofluorometric assay as previously described.13 All samples were analysed in duplicate, with a detection limit of 1.5 µg/L and intra- and interassay coefficients of variation of <5% and <7%, respectively. Plasma proBNP concentration was measured using a processing-independent assay (PIA). After incubation with the endoprotease trypsin (which cleaves intact proBNP and its N-terminal fragments at an arginyl residue in position 21), the proBNP 1–21 fragment was quantified with a radioimmunoassay specific for the N-terminal decapeptide. The analytical validation has been reported previously,11 but, in summary, measurements using this proBNP PIA are highly comparable with those of, for instance, the NT-proBNP assay on the Elecsys platform from Roche with a negligible systematic bias.
Other blood tests including high sensitivity C-reactive protein (hsCRP), blood glucose, glycated haemoglobin (HbA1c), lipids, and creatinine were assayed with routine laboratory methods. The estimated glomerular filtration rate was calculated on the basis of serum creatinine, age, and gender using the MDRD (Modification of Diet in Renal Disease) formula.14
Statistics
In Table 1, comparisons between groups were performed by χ2 test for dichotomous variables and Student's t-test or Mann–Whitney test for continuous variables dependent on distribution. The relationship between adiponectin and plasma proBNP was analysed with linear regression using regression splines at plasma proBNP quartiles and logarithmically transformed adiponectin and proBNP.
| Variables | No HF (n = 5303) | HF (n = 271) | P-value |
|---|---|---|---|
| Age (years)a | 57 ± 16 | 73 ± 10 | <0.001 |
| Male sex | 41.3% | 45.5% | 0.162 |
| Adiponectin (mg/L)b | 9.6 (6.8–13.7) | 11.7 (7.8–17.1) | <0.001 |
| Plasma proBNP (pmol/L)b | 17 (7–30) | 37 (20–67) | <0.001 |
| Diabetes | 7% | 15% | <0.001 |
| Blood glucose (mmol/L)a | 5.4 (4.9–6.1) | 5.7 (5.2–6.6) | <0.001 |
| Haemoglobin A1c (%)b | 5.8% (5.5–6.2%) | 6.0% (5.7–5.5%) | <0.001 |
| Hypertension | 46% | 85% | <0.001 |
| Systolic blood pressure (mmHg)a | 136 ± 23 | 155 ± 21 | <0.001 |
| Diastolic blood pressure (mmHg)a | 79 ± 12 | 81 ± 14 | 0.009 |
| Hypercholesterolaemia | 6% | 9% | 0.012 |
| Total cholesterol (mmol/L)b | 5.4 (4.7–6.3) | 5.7 (5.0–6.4) | 0.003 |
| HDL cholesterol (mmol/L)b | 1.4 (1.1–1.8) | 1.4 (1.1–1.9) | 0.853 |
| LDL cholesterol (mmol/L)b | 3.4 (2.8–4.2) | 3.5 (2.9–4.2) | 0.098 |
| Triglyceride (mmol/L)b | 1.3 (0.9–1.8) | 1.3 (1.0–1.9) | 0.062 |
| Current smoking | 33% | 33% | 0.997 |
| Physically active (lenient) | 41% | 25% | <0.001 |
| Body mass index (kg/m2) | 25.7 ± 4.3 | 27.3 ± 5.2 | <0.001 |
| Waist–hip ratio | 0.86 ± 0.09 | 0.91 ± 0.09 | <0.001 |
| High sensitivity C-reactive protein (mg/L)b | 1.4 (0.6–3.2) | 2.6 (1.3–5.7) | <0.001 |
| Creatinine (µmol/L)a | 80 ± 14 | 84 ± 21 | 0.001 |
| Estimated glomerular filtration rate (mL/min)b | 86 (68–106) | 67 (51–88) | <0.001 |
- HF, heart failure.
- Dichotomous variables are presented in %, Gaussian distributed as mean ± standard deviationa and non-Gaussian distributed as median (interquartile range)b.
The association of adiponectin with incident HF was examined by Kaplan–Meier curves and Cox proportional hazards regression analyses. Deviation from linearity was determined by simultaneous assessment of linear and quadratic effects. Evaluation of first-order interactions was carried out in the final model, adjusting for multiple testing by the Bonferroni method. Mis-specification of the functional form of the covariates and the assumption of proportional hazards were evaluated by plots of the cumulative martingale residuals. P-values <5% on two-sided tests were considered significant. All statistical calculations were performed using the SAS statistical software (SAS for Windows, release 9.2, SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
The geometric mean of plasma adiponectin was 9.8 mg/L (range 3.4–27.8) and for plasma proBNP it was 13 ng/L (range 1–178). During the 8.5 years (IQR 8.0–9.1 years) of follow-up, 271 participants (3%) developed symptomatic HF. Table 1 describes baseline characteristics according to the subsequent development of HF. As expected, participants developing HF had higher levels of plasma proBNP and adiponectin; however, they were also older, and had higher blood pressure and increased hsCRP, whereas they had a lower estimated glomerular filtration rate and were less physically active.
Plasma adiponectin and pro brain natriuretic peptide
It is well established that plasma adiponectin is dependent on age and gender, and also varies according to several metabolic disorders.15 However, we found that adiponectin was also strongly associated with plasma proBNP (r = 0.27, P < 0.001). Importantly, the use of regression splines showed that plasma proBNP contributed much more to adiponectin levels when elevated beyond the median (at log2 plasma proBNP = 4.2), which corresponds to pathological levels. After the inclusion of the significant regression spline (P < 0.001), increasing plasma proBNP below the median was still associated with increasing adiponectin (β-coefficient 0.075 per doubling of plasma proBNP, P < 0.001). The small β-coefficient shows that when plasma proBNP levels are low, it only contributes slightly to plasma adiponectin. In contrast, when plasma proBNP levels increased beyond the median, the contribution to plasma adiponectin was significantly larger (β-coefficient 0.273 per doubling of plasma proBNP, P < 0.001) (Figure 1). Importantly, this non-linear association persisted (P < 0.001) after adjustment for all baseline variables (listed in Table 1). Thus, when plasma proBNP levels are increased beyond the median, the contribution to plasma adiponectin is about four times the contribution at lower proBNP levels.

Table 2 shows the six variables contributing the most to the variation in plasma adiponectin levels. As seen, plasma proBNP explained more of the variation in plasma adiponectin than diabetes or hsCRP, and about the same as gender and visceral fat (measured by the waist–hip ratio).
| Variable | β-coefficient | Contribution to plasma adiponectin variation, % | P-value |
|---|---|---|---|
| Model | – | – | <0.001 |
| Age (per 1 year) | 0.01 | 23.0% | <0.001 |
| HDL (per 1 mmol/L) | 0.44 | 20.5% | <0.001 |
| Waist–hip ratio (per 1) | −1.35 | 11.4% | <0.001 |
| Gender (male) | −0.21 | 9.6% | <0.001 |
| Plasma proBNP (per doubling) | 0.01/0.14* | 9.0% | <0.001 |
| hsCRP (per doubling) | −0.04 | 4.4% | <0.001 |
- All variables from Table 1 were included in the model.
- All continuous variables non-Gaussian distributed including adiponectin, HDL, high sensitivity C-reactive protein (hsCRP), and plasma proBNP were entered in the model logarithmically transformed.
- *Below/above the median
Plasma adiponectin and pro brain natriuretic peptide and risk of heart failure
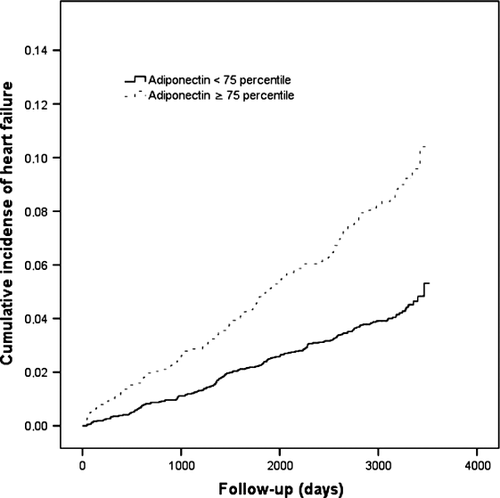
Kaplan–Meier curves demonstrated a significant association between plasma adiponectin and incident HF (above vs. below the 75th percentile, P < 0.001) (Figure 2). The result was confirmed in both univariable and multivariable Cox regression analyses. After adjustment for all baseline variables listed in Table 1 (plasma proBNP excluded), adiponectin remained a significantly independent predictor of HF; each increase of 1 standard deviation (SD) in adiponectin was associated with a hazard ratio (HR) of 1.20 [95% confidence interval (CI) 1.06–1.30; P = 0.003) (Table 3).
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Adiponectin (per SD increase) | 1.39 | 1.28–1.51 | <0.001 | 1.20 | 1.06–1.36 | 0.003 | 1.08 | 0.95–1.23 | 0.26 |
| Plasma proBNP (per SD increase ) | 1.63 | 1.56–1.70 | <0.001 | 1.40 | 1.29–1.51 | <0.001 | 1.37 | 1.27–1.49 | <0.001 |
- Model 1: univariable (adiponectin or plasma proBNP).
- Model 2: adiponectin or plasma proBNP + age, gender, current smoking, total cholesterol, HDL, LDL, triglycerides, hypertension, systolic and diastolic blood pressure, diabetes, glycated haemoglobin, blood glucose, estimated glomerular filtration rate, high sensitivity C-reactive protein, physical activity, waist–hip ratio, and body mass index.
- Model 3: Model 2 but including both adiponectin and plasma proBNP.
- CI, confidence interval; HR, hazard ratio; SD, standard deviation.
However, when plasma proBNP was included in the model, the association between adiponectin and HF vanished (P = 0.26) (Table 3). Importantly we repeated the analyses using log2-transformed adiponectin and plasma proBNP, retrieving similar results.
This finding demonstrates that plasma proBNP is a very strong confounder for the association between adiponectin and HF, and that much of the association between adiponectin and HF is explained by the relationship with plasma proBNP. In contrast, plasma proBNP was strongly associated with incident HF, in both univariable and multivariable models including adiponectin (Table 3). We tested for a possible interaction between adiponectin and plasma proBNP; however, the interaction term between adiponectin and proBNP dichotomized according to the median was non-significant (P = 0.98). No interactions with other baseline variables were found.
Discussion
The present study indicates that concomitantly elevated plasma proBNP levels may explain the positive association between plasma adiponectin and the future risk of incident HF in the general population. Our findings suggest that adiponectin may be a mediator of proBNP-related effects.
In spite of its beneficial effects (including anti-inflammatory, antiatherogenic, and insulin-sensitizing effects), several studies have documented that adiponectin levels are elevated in patients with HF.16, 17 In patients with HF, increased adiponectin levels are associated with both systolic and diastolic dysfunction,18 and reduced functional capacity,19 and increases with HF severity.20 In accordance with this, high adiponectin consistently predicts a poor prognosis in these patients.3, 21-23
It has been proposed that adiponectin is up-regulated in a counter-regulatory fashion, thus possibly reflecting disease severity. However, the mechanism through which HF stimulates adiponectin production in adipose tissue has not been clarified. To the best of our knowledge, no studies have investigated adiponectin and incident HF in a healthy population.
There is increasing evidence of cross-talk between the heart and adipose tissue, emphasizing the role of the heart in regulation of total body metabolism. NP has emerged as the prime candidate for a mediator of such a cross-talk due to its strong lipolytic effects.24 New experimental data suggest that NP may cause weight loss due to increased oxidative capacity and energy uncoupling in skeletal muscle and adipose tissue.25 In adipose tissue, NP also promotes brown adipocyte functions.26 Together, these studies indicate that NP plays an important role in regulating metabolism and adipose tissue.
Natriuretic peptide and adiponectin share several striking similarities in HF; they both show beneficial effects in experimental models and are associated inversely with visceral fat mass; however, plasma levels are elevated in HF and are associated with a poor prognosis.7 In fact, new data indicate that NP may regulate adiponectin directly.
Tsukamoto et al. demonstrated that incubation of cultured adipocytes with BNP or atrial natriuretic peptide (ANP) increased adiponectin mRNA expression and secretion.9 Furthermore, treatment with pathological concentrations of ANP in patients with HF increased plasma adiponectin, which after the end of treatment returned to pre-treatment levels.9, 27 In healthy subjects, infusion of ANP also increased plasma adiponectin.28 Recently ANP was demonstrated to activate AMP-activated protein kinase (AMPK),29 which is the main signalling pathway of adiponectin, and Birkenfeld et al.28 proposed that ANP activates AMPK through adiponectin. In experimental models, infusion of adiponectin results in weight loss by enhancing energy expenditure, and in humans adiponectin is inversely associated with body mass index (BMI) and can increase after weight loss.30 Thus in patients with HF, chronically elevated NP, through activation of AMPK, can lead to chronically elevated adiponectin, which further contributes to cardiac cachexia, a severe symptom of progressing HF.
We found a non-linear association between plasma adiponectin and proBNP, demonstrating that at pathological plasma levels, proBNP influenced plasma adiponectin levels much more than at lower, non-pathological plasma levels; however, the cause of this relationship is unknown. NP reduces sympathetic nervous activity which inhibits adiponectin secretion. Thus, in HF patients, who are characterized by increased sympathetic activity, NP stimulation of adiponectin secretion could be even larger. This could, at least partly, explain the increased contribution to plasma adiponectin when plasma proBNP becomes elevated.
The NP receptor C, known as the clearance receptor, removes NP from the circulation. Interestingly it is found in adipose tissue and is regulated by fasting.
It is possible that the clearance receptor is able to inhibit most of the signalling from NP in adipose tissue at lower plasma levels, whereas at higher plasma levels increasing activation of NP receptor A leads to increased AMPK; however, more research in this area is needed.
An independent association between adiponectin and NT-proBNP has previously been described in healthy humans.10, 31 That NPs are important for the prognostic value of adiponectin has also been shown in healthy subjects; Wannemethee et al. demonstrated an independent association between high adiponectin, CAD, and cardiovascular-related mortality; however, after including NT-proBNP in the models, the association vanished.21 We were able to extend these findings to a healthy population, demonstrating that at least a part of the positive association between adiponectin and risk of HF appears to be explained by concomitantly elevated plasma proBNP.
The clinical implications of our findings suggest that due to the ability of NPs to increase total plasma adiponectin coupled with regulation of fat mass and energy uncoupling in skeletal muscle and adipose tissue, raising NP levels could be advantageous in the management of obesity, diabetes, and the metabolic syndrome. Interestingly, in theory it is possible to increase adiponectin with neural endopeptidase inhibitors, which increase plasma levels of NP due to inhibition of degradation.32 However, to our knowledge, this has never been investigated.
Limitations and strengths
The present findings are based on an epidemiological approach including the use of nationwide registries for ascertainment of HF. Though highly validated, these findings cannot prove causality. Moreover, the aetiology of HF (CAD or not) was not established. The immunoassay used in our study to detect adiponectin has been demonstrated to measure all three major forms of adiponectin.33 In the present study, no attempts were made to measure the different molecular adiponectin forms separately; however, it is generally accepted that total adiponectin provides the most information, with regard to HF.3 Study strengths include the large number of participants and the long follow-up time.
In conclusion, plasma adiponectin and proBNP are strongly associated. Increasing plasma adiponectin is associated with increased risk of HF. However, concomitantly elevated proBNP levels appear to explain the positive association between adiponectin and risk of HF.
Acknowledgements
We would like to thank Karen Mathiassen and Hanne Pedersen, Medical Research Laboratory, Department of Clinical Medicine, Faculty of Health, Aarhus University for invaluable technical assistance.
Funding
The study was supported by Snedkermester Sophus Jacobsen og Hustru Astrid Jacobsens Foundation, the P. Carl Petersen Foundation, and the Danish Heart Foundation.
Conflict of interest: none declared.




