2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC
ESC Committee for Practice Guidelines (CPG) and National Cardiac Societies document reviewers: listed in the Appendix.:
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), European Association for Cardiovascular Prevention and Rehabilitation (EACPR), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
Councils: Council on Cardiovascular Nursing and Allied Professions, Council for Cardiology Practice, Council on Cardiovascular Primary Care, Council on Hypertension.
Working Groups: Cardiovascular Pharmacotherapy, Cardiovascular Surgery, Myocardial and Pericardial Diseases, Myocardial Function, Pulmonary Circulation and Right Ventricular Function, Valvular Heart Disease.
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to John Wiley & Sons, the publisher of the European Journal of Heart Failure and the party authorized to handle such permissions on behalf of the ESC ([email protected]).
Disclaimer: . The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and, where appropriate and/or necessary, the patient's caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
Table of contents
-
1. Preamble 3
-
2. Introduction 7
-
3. Definition, epidemiology and prognosis 8
-
3.1 Definition of heart failure 8
-
3.2 Terminology 9
-
3.2.1 Heart failure with preserved, mid-range and reduced ejection fraction 9
-
3.2.2 Terminology related to the time-course of heart failure 9
-
3.2.3 Terminology related to the symptomatic severity of heart failure 10
-
3.3 Epidemiology, aetiology and natural history of heart failure 10
-
3.4 Prognosis 10
-
4. Diagnosis 10
-
4.1 Symptoms and signs 10
-
4.2 Essential initial investigations: natriuretic peptides, electrocardiogram, echocardiography 11
-
4.3 Algorithm for the diagnosis of heart failure 12
-
4.3.1 Algorithm for the diagnosis of heart failure in non-acute setting 12
-
4.3.2 Diagnosis of heart failure with preserved ejection fraction 12
-
5. Cardiac imaging and other diagnostic tests 14
-
5.1 Chest X-ray 14
-
5.2 Transthoracic echocardiography 14
-
5.2.1 Assessment of left ventricular systolic function 14
-
5.2.2 Assessment of left ventricular diastolic function 15
-
5.2.3 Assessment of right ventricular function and pulmonary arterial pressure 15
-
5.3 Transoesophageal echocardiography 15
-
5.4 Stress echocardiography 15
-
5.5 Cardiac magnetic resonance 15
-
5.6 Single-photon emission computed tomography and radionuclide ventriculography 15
-
5.7 Positron emission tomography 15
-
5.8 Coronary angiography 16
-
5.9 Cardiac computed tomography 16
-
5.10 Other diagnostic tests 17
-
5.10.1 Genetic testing in heart failure 17
-
6. Delaying or preventing the development of overt heart failure or preventing death before the onset of symptoms 18
-
7. Pharmacological treatment of heart failure with reduced ejection fraction 19
-
7.1 Objectives in the management of heart failure 19
-
7.2 Treatments recommended in all symptomatic patients with heart failure with reduced ejection fraction 20
-
7.2.1 Angiotensin-converting enzyme inhibitors 20
-
7.2.2 Beta-blockers 20
-
7.2.3 Mineralocorticoid/aldosterone receptor antagonists 20
-
7.3 Other treatments recommended in selected patients with symptomatic heart failure with reduced ejection fraction 20
-
7.3.1 Diuretics 20
-
7.3.2 Angiotensin receptor neprilysin inhibitor 23
-
7.3.3 If-channel inhibitor 24
-
7.3.4 Angiotensin II type I receptor blockers 24
-
7.3.5 Combination of hydralazine and isosorbide dinitrate 24
-
7.4 Other treatments with less-certain benefits in patients with symptomatic heart failure with reduced ejection fraction 24
-
7.4.1 Digoxin and other digitalis glycosides 24
-
7.4.2 n-3 polyunsaturated fatty acids 25
-
7.5 Treatments not recommended (unproven benefit) in patients with symptomatic heart failure with reduced ejection fraction 25
-
7.5.1 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (‘statins') 25
-
7.5.2 Oral anticoagulants and antiplatelet therapy 25
-
7.5.3 Renin inhibitors 25
-
7.6 Treatments not recommended (believed to cause harm) in patients with symptomatic heart failure with reduced ejection fraction 26
-
7.6.1 Calcium-channel blockers 26
-
8. Non-surgical device treatment of heart failure with reduced ejection fraction 26
-
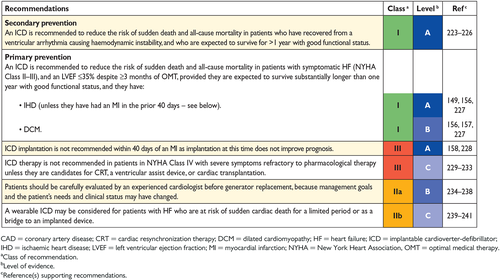
8.1 Implantable cardioverter-defibrillator 26
-
8.1.1 Secondary prevention of sudden cardiac death 26
-
8.1.2 Primary prevention of sudden cardiac death 27
-
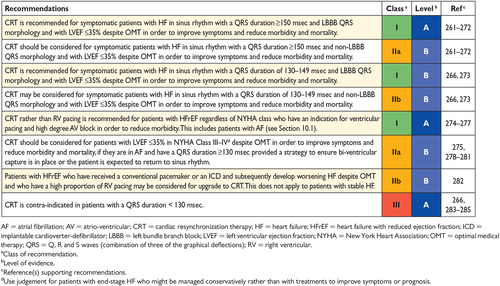
8.2 Cardiac resynchronization therapy 28
-
8.3 Other implantable electrical devices 29
-
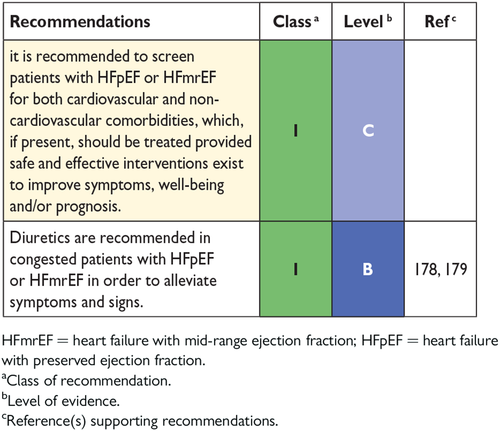
9. Treatment of heart failure with preserved ejection fraction 29
-
9.1 Effect of treatment on symptoms in heart failure with preserved ejection fraction 30
-
9.2 Effect of treatment on hospitalization for heart failure in heart failure with preserved ejection fraction 30
-
9.3 Effect of treatment on mortality in heart failure with preserved ejection fraction 30
-
9.4 Other considerations 30
-
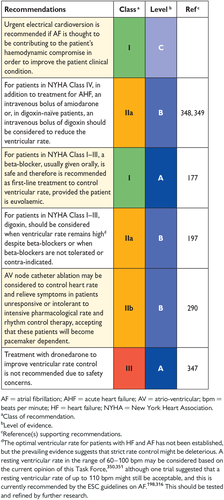
10. Arrhythmias and conductance disturbances 30
-
10.1 Atrial fibrillation 31
-
10.1.1 Prevention of atrial fibrillation in patients with heart failure 31
-
10.1.2 Management of new-onset, rapid atrial fibrillation in patients with heart failure 31
-
10.1.3 Rate control 31
-
10.1.4 Rhythm control 32
-
10.1.5 Thromboembolism prophylaxis 33
-
10.2 Ventricular arrhythmias 33
-
10.3 Symptomatic bradycardia, pauses and atrio-ventricular block 34
-
11. Co-morbidities 35
-
11.1 Heart failure and co-morbidities 35
-
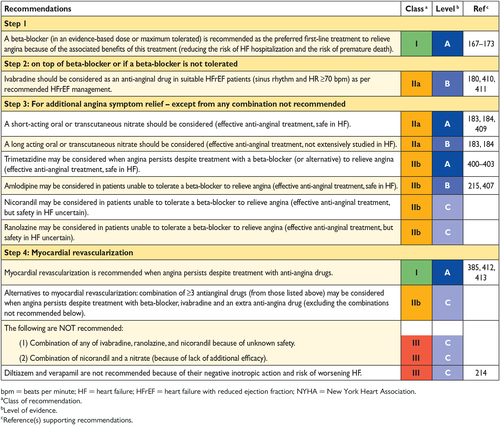
11.2 Angina and coronary artery disease 35
-
11.2.1 Pharmacological management 35
-
11.2.2 Myocardial revascularization 35
-
11.3 Cachexia and sarcopenia 36
-
11.4 Cancer 36
-
11.5 Central nervous system (including depression, stroke and autonomic dysfunction) 37
-
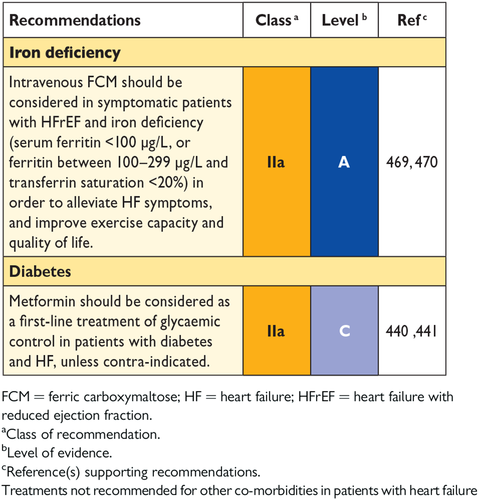
11.6 Diabetes 37
-
11.7 Erectile dysfunction 38
-
11.8 Gout and arthritis 38
-
11.9 Hypokalaemia and hyperkalaemia 38
-
11.10 Hyperlipidaemia 38
-
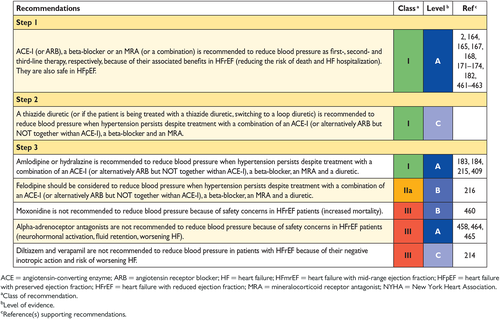
11.11 Hypertension 38
-
11.12 Iron deficiency and anaemia 39
-
11.13 Kidney dysfunction (including chronic kidney disease, acute kidney injury, cardio-renal syndrome, and prostatic obstruction) 40
-
11.14 Lung disease (including asthma and chronic obstructive pulmonary disease) 41
-
11.15 Obesity 41
-
11.16 Sleep disturbance and sleep-disordered breathing 41
-
11.17 Valvular heart disease 42
-
11.17.1 Aortic stenosis 42
-
11.17.2 Aortic regurgitation 42
-
11.17.3 Mitral regurgitation 42
-
11.17.4 Tricuspid regurgitation 42
-
12. Acute heart failure 43
-
12.1 Definition and classification 43
-
12.2 Diagnosis and initial prognostic evaluation 44
-
12.3 Management 48
-
12.3.1 Identification of precipitants/causes leading to decompensation which need urgent management 48
-
12.3.2 Criteria for hospitalization in ward vs. intensive/coronary care unit 49
-
12.3.3 Management of the early phase 49
-
12.3.4 Management of patients with cardiogenic shock 54
-
12.4 Management of evidence-based oral therapies 54
-
12.5 Monitoring of clinical status of patients hospitalized due to acute heart failure 55
-
12.6 Criteria for discharge from hospital and follow-up in high-risk period 55
-
12.7 Goals of treatment during the different stages of management of acute heart failure 55
-
13. Mechanical circulatory support and heart transplantation 56
-
13.1 Mechanical circulatory support 56
-
13.1.1 Mechanical circulatory support in acute heart failure 56
-
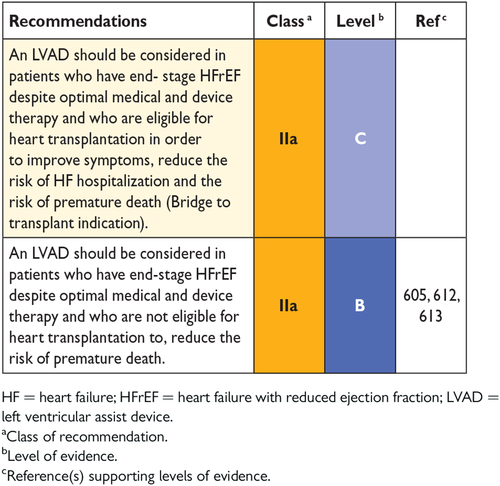
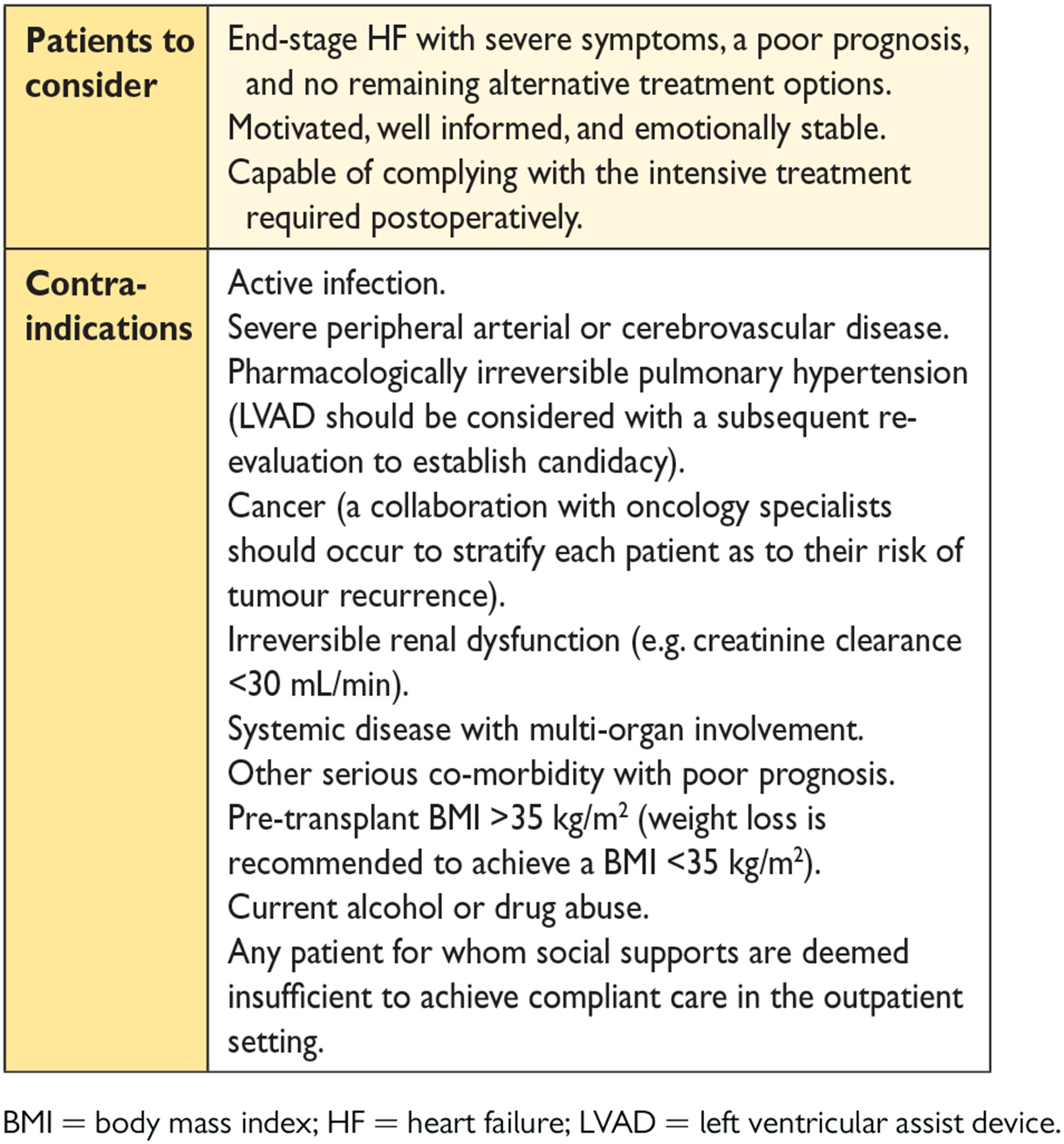
13.1.2 Mechanical circulatory support in end-stage chronic heart failure 56
-
13.2 Heart transplantation 58
-
14. Multidisciplinary team management 59
-
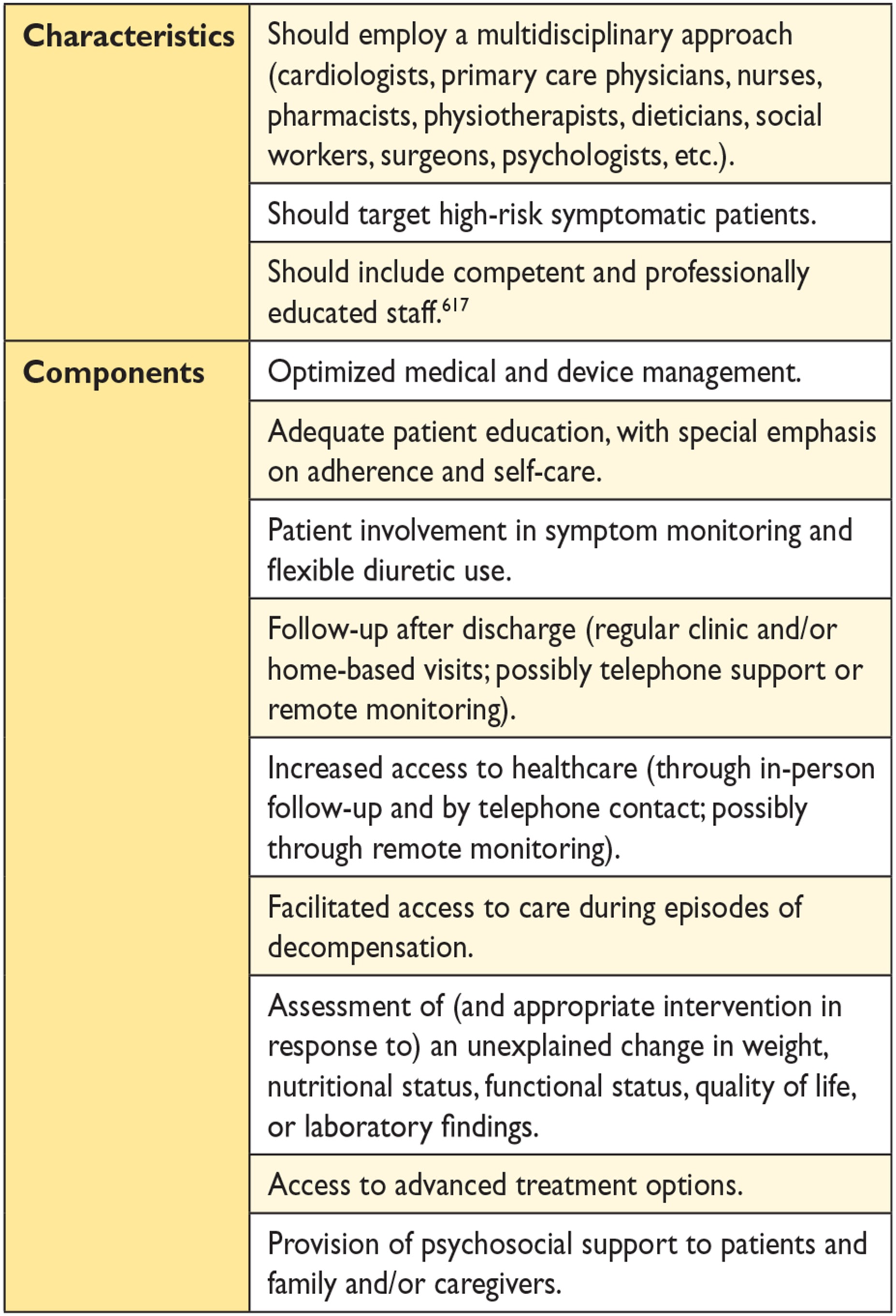
14.1 Organization of care 59
-
14.2 Discharge planning 61
-
14.3 Lifestyle advice 61
-
14.4 Exercise training 61
-
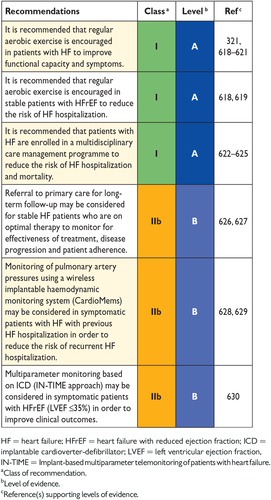
14.5 Follow-up and monitoring 61
-
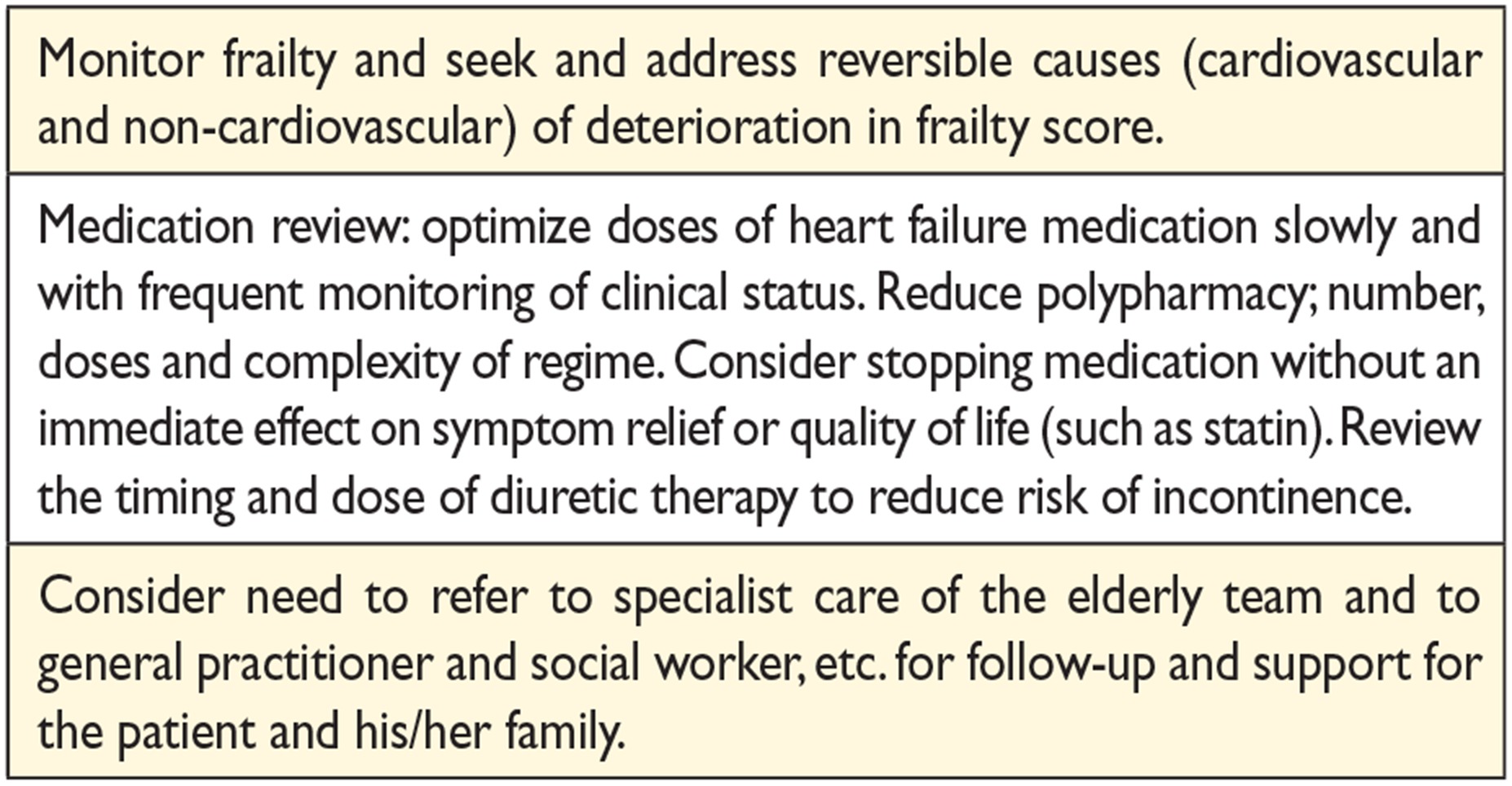
14.6 The older adult, frailty and cognitive impairment 62
-
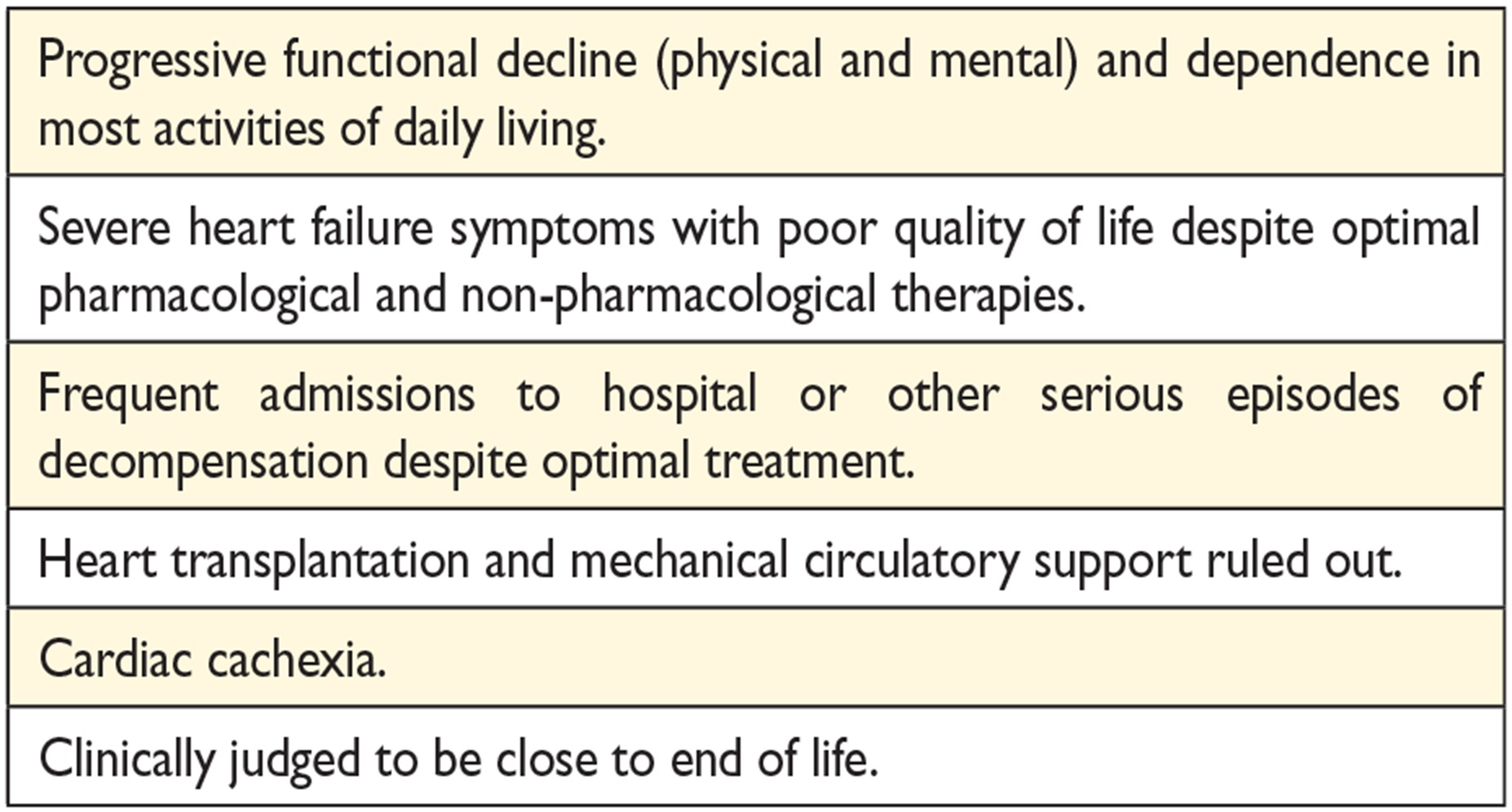
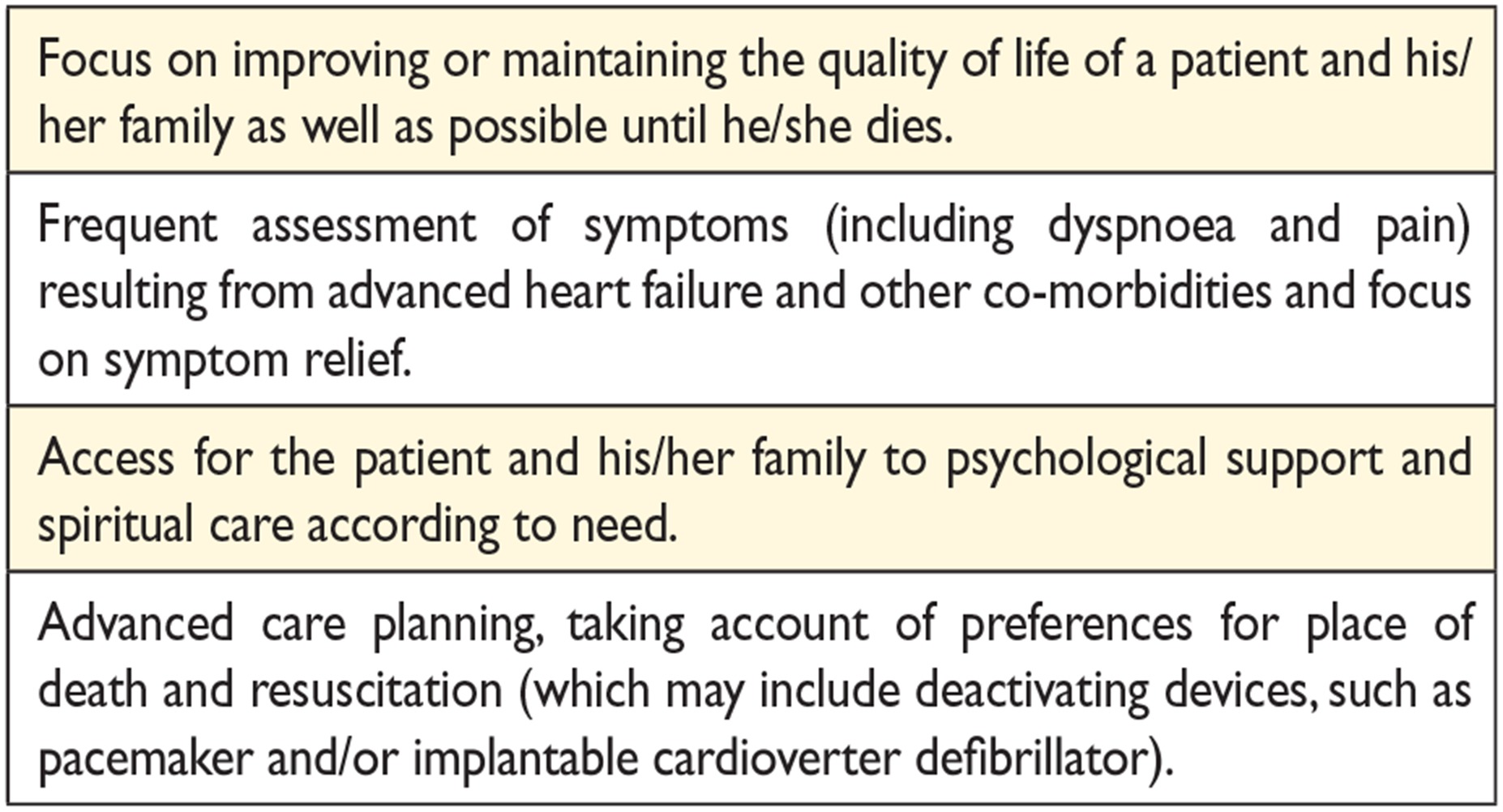
14.7 Palliative/end-of-life care 62
-
15. Gaps in evidence 63
-
16. To do and not to do messages from the Guidelines 64
-
17. Web Addenda 65
-
18. Appendix 1 66
-
19. References 66
Abbreviations and acronyms
-
- ACC/AHA
-
- American College of Cardiology/American Heart Association
-
- ACCF/AHA
-
- American College of Cardiology Foundation/American Heart Association
-
- ACE
-
- angiotensin-converting enzyme
-
- ACEI
-
- angiotensin-converting enzyme inhibitor
-
- ACS
-
- acute coronary syndrome
-
- AF
-
- atrial fibrillation
-
- AHF
-
- acute heart failure
-
- AHI
-
- apnoea/hypopnoea index
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- AKI
-
- acute kidney injury
-
- Aldo-DHF
-
- aldosterone receptor blockade in diastolic heart failure
-
- AL
-
- amyloid light chain
-
- ALT
-
- alanine aminotransferase
-
- AMI
-
- acute myocardial infarction
-
- AMICA
-
- Atrial fibrillation Management In Congestive heart failure with Ablation
-
- ANP
-
- A-type natriuretic peptide
-
- ANS
-
- autonomic nervous system
-
- ARB
-
- angiotensin receptor blocker
-
- ARNI
-
- angiotensin receptor neprilysin inhibitor
-
- ARVC
-
- arrhythmogenic right ventricular cardiomyopathy
-
- AST
-
- aspartate aminotransferase
-
- ASV
-
- assisted servo-ventilation
-
- ATLAS
-
- Assessment of Treatment with Lisinopril And Survival
-
- ATTR
-
- transthyretin-mediated amyloidosis
-
- AV
-
- atrio-ventricular
-
- AVP
-
- arginine vasopressin
-
- b.i.d.
-
- bis in die (twice daily)
-
- BioPACE
-
- Biventricular Pacing for Atrio-ventricular Block to Prevent Cardiac Desynchronization
-
- BiPAP
-
- bilevel positive airway pressure
-
- BiVAD
-
- biventricular assist device
-
- BLOCK-HF
-
- Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrio-ventricular Block
-
- BMI
-
- body mass index
-
- BNP
-
- B-type natriuretic peptide
-
- BP
-
- blood pressure
-
- bpm
-
- beats per minute
-
- BSA
-
- body surface area
-
- BTB
-
- bridge to bridge
-
- BTC
-
- bridge to candidacy
-
- BTD
-
- bridge to decision
-
- BTR
-
- bridge to recovery
-
- BTT
-
- bridge to transplantation
-
- BUN
-
- blood urea nitrogen
-
- CABANA
-
- Catheter ABlation versus ANtiarrhythmic drug therapy for Atrial fibrillation
-
- CABG
-
- coronary artery bypass graft/grafting
-
- CAD
-
- coronary artery disease
-
- CARE-HF
-
- CArdiac REsynchronization in Heart Failure
-
- CASTLE-AF
-
- Catheter Ablation versus Standard conventional Treatment in patients with LEft ventricular dysfunction and Atrial Fibrillation
-
- CCB
-
- calcium-channel blocker
-
- CCM
-
- cardiac contractility modulation
-
- CCS
-
- Canadian Cardiovascular Society
-
- CCU
-
- coronary care unit
-
- CHA2DS2-VASc
-
- Congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female)
-
- CHARM-Alternative
-
- Candesartan in heart failure assessment of reduction in mortality and morbidity
-
- CHARM-Added
-
- Candesartan Cilexetil in Heart Failure Assessment of Reduction in Mortality and Morbidity
-
- CHARM-Preserved
-
- Candesartan Cilexetil in Heart Failure Assessment of Reduction in Mortality and Morbidity
-
- CI
-
- cardiac index
-
- CI-AKI
-
- contrast-induced acute kidney injury
-
- CIBIS II
-
- Cardiac Insufficiency Bisoprolol Study II
-
- CK
-
- creatine kinase
-
- CKD
-
- chronic kidney disease
-
- CK-MB
-
- creatine kinase MB
-
- CMP
-
- cardiomyopathy
-
- CMR
-
- cardiac magnetic resonance
-
- COMPANION
-
- Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure
-
- CONFIRM-HF
-
- Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure
-
- CONSENSUS
-
- Cooperative North Scandinavian Enalapril Survival Study
-
- COPD
-
- chronic obstructive pulmonary disease
-
- COPERNICUS
-
- Carvedilol Prospective Randomized Cumulative Survival
-
- COX-2 inhibitor
-
- cyclooxygenase-2 inhibitor
-
- CPAP
-
- continuous positive airway pressure
-
- CPG
-
- Committee for Practice Guidelines
-
- CRT
-
- cardiac resynchronization therapy
-
- CRT-D
-
- defibrillator with cardiac resynchronization therapy
-
- CRT-P
-
- pacemaker with cardiac resynchronization therapy
-
- CSA
-
- central sleep apnoea
-
- CSR
-
- Cheyne-Stokes respiration
-
- CT
-
- computed tomography
-
- CYP3A4
-
- cytochrome P450 3A4
-
- DCM
-
- dilated cardiomyopathy
-
- DES
-
- desmin
-
- DHA
-
- docosahexaenoic acid
-
- DIG-PEF
-
- ancillary Digitalis Investigation Group trial
-
- DNA
-
- deoxyribonucleic acid
-
- DOSE
-
- Diuretic Optimization Strategies Evaluation
-
- DPD
-
- 3,3-diphosphono-1,2-propanodicarboxylic acid
-
- DPP4i
-
- dipeptidyl peptidase-4 inhibitor
-
- DT
-
- destination therapy
-
- e′
-
- early diastolic tissue velocity
-
- ECG
-
- electrocardiogram
-
- Echo-CRT
-
- Echocardiography Guided Cardiac Resynchronization Therapy
-
- ECLS
-
- extracorporeal life support
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- ED
-
- emergency department
-
- EF
-
- ejection fraction
-
- eGFR
-
- estimated glomerular filtration rate
-
- EHRA
-
- European Heart Rhythm Association
-
- EMA
-
- European Medicines Agency
-
- EMB
-
- endomyocardial biopsy
-
- EMF
-
- endomyocardial fibrosis
-
- EMPHASIS-HF
-
- Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure
-
- EPA
-
- eicosapentaenoic acid
-
- EPHESUS
-
- Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study
-
- ESC
-
- European Society of Cardiology
-
- EU
-
- European Union
-
- EULAR
-
- European League Against Rheumatism
-
- Ex-DHF
-
- Exercise training in Diastolic Heart Failure
-
- FACIT-Pal
-
- Functional Assessment of Chronic Illness Therapy - Palliative Care
-
- FAIR-HF
-
- Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure
-
- FCM
-
- ferric carboxymaltose
-
- FiO2
-
- fraction of inspired oxygen
-
- GFR
-
- glomerular filtration rate
-
- GGTP
-
- gamma-glutamyl transpeptidase
-
- GH
-
- growth hormone
-
- GLS
-
- global longitudinal strain
-
- GLP-1
-
- glucagon-like peptide 1
-
- HAS-BLED
-
- Hypertension, Abnormal renal/liver function (1 point each), Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly (1 point each)
-
- HbA1c
-
- glycated haemoglobin
-
- HCM
-
- hypertrophic cardiomyopathy
-
- HES
-
- hypereosinophilic syndrome
-
- HF
-
- heart failure
-
- HFA
-
- Heart Failure Association
-
- HFmrEF
-
- heart failure with mid-range ejection fraction
-
- HFpEF
-
- heart failure with preserved ejection fraction
-
- HFrEF
-
- heart failure with reduced ejection fraction
-
- H-ISDN
-
- hydralazine and isosorbide dinitrate
-
- HIV/AIDS
-
- human immunodeficiency virus/acquired immune deficiency syndrome
-
- HR
-
- heart rate
-
- Hs troponin
-
- high sensitivity troponin
-
- IABP
-
- intra-aortic balloon pump
-
- IABP-SHOCK
-
- IntraAortic Balloon Pump in Cardiogenic Shock
-
- IABP-SHOCK II
-
- IntraAortic Balloon Pump in Cardiogenic Shock II
-
- ICD
-
- implantable cardioverter-defibrillator
-
- ICU
-
- intensive care unit
-
- IHD
-
- ischaemic heart disease
-
- IL
-
- interleukin
-
- INH
-
- Interdisciplinary Network for Heart Failure
-
- INTERMACS
-
- Interagency Registry for Mechanically Assisted Circulatory Support
-
- IN-TIME
-
- Implant-based multiparameter telemonitoring of patients with heart failure
-
- IPD
-
- individual patient data
-
- I-PRESERVE
-
- Irbesartan in Heart Failure with Preserved Ejection Fraction Study
-
- i.v.
-
- intravenous
-
- IVC
-
- inferior vena cava
-
- IVRT
-
- isovolumetric relaxation time
-
- KCCQ
-
- Kansas City Cardiomyopathy Questionnaire
-
- LA
-
- left atrial/atrium
-
- LAE
-
- left atrial enlargement
-
- LAVI
-
- left atrial volume index
-
- LBBB
-
- left bundle branch block
-
- LGE
-
- late gadolinium enhancement
-
- LMNA
-
- lamin A/C
-
- LMWH
-
- low-molecular-weight heparin
-
- LV
-
- left ventricular/left ventricle
-
- LVAD
-
- left ventricular assist device
-
- LVEDP
-
- left ventricular end diastolic pressure
-
- LVEDV
-
- left ventricular end diastolic volume
-
- LVEF
-
- left ventricular ejection fraction
-
- LVESV
-
- left ventricular end systolic volume
-
- LVID
-
- left ventricular internal dimension
-
- LVMI
-
- left ventricular mass index
-
- LVSD
-
- left ventricular systolic dysfunction
-
- MADIT-CRT
-
- Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy
-
- MCS
-
- mechanical circulatory support
-
- MERIT-HF
-
- Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure
-
- MR
-
- mineralocorticoid receptor/magnetic resonance
-
- MRA
-
- mineralocorticoid receptor antagonist
-
- MR-proANP
-
- mid-regional pro A-type natriuretic peptide
-
- MV
-
- mitral valve
-
- MV A-Wave
-
- mitral valve late diastolic flow
-
- MV E-Wave
-
- mitral valve early diastolic flow
-
- MYBPC3
-
- cardiac myosin binding protein C
-
- MYH7
-
- cardiac β-myosin heavy chain
-
- n-3 PUFA
-
- n-3 polyunsaturated fatty acid
-
- NEP
-
- neprilysin
-
- NOAC
-
- non-vitamin K antagonist oral anticoagulant
-
- NP
-
- natriuretic peptide
-
- NPPV
-
- non-invasive positive pressure ventilation
-
- NSAID
-
- non-steroidal anti-inflammatory drug
-
- NSTE-ACS
-
- non-ST elevation acute coronary syndrome
-
- NT-proBNP
-
- N-terminal pro-B type natriuretic peptide
-
- NYHA
-
- New York Heart Association
-
- o.d.
-
- omne in die (once daily)
-
- OMT
-
- optimal medical therapy
-
- OSA
-
- obstructive sleep apnoea
-
- PaCO2
-
- partial pressure of carbon dioxide in arterial blood
-
- PAH
-
- pulmonary arterial hypertension
-
- PaO2
-
- partial pressure of oxygen in arterial blood
-
- PARADIGM-HF
-
- Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial
-
- PARAMOUNT
-
- LCZ696 Compared to Valsartan in Patients With Chronic Heart Failure and Preserved Left-ventricular Ejection Fraction
-
- PCI
-
- percutaneous coronary intervention
-
- PCWP
-
- pulmonary capillary wedge pressure
-
- PDE5I
-
- phosphodiesterase 5 inhibitor
-
- Peak VO2
-
- peak oxygen uptake
-
- PEP-CHF
-
- Perindopril in Elderly People with Chronic Heart Failure
-
- PET
-
- positron emission tomography
-
- PLN
-
- phospholamban
-
- PPV
-
- positive pressure ventilation
-
- PRISMA 7
-
- seven-item, self-completion questionnaire to identify older adults with moderate to severe disabilities
-
- PROTECT II
-
- Prospective, Multi-center, Randomized Controlled Trial of the IMPELLA RECOVER LP 2.5 System Versus Intra Aortic Balloon Pump (IABP) in Patients Undergoing Non Emergent High Risk PCI
-
- PS-PEEP
-
- pressure-support positive end-expiratory pressure
-
- PV
-
- pulmonary vein
-
- PVR
-
- pulmonary vascular resistance
-
- QALY
-
- quality-adjusted life year
-
- QRS
-
- Q, R, and S waves (combination of three of the graphical deflections)
-
- RA
-
- right atrium/atrial
-
- RAAS
-
- renin–angiotensin–aldosterone system
-
- RAFT
-
- Resynchronization-Defibrillation for Ambulatory Heart Failure Trial
-
- RALES
-
- Randomized Aldactone Evaluation Study
-
- RCT
-
- randomized controlled trial
-
- RELAX
-
- Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure
-
- REVERSE
-
- REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction
-
- RV
-
- right ventricular/ventricle
-
- RVAD
-
- right ventricular assist device
-
- SADHART
-
- Sertraline Antidepressant Heart Attack Randomized Trial
-
- SAVE
-
- Survival After Veno-arterial ECMO
-
- SBP
-
- systolic blood pressure
-
- SCD-HeFT
-
- Sudden Cardiac Death in Heart Failure Trial
-
- SDB
-
- sleep-disordered breathing
-
- SENIORS
-
- Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisations in Seniors with Heart Failure
-
- SERVE-HF
-
- Treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive Servo-ventilation in patients with chronic heart failure
-
- SHIFT
-
- Systolic Heart failure treatment with the If inhibitor ivabradine Trial
-
- SIGNIFY
-
- Study Assessing the Morbidity–Mortality Benefits of the If Inhibitor Ivabradine in Patients with Coronary Artery Disease
-
- SOLVD
-
- Studies of Left Ventricular Dysfunction
-
- SPECT
-
- single-photon emission computed tomography
-
- SpO2
-
- transcutaneous oxygen saturation
-
- SPPB
-
- Short Physical Performance Battery
-
- SPRINT
-
- Systolic Blood Pressure Intervention Trial
-
- STEMI
-
- ST segment elevation myocardial infarction
-
- STICH
-
- Surgical Treatment for Ischemic Heart Failure
-
- STS
-
- structured telephone support
-
- TAPSE
-
- tricuspid annular plane systolic excursion
-
- TAVI
-
- transaortic valve implantation
-
- TDI
-
- tissue Doppler imaging
-
- TECOS
-
- Trial Evaluating Cardiovascular Outcomes with Sitagliptin
-
- TEHAF
-
- Telemonitoring in Patients with Heart Failure
-
- Tele-HF
-
- Telemonitoring to Improve Heart Failure Outcomes
-
- TIA
-
- transient ischaemic attack
-
- TIBC
-
- total iron-binding capacity
-
- t.i.d.
-
- ter in die (three times a day)
-
- TIM-HF
-
- Telemedical Interventional Monitoring in Heart Failure
-
- TOE
-
- transoesophageal echocardiography
-
- TOPCAT
-
- Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
-
- TR
-
- tricuspid regurgitation
-
- TRV
-
- tricuspid regurgitation velocity
-
- TSAT
-
- transferrin saturation
-
- TSH
-
- thyroid-stimulating hormone
-
- TTE
-
- transthoracic echocardiography
-
- TTN
-
- titin
-
- ULT
-
- urate lowering therapy
-
- VAD
-
- ventricular assist device
-
- Val-HeFT
-
- Valsartan Heart Failure Trial
-
- VE-VCO2
-
- ventilatory equivalent ratio for carbon dioxide
-
- VT
-
- ventricular tachycardia
-
- VV interval
-
- interventricular pacing interval
-
- WBC
-
- white blood cells
-
- WISH
-
- Weight Monitoring in Patients with Severe Heart Failure
-
- WRF
-
- worsening renal function
1 Preamble
Guidelines summarize and evaluate all available evidence on a particular issue at the time of the writing process, with the aim of assisting health professionals in selecting the best management strategies for an individual patient with a given condition, taking into account the impact on outcome, as well as the risk–benefit ratio of particular diagnostic or therapeutic means. Guidelines and recommendations should help health professionals to make decisions in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of Guidelines have been issued in recent years by the European Society of Cardiology (ESC) as well as by other societies and organisations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
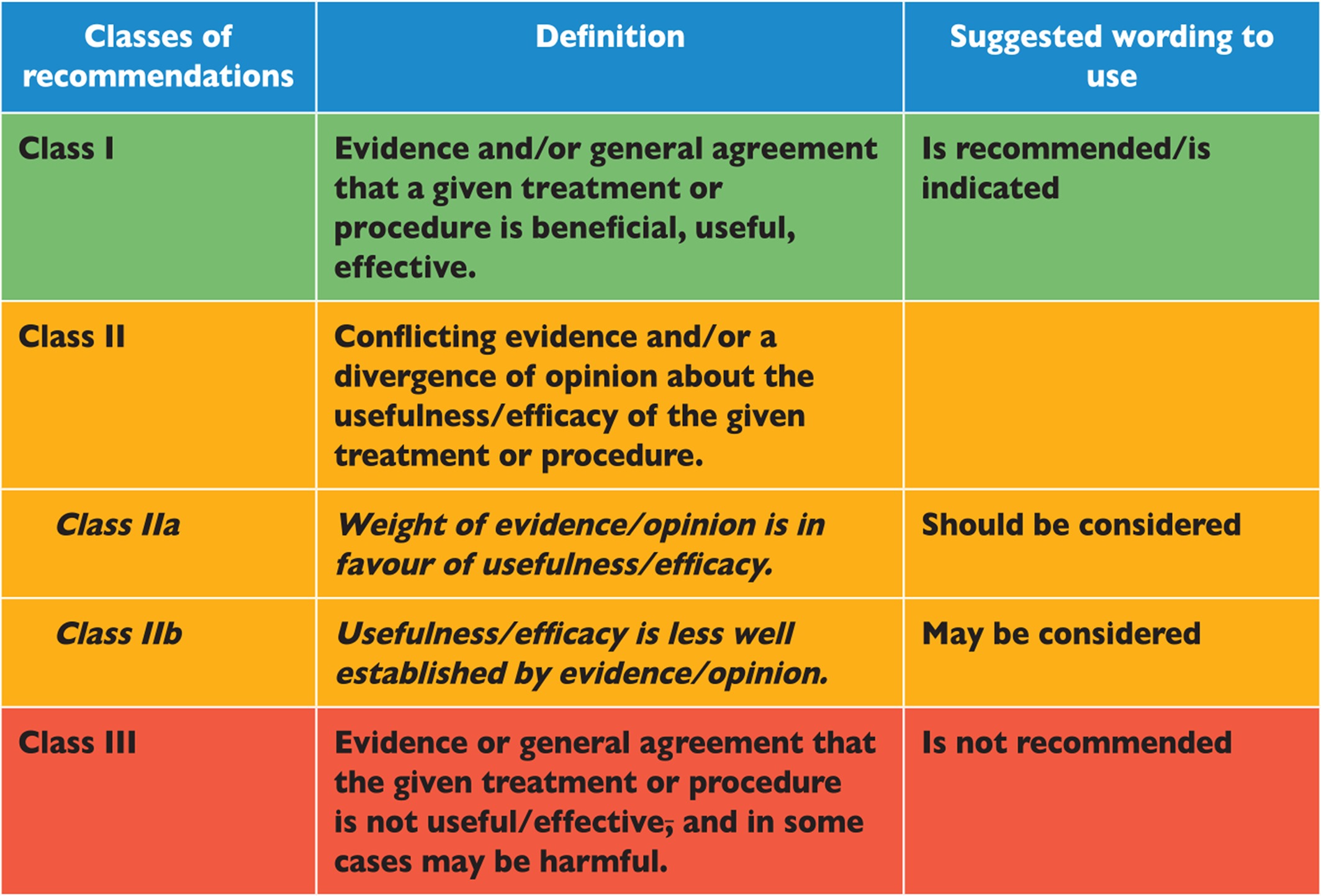
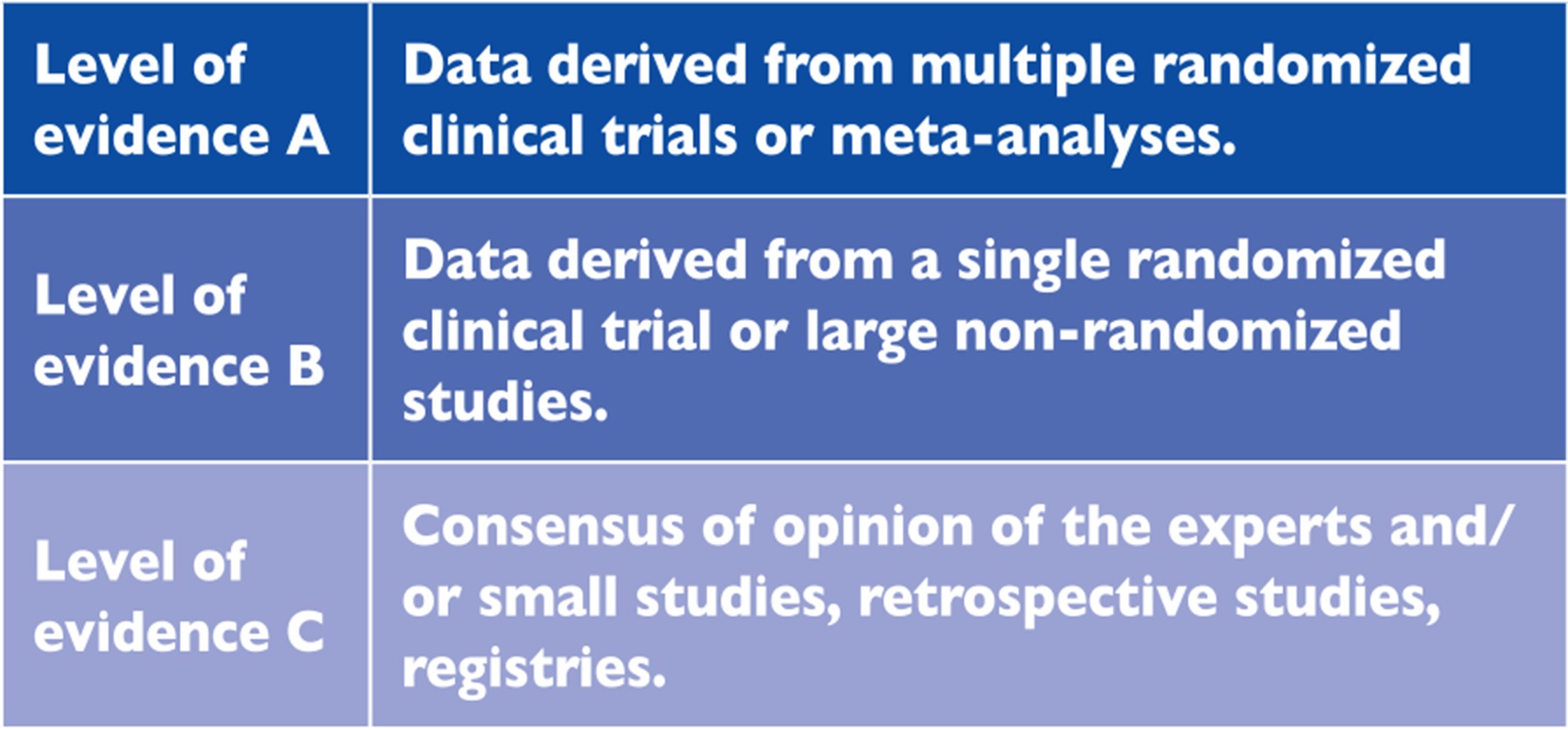
Members of this Task Force were selected by the ESC to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk-benefit ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined in Tables 1.1 and 1.2.
The experts of the writing and reviewing panels provided declarations of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period must be notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines produced by task forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines covers not only integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guidelines versions, summary slides, booklets with essential messages, summary cards for non-specialists, and an electronic version for digital applications (smartphones, etc.) are produced. These versions are abridged and thus, if needed, one should always refer to the full text version, which is freely available on the ESC website. The National Cardiac Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2 Introduction
The aim of all the ESC Guidelines is to help health professionals to make decisions in their everyday life based on the best available evidence. We will soon be celebrating the 30th anniversary of clinical trials that for the first time incontrovertibly demonstrated that the miserable outcome of patients with heart failure (HF) can be markedly improved.2 Since then, in the area of HF management we have witnessed and celebrated numerous highs, which have definitely outnumbered several lows, all of which have allowed us to unravel the pathophysiology of this clinical syndrome, but more importantly has led to better care of our patients.3 In the year 2016, no one would any longer dispute that, by applying all evidence-based discoveries, HF is now becoming a preventable and treatable disease.
-
a new term for patients with HF and a left ventricular ejection fraction (LVEF) that ranges from 40 to 49% — ‘HF with mid-range EF (HFmrEF)’; we believe that identifying HFmrEF as a separate group will stimulate research into the underlying characteristics, pathophysiology and treatment of this population;
-
clear recommendations on the diagnostic criteria for HF with reduced EF (HFrEF), HFmrEF and HF with preserved EF (HFpEF);
-
a new algorithm for the diagnosis of HF in the non-acute setting based on the evaluation of HF probability;
-
recommendations aimed at prevention or delay of the development of overt HF or the prevention of death before the onset of symptoms;
-
indications for the use of the new compound sacubitril/valsartan, the first in the class of angiotensin receptor neprilysin inhibitors (ARNIs);
-
modified indications for cardiac resynchronization therapy (CRT);
-
the concept of an early initiation of appropriate therapy going along with relevant investigations in acute HF that follows the ‘time to therapy’ approach already well established in acute coronary syndrome (ACS);
-
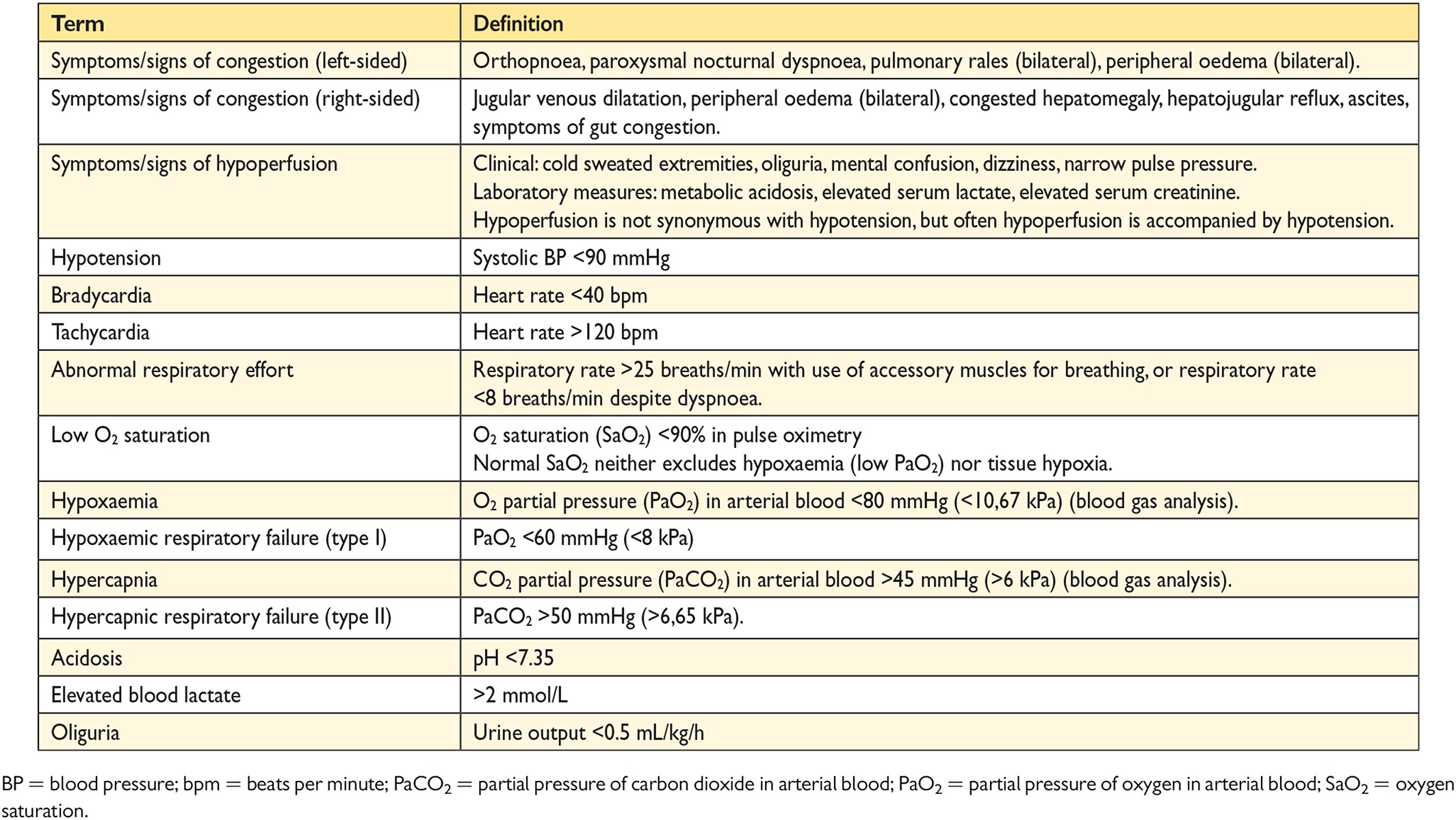
a new algorithm for a combined diagnosis and treatment approach of acute HF based on the presence/absence of congestion/hypoperfusion.
This document is the result of extensive interactions between the Task Force, the review team and the ESC Committee for Practice Guidelines. It represents a consensus of opinion of all of the experts involved in its development. Concurrently to the development of the 2016 ESC Guidelines on HF, the group writing the “2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure” independently developed its recommendations on new pharmacotherapy for Heart Failure. Both working groups/Task Force independently surveyed the evidence, arrived at similar conclusions, and constructed similar, but not identical, recommendations. Given the concordance, the respective organizations simultaneously issued aligned recommendations on the use of these new treatments to minimize confusion and improve the care of patients with HF.
3 Definition, epidemiology and prognosis
3.1 Definition of heart failure
HF is a clinical syndrome characterized by typical symptoms (e.g. breathlessness, ankle swelling and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles and peripheral oedema) caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.
The current definition of HF restricts itself to stages at which clinical symptoms are apparent. Before clinical symptoms become apparent, patients can present with asymptomatic structural or functional cardiac abnormalities [systolic or diastolic left ventricular (LV) dysfunction], which are precursors of HF. Recognition of these precursors is important because they are related to poor outcomes, and starting treatment at the precursor stage may reduce mortality in patients with asymptomatic systolic LV dysfunction4, 5 (for details see Section 6).
Demonstration of an underlying cardiac cause is central to the diagnosis of HF. This is usually a myocardial abnormality causing systolic and/or diastolic ventricular dysfunction. However, abnormalities of the valves, pericardium, endocardium, heart rhythm and conduction can also cause HF (and more than one abnormality is often present). Identification of the underlying cardiac problem is crucial for therapeutic reasons, as the precise pathology determines the specific treatment used (e.g. valve repair or replacement for valvular disease, specific pharmacological therapy for HF with reduced EF, reduction of heart rate in tachycardiomyopathy, etc).
3.2 Terminology
3.2.1 Heart failure with preserved, mid-range and reduced ejection fraction
The main terminology used to describe HF is historical and is based on measurement of the LVEF. HF comprises a wide range of patients, from those with normal LVEF [typically considered as ≥50%; HF with preserved EF (HFpEF)] to those with reduced LVEF [typically considered as <40%; HF with reduced EF (HFrEF)] (Table 3.1). Patients with an LVEF in the range of 40–49% represent a ‘grey area’, which we now define as HFmrEF (Table 3.1). Differentiation of patients with HF based on LVEF is important due to different underlying aetiologies, demographics, co-morbidities and response to therapies.6 Most clinical trials published after 1990 selected patients based on LVEF [usually measured using echocardiography, a radionuclide technique or cardiac magnetic resonance (CMR)], and it is only in patients with HFrEF that therapies have been shown to reduce both morbidity and mortality.
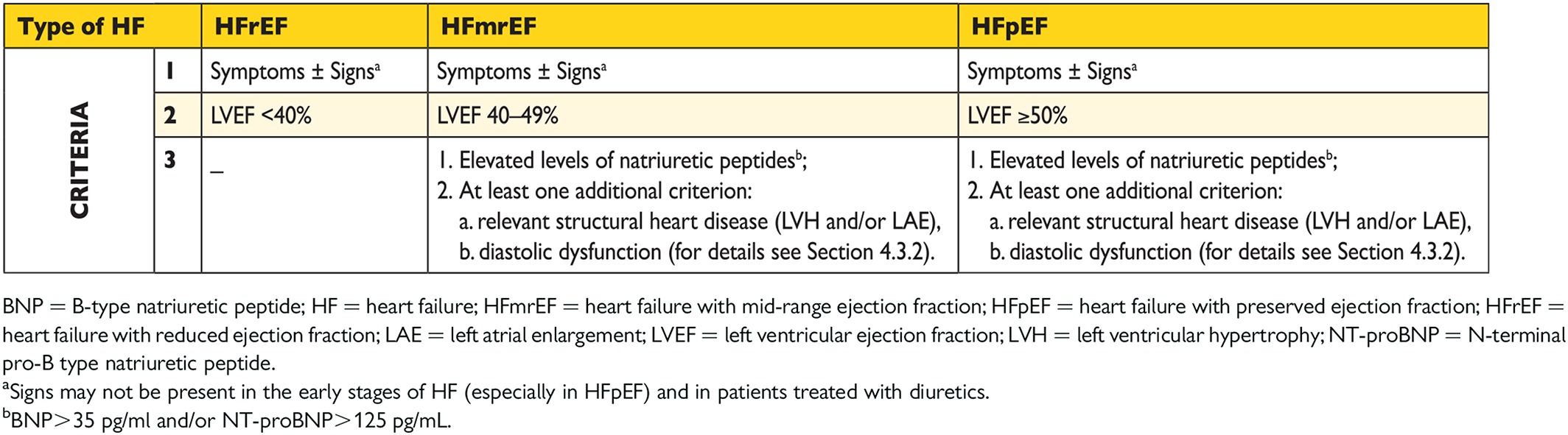
The diagnosis of HFpEF is more challenging than the diagnosis of HFrEF. Patients with HFpEF generally do not have a dilated LV, but instead often have an increase in LV wall thickness and/or increased left atrial (LA) size as a sign of increased filling pressures. Most have additional ‘evidence’ of impaired LV filling or suction capacity, also classified as diastolic dysfunction, which is generally accepted as the likely cause of HF in these patients (hence the term ‘diastolic HF’). However, most patients with HFrEF (previously referred to as ‘systolic HF’) also have diastolic dysfunction, and subtle abnormalities of systolic function have been shown in patients with HFpEF. Hence the preference for stating preserved or reduced LVEF over preserved or reduced ‘systolic function’.
In previous guidelines it was acknowledged that a grey area exists between HFrEF and HFpEF.7 These patients have an LVEF that ranges from 40 to 49%, hence the term HFmrEF. Identifying HFmrEF as a separate group will stimulate research into the underlying characteristics, pathophysiology and treatment of this group of patients. Patients with HFmrEF most probably have primarily mild systolic dysfunction, but with features of diastolic dysfunction (Table 3.1).
Patients without detectable LV myocardial disease may have other cardiovascular causes for HF (e.g. pulmonary hypertension, valvular heart disease, etc.). Patients with non-cardiovascular pathologies (e.g. anaemia, pulmonary, renal or hepatic disease) may have symptoms similar or identical to those of HF and each may complicate or exacerbate the HF syndrome.
3.2.2 Terminology related to the time course of heart failure
In these guidelines, the term HF is used to describe the symptomatic syndrome, graded according to the New York Heart Association (NYHA) functional classification (see Section 3.2.3 and Web Table 3.2), although a patient can be rendered asymptomatic by treatment. In these guidelines, a patient who has never exhibited the typical symptoms and/or signs of HF and with a reduced LVEF is described as having asymptomatic LV systolic dysfunction. Patients who have had HF for some time are often said to have ‘chronic HF’. A treated patient with symptoms and signs that have remained generally unchanged for at least 1 month is said to be ‘stable’. If chronic stable HF deteriorates, the patient may be described as ‘decompensated’ and this may happen suddenly or slowly, often leading to hospital admission, an event of considerable prognostic importance. New-onset (‘de novo’) HF may also present acutely, for example, as a consequence of acute myocardial infarction (AMI), or in a subacute (gradual) fashion, for example, in patients with a dilated cardiomyopathy (DCM), who often have symptoms for weeks or months before the diagnosis becomes clear. Although symptoms and signs of HF may resolve, the underlying cardiac dysfunction may not, and patients remain at the risk of recurrent ‘decompensation’.
Occasionally, however, a patient may have HF due to a problem that resolves completely (e.g. acute viral myocarditis, takotsubo cardiomyopathy or tachycardiomyopathy). Other patients, particularly those with ‘idiopathic’ DCM, may also show substantial or even complete recovery of LV systolic function with modern disease-modifying therapy [including angiotensin-converting enzyme inhibitor (ACEI), beta-blocker, mineralocorticoid receptor antagonist (MRA), ivabradine and/or CRT]. ‘Congestive HF’ is a term that is sometimes used, and may describe acute or chronic HF with evidence of volume overload. Many or all of these terms may be accurately applied to the same patient at different times, depending upon their stage of illness.
3.2.3 Terminology related to the symptomatic severity of heart failure
The NYHA functional classification (Web Table 3.2) has been used to describe the severity of symptoms and exercise intolerance. However, symptom severity correlates poorly with many measures of LV function; although there is a clear relationship between the severity of symptoms and survival, patients with mild symptoms may still have an increased risk of hospitalization and death.8-10
Sometimes the term ‘advanced HF’ is used to characterize patients with severe symptoms, recurrent decompensation and severe cardiac dysfunction.11 The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) classification describes stages of HF development based on structural changes and symptoms (Web Table 3.3).12 The Killip classification may be used to describe the severity of the patient's condition in the acute setting after myocardial infarction (see Section 12).13
3.3 Epidemiology, aetiology and natural history of heart failure
The prevalence of HF depends on the definition applied, but is approximately 1–2% of the adult population in developed countries, rising to ≥10% among people >70 years of age.14-17 Among people >65 years of age presenting to primary care with breathlessness on exertion, one in six will have unrecognized HF (mainly HFpEF).18, 19 The lifetime risk of HF at age 55 years is 33% for men and 28% for women.16 The proportion of patients with HFpEF ranges from 22 to 73%, depending on the definition applied, the clinical setting (primary care, hospital clinic, hospital admission), age and sex of the studied population, previous myocardial infarction and the year of publication.17, 18, 20-30
Data on temporal trends based on hospitalized patients suggest that the incidence of HF may be decreasing, more for HFrEF than for HFpEF.31, 32 HFpEF and HFrEF seem to have different epidemiological and aetiological profiles. Compared with HFrEF, patients with HFpEF are older, more often women and more commonly have a history of hypertension and atrial fibrillation (AF), while a history of myocardial infarction is less common.32, 33 The characteristics of patients with HFmrEF are between those with HFrEF and HFpEF,34 but further studies are needed to better characterize this population.
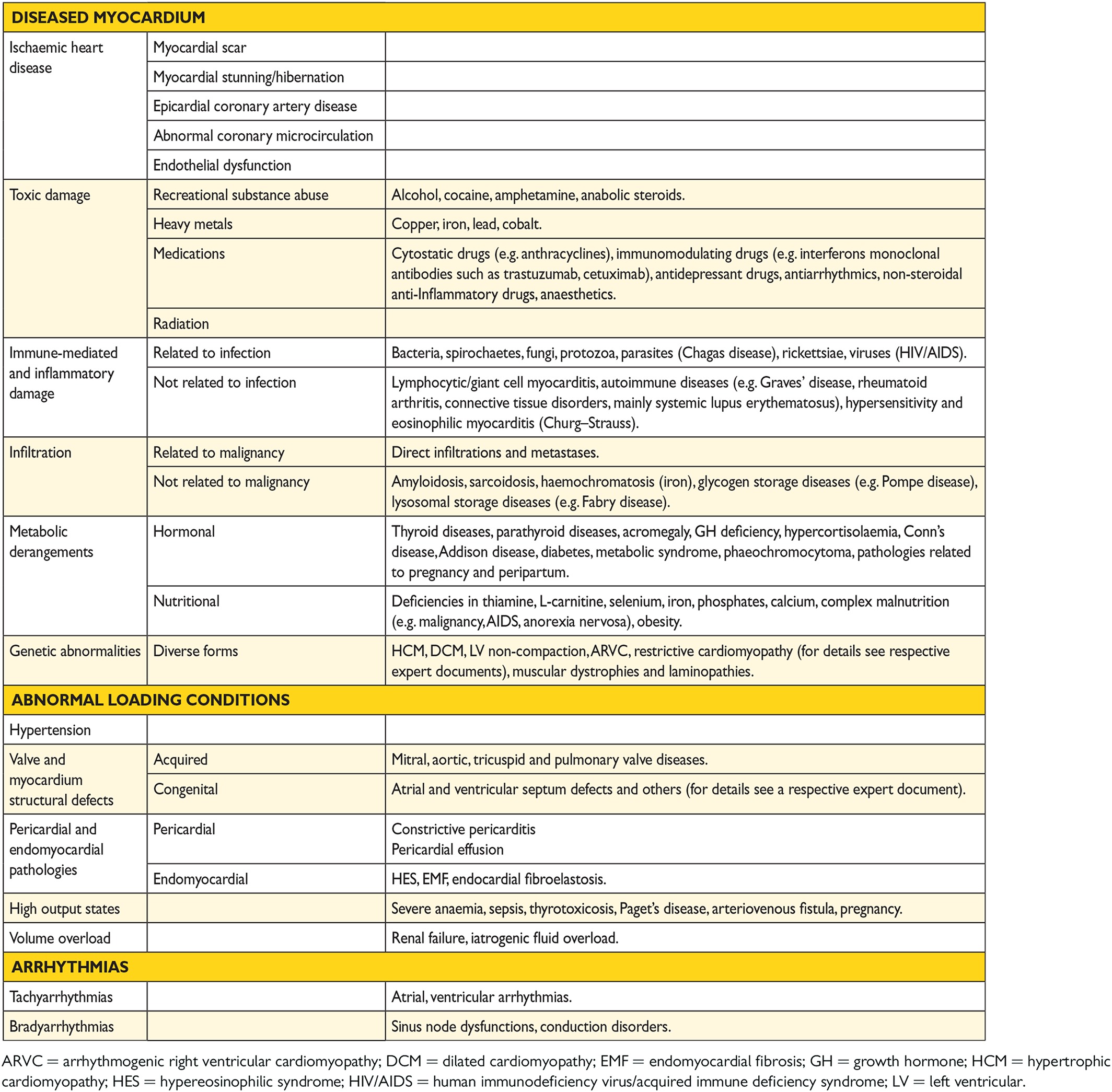
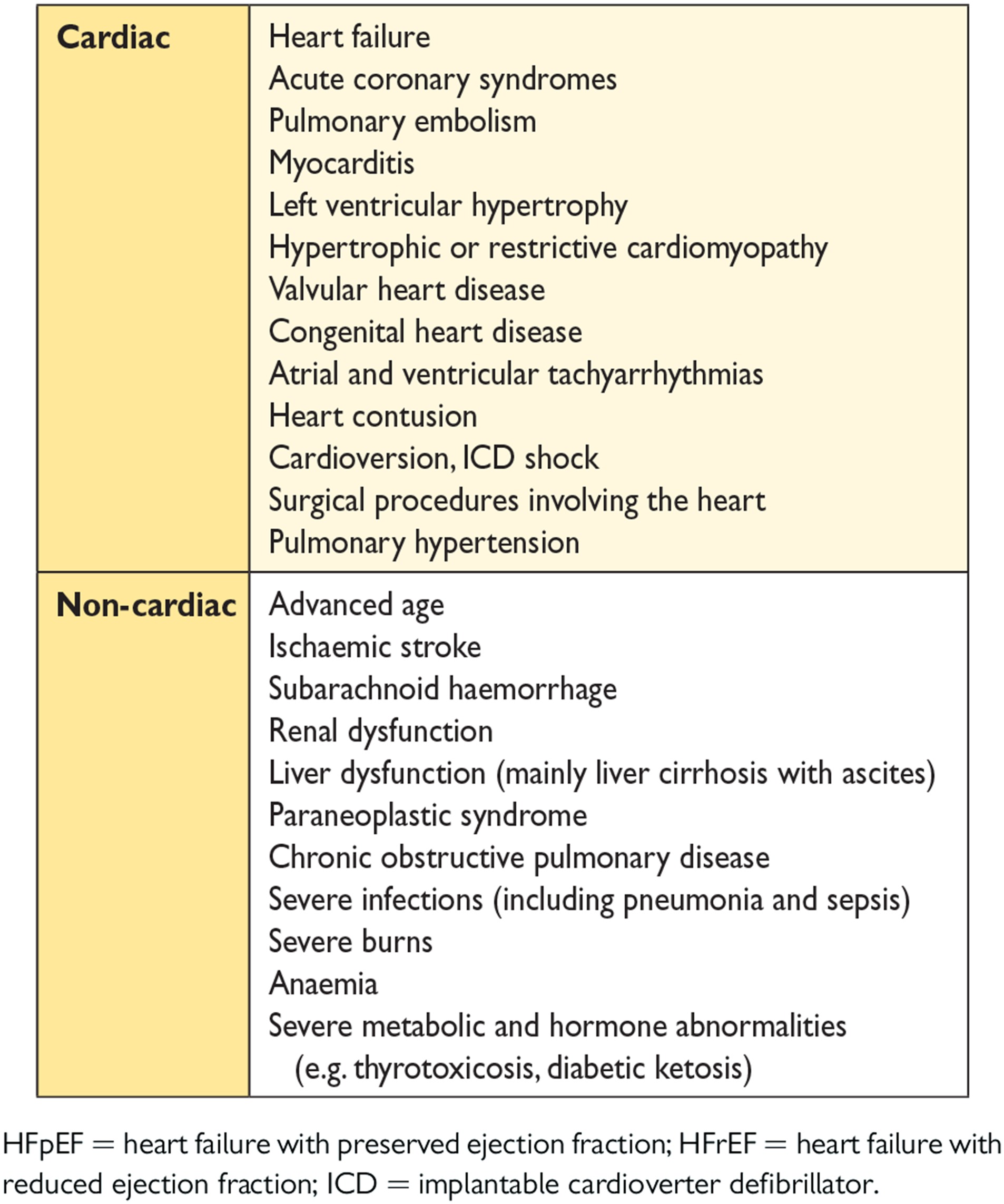
The aetiology of HF is diverse within and among world regions. There is no agreed single classification system for the causes of HF, with much overlap between potential categories (Table 3.4). Many patients will have several different pathologies—cardiovascular and non-cardiovascular—that conspire to cause HF. Identification of these diverse pathologies should be part of the diagnostic workup, as they may offer specific therapeutic opportunities.
Many patients with HF and ischaemic heart disease (IHD) have a history of myocardial infarction or revascularization. However, a normal coronary angiogram does not exclude myocardial scar (e.g. by CMR imaging) or impaired coronary microcirculation as alternative evidence for IHD.
In clinical practice, a clear distinction between acquired and inherited cardiomyopathies remains challenging. In most patients with a definite clinical diagnosis of HF, there is no confirmatory role for routine genetic testing, but genetic counselling is recommended in patients with hypertrophic cardiomyopathy (HCM), ‘idiopathic’ DCM or arrhythmogenic right ventricular cardiomyopathy (ARVC) (see Section 5.10.1), since the outcomes of these tests may have clinical implications.
Over the last 30 years, improvements in treatments and their implementation have improved survival and reduced the hospitalization rate in patients with HFrEF, although the outcome often remains unsatisfactory. The most recent European data (ESC-HF pilot study) demonstrate that 12-month all-cause mortality rates for hospitalized and stable/ambulatory HF patients were 17% and 7%, respectively, and the 12-month hospitalization rates were 44% and 32%, respectively.35 In patients with HF (both hospitalized and ambulatory), most deaths are due to cardiovascular causes, mainly sudden death and worsening HF. All-cause mortality is generally higher in HFrEF than HFpEF.35, 36 Hospitalizations are often due to non-cardiovascular causes, particularly in patients with HFpEF. Hospitalization for cardiovascular causes did not change from 2000 to 2010, whereas those with non-cardiovascular causes increased.31
3.4 Prognosis
Estimation of prognosis for morbidity, disability and death helps patients, their families and clinicians decide on the appropriate type and timing of therapies (in particular, decisions about a rapid transition to advanced therapies) and assists with planning of health and social services and resources.
Numerous prognostic markers of death and/or HF hospitalization have been identified in patients with HF (Web Table 3.5). However, their clinical applicability is limited and precise risk stratification in HF remains challenging.
In recent decades, several multivariable prognostic risk scores have been developed for different populations of patients with HF,36-41 and some are available as interactive online applications. Multivariable risk scores may help predict death in patients with HF, but remain less useful for the prediction of subsequent HF hospitalizations.37, 38 A systematic review examining 64 prognostic models37 along with a meta-analysis and meta-regression study of 117 prognostic models38 revealed only a moderate accuracy of models predicting mortality, whereas models designed to predict the combined endpoint of death or hospitalization, or only hospitalization, had an even poorer discriminative ability.
4 Diagnosis
4.1 Symptoms and signs
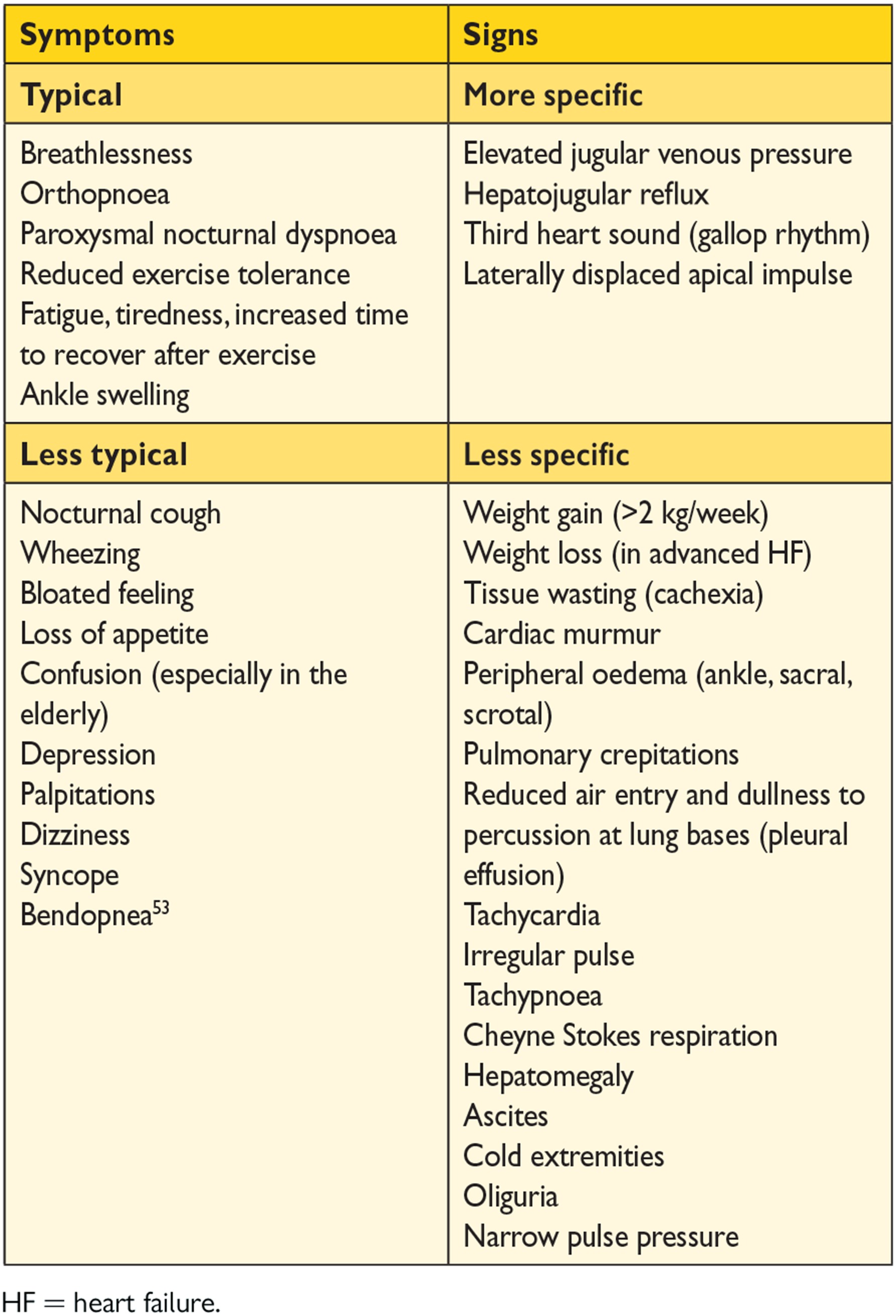
Symptoms are often non-specific and do not, therefore, help discriminate between HF and other problems (Table 4.1).42-46 Symptoms and signs of HF due to fluid retention may resolve quickly with diuretic therapy. Signs, such as elevated jugular venous pressure and displacement of the apical impulse, may be more specific, but are harder to detect and have poor reproducibility.18, 46, 47 Symptoms and signs may be particularly difficult to identify and interpret in obese individuals, in the elderly and in patients with chronic lung disease.48-50 Younger patients with HF often have a different aetiology, clinical presentation and outcome compared with older patients.51, 52
A detailed history should always be obtained. HF is unusual in an individual with no relevant medical history (e.g. a potential cause of cardiac damage), whereas certain features, particularly previous myocardial infarction, greatly increase the likelihood of HF in a patient with appropriate symptoms and signs.42-45
At each visit, symptoms and signs of HF need to be assessed, with particular attention to evidence of congestion. Symptoms and signs are important in monitoring a patient's response to treatment and stability over time. Persistence of symptoms despite treatment usually indicates the need for additional therapy, and worsening of symptoms is a serious development (placing the patient at risk of urgent hospital admission and death) and merits prompt medical attention.
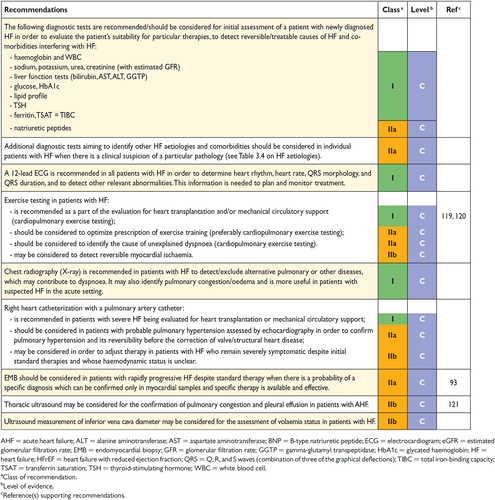
4.2 Essential initial investigations: natriuretic peptides, electrocardiogram and echocardiography
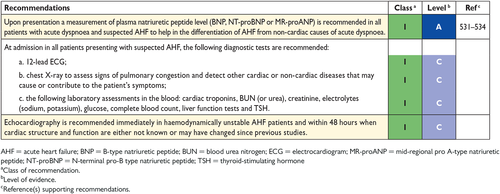
The plasma concentration of natriuretic peptides (NPs) can be used as an initial diagnostic test, especially in the non-acute setting when echocardiography is not immediately available. Elevated NPs help establish an initial working diagnosis, identifying those who require further cardiac investigation; patients with values below the cut-point for the exclusion of important cardiac dysfunction do not require echocardiography (see also Section 4.3 and Section 12). Patients with normal plasma NP concentrations are unlikely to have HF. The upper limit of normal in the non-acute setting for B-type natriuretic peptide (BNP) is 35 pg/mL and for N-terminal pro-BNP (NT-proBNP) it is 125 pg/mL; in the acute setting, higher values should be used [BNP < 100 pg/mL, NT-proBNP < 300 pg/mL and mid-regional pro A-type natriuretic peptide (MR-proANP) < 120 pmol/L]. Diagnostic values apply similarly to HFrEF and HFpEF; on average, values are lower for HFpEF than for HFrEF.54, 55 At the mentioned exclusionary cut-points, the negative predictive values are very similar and high (0.94–0.98) in both the non-acute and acute setting, but the positive predictive values are lower both in the non-acute setting (0.44–0.57) and in the acute setting (0.66–0.67).54, 56-61 Therefore, the use of NPs is recommended for ruling-out HF, but not to establish the diagnosis.
There are numerous cardiovascular and non-cardiovascular causes of elevated NPs that may weaken their diagnostic utility in HF. Among them, AF, age and renal failure are the most important factors impeding the interpretation of NP measurements.55 On the other hand, NP levels may be disproportionally low in obese patients62 (see also Section 12.2 and Table 12.2).
An abnormal electrocardiogram (ECG) increases the likelihood of the diagnosis of HF, but has low specificity.18, 46, 63, 64 Some abnormalities on the ECG provide information on aetiology (e.g. myocardial infarction), and findings on the ECG might provide indications for therapy (e.g. anticoagulation for AF, pacing for bradycardia, CRT if broadened QRS complex) (see Sections 8 and 10). HF is unlikely in patients presenting with a completely normal ECG (sensitivity 89%).43 Therefore, the routine use of an ECG is mainly recommended to rule out HF.
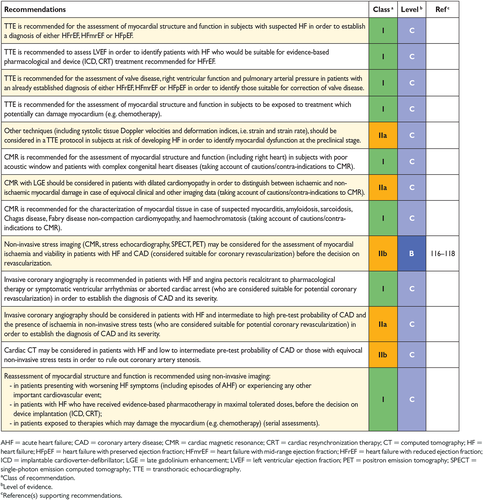
Echocardiography is the most useful, widely available test in patients with suspected HF to establish the diagnosis. It provides immediate information on chamber volumes, ventricular systolic and diastolic function, wall thickness, valve function and pulmonary hypertension.65-74 This information is crucial in establishing the diagnosis and in determining appropriate treatment (see Sections 5.2–5.4 for details on echocardiography).
The information provided by careful clinical evaluation and the above mentioned tests will permit an initial working diagnosis and treatment plan in most patients. Other tests are generally required only if the diagnosis remains uncertain (e.g. if echocardiographic images are suboptimal or an unusual cause of HF is suspected) (for details see Sections 5.5–5.10).
4.3 Algorithm for the diagnosis of heart failure
4.3.1 Algorithm for the diagnosis of heart failure in the non-acute setting
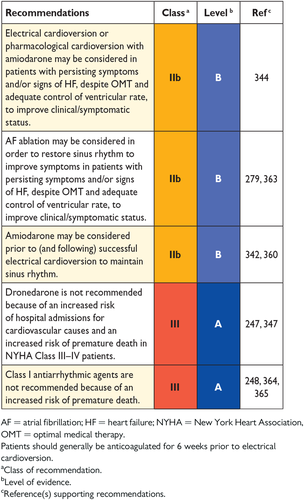
An algorithm for the diagnosis of HF in the non-acute setting is shown in Figure 4.1. The diagnosis of HF in the acute setting is discussed in Section 12.
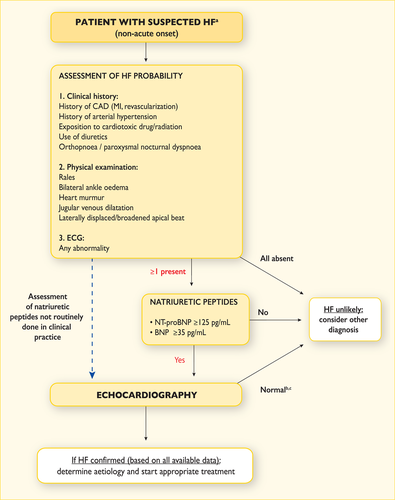
Diagnostic algorithm for a diagnosis of heart failure of non-acute onset
BNP = B-type natriuretic peptide; CAD = coronary artery disease; HF = heart failure; MI = myocardial infarction; NT-proBNP = N-terminal pro-B type natriuretic peptide.
aPatient reporting symptoms typical of HF (see Table 4.1).
bNormal ventricular and atrial volumes and function.
cConsider other causes of elevated natriuretic peptides (Table 12.2).
For patients presenting with symptoms or signs for the first time, non-urgently in primary care or in a hospital outpatient clinic (Table 4.1), the probability of HF should first be evaluated based on the patient's prior clinical history [e.g. coronary artery disease (CAD), arterial hypertension, diuretic use], presenting symptoms (e.g. orthopnoea), physical examination (e.g. bilateral oedema, increased jugular venous pressure, displaced apical beat) and resting ECG. If all elements are normal, HF is highly unlikely and other diagnoses need to be considered. If at least one element is abnormal, plasma NPs should be measured, if available, to identify those who need echocardiography (an echocardiogram is indicated if the NP level is above the exclusion threshold or if circulating NP levels cannot be assessed).55-60, 75-78
4.3.2 Diagnosis of heart failure with preserved ejection fraction
The diagnosis of HFpEF remains challenging. LVEF is normal and signs and symptoms for HF (Table 4.1) are often non-specific and do not discriminate well between HF and other clinical conditions. This section summarizes practical recommendations necessary for proper diagnosis of this clinical entity in clinical practice.
-
The presence of symptoms and/or signs of HF (see Table 4.1)
-
A ‘preserved’ EF (defined as LVEF ≥50% or 40–49% for HFmrEF)
-
Elevated levels of NPs (BNP >35 pg/mL and/or NT-proBNP >125 pg/mL)
-
Objective evidence of other cardiac functional and structural alterations underlying HF (for details, see below)
-
In case of uncertainty, a stress test or invasively measured elevated LV filling pressure may be needed to confirm the diagnosis (for details, see below).
The next step comprises an advanced workup in case of initial evidence of HFpEF/HFmrEF and consists of objective demonstration of structural and/or functional alterations of the heart as the underlying cause for the clinical presentation. Key structural alterations are a left atrial volume index (LAVI) >34 mL/m2 or a left ventricular mass index (LVMI) ≥115 g/m² for males and ≥95 g/m² for females.65, 67, 72 Key functional alterations are an E/e′ ≥13 and a mean e' septal and lateral wall <9 cm/s.65, 67, 70, 72, 80-84 Other (indirect) echocardiographically derived measurements are longitudinal strain or tricuspid regurgitation velocity (TRV).72, 82 An overview of normal and abnormal values for echocardiographic parameters related to diastolic function is presented in Web Table 4.3. Not all of the recommended values are identical to those published in previous guidelines, because of the inclusion of new data published in recent reports, in particular by Cabarello et al.70
A diastolic stress test can be performed with echocardiography, typically using a semi-supine bicycle ergometer exercise protocol with assessment of LV (E/e′) and pulmonary artery pressures (TRV), systolic dysfunction (longitudinal strain), stroke volume and cardiac output changes with exercise.85, 86 Different dynamic exercise protocols are available, with semi-supine bicycle ergometry and echocardiography at rest and submaximal exercise being used most often.85 Exercise-induced increases in E/e′ beyond diagnostic cut-offs (i.e. >13), but also other indirect measures of systolic and diastolic function, such as longitudinal strain or TRV, are used. Alternatively, invasive haemodynamics at rest with assessment of filling pressures [pulmonary capillary wedge pressure (PCWP) ≥15 mmHg or left ventricular end diastolic pressure (LVEDP) ≥16 mmHg] followed by exercise haemodynamics if below these thresholds, with assessment of changes in filling pressures, pulmonary artery systolic pressure, stroke volume and cardiac output, can be performed.87
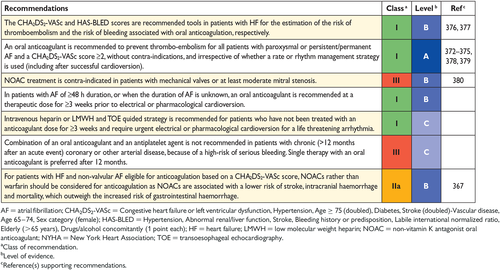
The diagnosis of HFpEF in patients with AF is difficult. Since AF is associated with higher NP levels, the use of NT-proBNP or BNP for diagnosing HFpEF probably needs to be stratified by the presence of sinus rhythm (with lower cut-offs) vs. AF (higher cut-offs). LAVI is increased by AF, and functional parameters of diastolic dysfunction are less well established in AF, and other cut-off values probably apply. On the other hand, AF might be a sign of the presence of HFpEF, and patients with AF and HFpEF often have similar patient characteristics. In addition, patients with HFpEF and AF might have more advanced HF compared with patients with HFpEF and sinus rhythm.
Patients with HFpEF are a heterogeneous group with various underlying aetiologies and pathophysiological abnormalities. Based on specific suspected causes, additional tests can be performed (Web Table 4.4).71, 88-94 However, they can only be recommended if the results might affect management.
5 Cardiac imaging and other diagnostic tests
Cardiac imaging plays a central role in the diagnosis of HF and in guiding treatment. Of several imaging modalities available, echocardiography is the method of choice in patients with suspected HF, for reasons of accuracy, availability (including portability), safety and cost.68, 69, 72 Echocardiography may be complemented by other modalities, chosen according to their ability to answer specific clinical questions and taking account of contraindications to and risks of specific tests.71, 73
In general, imaging tests should only be performed when they have a meaningful clinical consequence. The reliability of the outcomes is highly dependent on the imaging modality, the operator and centre experience and imaging quality. Normal values may vary with age, sex and imaging modality.
5.1 Chest X-ray
A chest X-ray is of limited use in the diagnostic work-up of patients with suspected HF. It is probably most useful in identifying an alternative, pulmonary explanation for a patient's symptoms and signs, i.e. pulmonary malignancy and interstitial pulmonary disease, although computed tomography (CT) of the chest is currently the standard of care. For the diagnosis of asthma or chronic obstructive pulmonary disease (COPD), pulmonary function testing with spirometry is needed. The chest X-ray may, however, show pulmonary venous congestion or oedema in a patient with HF, and is more helpful in the acute setting than in the non-acute setting.49, 64 It is important to note that significant LV dysfunction may be present without cardiomegaly on the chest X-ray.49, 64
5.2 Transthoracic echocardiography
Echocardiography is a term used here to refer to all cardiac ultrasound imaging techniques, including two-dimensional/three-dimensional echocardiography, pulsed and continuous wave Doppler, colour flow Doppler, tissue Doppler imaging (TDI) contrast echocardiography and deformation imaging (strain and strain rate).
Transthoracic echocardiography (TTE) is the method of choice for assessment of myocardial systolic and diastolic function of both left and right ventricles.
5.2.1 Assessment of left ventricular systolic function
For measurement of LVEF, the modified biplane Simpson's rule is recommended. LV end diastolic volume (LVEDV) and LV end systolic volume (LVESV) are obtained from apical four- and two-chamber views. This method relies on accurate tracing of endocardial borders. In case of poor image quality, contrast agents should be used to improve endocardial delineation.72 Measurement of regional wall motion abnormalities might be particularly relevant for patients suspected of CAD or myocarditis.
The Teichholz and Quinones methods of calculating LVEF from linear dimensions, as well as a measurement of fractional shortening, are not recommended, as they may result in inaccuracies, particularly in patients with regional LV dysfunction and/or LV remodelling. Three-dimensional echocardiography of adequate quality improves the quantification of LV volumes and LVEF and has the best accuracy compared with values obtained through CMR.95
Doppler techniques allow the calculation of haemodynamic variables, such as stroke volume index and cardiac output, based on the velocity time integral at the LV outflow tract area.
In recent years, tissue Doppler parameters (S wave) and deformation imaging techniques (strain and strain rate) have been shown to be reproducible and feasible for clinical use, especially in detecting subtle abnormalities in systolic function in the preclinical stage; however, measurements may vary among vendors and software versions.74
5.2.2 Assessment of left ventricular diastolic function
LV diastolic dysfunction is thought to be the underlying pathophysiological abnormality in patients with HFpEF and perhaps HFmrEF, and thus its assessment plays an important role in diagnosis. Although echocardiography is at present the only imaging technique that can allow for the diagnosis of diastolic dysfunction, no single echocardiography variable is sufficiently accurate to be used in isolation to make a diagnosis of LV diastolic dysfunction. Therefore, a comprehensive echocardiography examination incorporating all relevant two-dimensional and Doppler data is recommended (see Section 4.3.2).
5.2.3 Assessment of right ventricular function and pulmonary arterial pressure
An obligatory element of echocardiography examination is the assessment of right ventricle (RV) structure and function, including RV and right atrial (RA) dimensions, an estimation of RV systolic function and pulmonary arterial pressure. Among parameters reflecting RV systolic function, the following measures are of particular importance: tricuspid annular plane systolic excursion (TAPSE; abnormal TAPSE <17 mm indicates RV systolic dysfunction) and tissue Doppler-derived tricuspid lateral annular systolic velocity (s′) (s′ velocity <9.5 cm/s indicates RV systolic dysfunction).72, 96 Systolic pulmonary artery pressure is derived from an optimal recording of maximal tricuspid regurgitant jet and the tricuspid systolic gradient, together with an estimate of RA pressure on the basis of inferior vena cava (IVC) size and its breathing-related collapse.97 RV size should be routinely assessed by conventional two-dimensional echocardiography using multiple acoustic windows, and the report should include both qualitative and quantitative parameters. In laboratories with experience in three-dimensional echocardiography, when knowledge of RV volumes may be clinically important, three-dimensional measurement of RV volumes is recommended.95 Three-dimensional speckle tracking echocardiography may be an additional quantitative method to assess RV function in specialised centres.98
5.3 Transoesophageal echocardiography
Transoesophageal echocardiography (TOE) is not needed in the routine diagnostic assessment of HF; however, it may be valuable in some clinical scenarios of patients with valve disease, suspected aortic dissection, suspected endocarditis or congenital heart disease and for ruling out intracavitary thrombi in AF patients requiring cardioversion. When the severity of mitral or aortic valve disease does not match the patient's symptoms using TTE alone, a TOE examination should be performed.
5.4 Stress echocardiography
Exercise or pharmacological stress echocardiography may be used for the assessment of inducible ischaemia and/or myocardium viability99 and in some clinical scenarios of patients with valve disease (e.g. dynamic mitral regurgitation, low-flow–low-gradient aortic stenosis).99, 100 There are also suggestions that stress echocardiography may allow the detection of diastolic dysfunction related to exercise exposure in patients with exertional dyspnoea, preserved LVEF and inconclusive diastolic parameters at rest.85, 86
5.5 Cardiac magnetic resonance
CMR is acknowledged as the gold standard for the measurements of volumes, mass and EF of both the left and right ventricles. It is the best alternative cardiac imaging modality for patients with non-diagnostic echocardiographic studies (particularly for imaging of the right heart) and is the method of choice in patients with complex congenital heart diseases.91, 101, 102
CMR is the preferred imaging method to assess myocardial fibrosis using late gadolinium enhancement (LGE) along with T1 mapping and can be useful for establishing HF aetiology.91, 103 For example, CMR with LGE allows differentiation between ischaemic and non-ischaemic origins of HF and myocardial fibrosis/scars can be visualized. In addition, CMR allows the characterization of myocardial tissue of myocarditis, amyloidosis, sarcoidosis, Chagas disease, Fabry disease non-compaction cardiomyopathy and haemochromatosis.91, 101, 103, 104
CMR may also be used for the assessment of myocardial ischaemia and viability in patients with HF and CAD (considered suitable for coronary revascularization). However, limited evidence from RCTs has failed to show that viability assessed by CMR or other means identified patients who obtained clinical benefit from revascularization.105-107
Clinical limitations of CMR include local expertise, lower availability and higher costs compared with echocardiography, uncertainty about safety in patients with metallic implants (including cardiac devices) and less reliable measurements in patients with tachyarrhythmias. Claustrophobia is an important limitation for CMR. Linear gadolinium-based contrast agents are contraindicated in individuals with a glomerular filtration rate (GFR) <30 mL/min/1.73m2, because they may trigger nephrogenic systemic fibrosis (this may be less of a concern with newer cyclic gadolinium-based contrast agents).108
5.6 Single-photon emission computed tomography and radionuclide ventriculography
Single-photon emission CT (SPECT) may be useful in assessing ischaemia and myocardial viability.109 Gated SPECT can also yield information on ventricular volumes and function, but exposes the patient to ionizing radiation. 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scintigraphy may be useful for the detection of transthyretin cardiac amyloidosis.110
5.7 Positron emission tomography
Positron emission tomography (PET) (alone or with CT) may be used to assess ischaemia and viability, but the flow tracers (N-13 ammonia or O-15 water) require an on-site cyclotron.92, 111 Rubidium is an alternative tracer for ischaemia testing with PET, which can be produced locally at relatively low cost. Limited availability, radiation exposure and cost are the main limitations.
5.8 Coronary angiography
Indications for coronary angiography in patients with HF are in concordance with the recommendations of other relevant ESC guidelines.112-114 Coronary angiography is recommended in patients with HF who suffer from angina pectoris recalcitrant to medical therapy,115 provided the patient is otherwise suitable for coronary revascularization. Coronary angiography is also recommended in patients with a history of symptomatic ventricular arrhythmia or aborted cardiac arrest. Coronary angiography should be considered in patients with HF and intermediate to high pre-test probability of CAD and the presence of ischaemia in non-invasive stress tests in order to establish the ischaemic aetiology and CAD severity.
5.9 Cardiac computed tomography
The main use of cardiac CT in patients with HF is as a non-invasive means to visualize the coronary anatomy in patients with HF with low intermediate pre-test probability of CAD or those with equivocal non-invasive stress tests in order to exclude the diagnosis of CAD, in the absence of relative contraindications. However, the test is only required when its results might affect a therapeutic decision.
5.10 Other diagnostic tests
5.10.1 Genetic testing in heart failure
Molecular genetic analysis in patients with cardiomyopathies is recommended when the prevalence of detectable mutations is sufficiently high and consistent to justify routine targeted genetic screening. Recommendations for genetic testing in patients with HF are based on the position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases.94 In most patients with a definite clinical diagnosis of HF, there is no confirmatory role for routine genetic testing to establish the diagnosis. Genetic counselling is recommended in patients with HCM, idiopathic DCM and ARVC. Restrictive cardiomyopathy and isolated non-compaction cardiomyopathies are of a possible genetic origin and should also be considered for genetic testing.
HCM is mostly inherited as an autosomal dominant disease with variable expressivity and age-related penetrance. Currently, more than 20 genes and 1400 mutations have been identified, most of which are located in the sarcomere genes encoding cardiac β-myosin heavy chain (MYH7) and cardiac myosin binding protein C (MYBPC3).88, 122
DCM is idiopathic in 50% of cases, about one-third of which are hereditary. There are already more than 50 genes identified that are associated with DCM. Many genes are related to the cytoskeleton. The most frequent ones are titin (TTN), lamin (LMNA) and desmin (DES).88, 123
ARVC is hereditary in most cases and is caused by gene mutations that encode elements of the desmosome. Desmosomal gene mutations explain 50% of cases and 10 genes are currently associated with the disease.124
Counselling should be performed by someone with sufficient knowledge of the specific psychological, social and medical implications of a diagnosis. Determination of the genotype is important, since some forms [e.g. mutations in LMNA and phospholamban (PLN)] are related to a poorer prognosis. DNA analysis could also be of help to establish the diagnosis of rare forms, such as mitochondrial cardiomyopathies. Screening of first-degree relatives for early detection is recommended from early adolescence onwards, although earlier screening may be considered depending on the age of disease onset in other family members.
Recently, the MOGE(S) classification of inherited cardiomyopathies has been proposed, which includes the morphofunctional phenotype (M), organ(s) involvement (O), genetic inheritance pattern (G), aetiological annotation (E), including genetic defect or underlying disease/substrate, and the functional status (S) of the disease.125
6 Delaying or preventing the development of overt heart failure or preventing death before the onset of symptoms
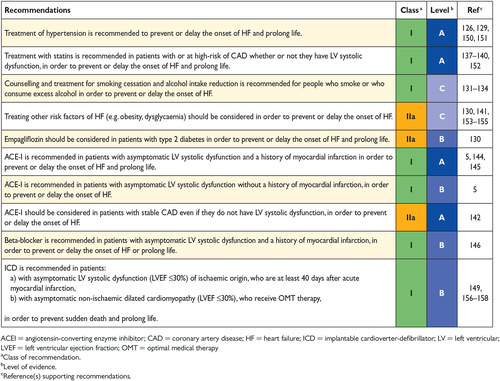
There is considerable evidence that the onset of HF may be delayed or prevented through interventions aimed at modifying risk factors for HF or treating asymptomatic LV systolic dysfunction (see recommendations table). Many trials show that control of hypertension will delay the onset of HF and some also show that it will prolong life.126-129 Different antihypertensive drugs [diuretics, ACEIs, angiotensin receptor blockers (ARBs), beta-blockers] have been shown to be effective, especially in older people, both in patients with and without a history of myocardial infarction.126-128 Along with the ongoing discussion on optimal target blood pressure values in hypertensive non-diabetic subjects, the recent SPRINT study has already demonstrated that treating hypertension to a lower goal [systolic blood pressure (SBP) <120 mmHg vs. <140 mmHg] in older hypertensive subjects (≥75 years of age) or high-risk hypertensive patients reduces the risk of cardiovascular disease, death and hospitalization for HF.129
Recently, empaglifozin (an inhibitor of sodium-glucose cotransporter 2), has been shown to improve outcomes (including the reduction of mortality and HF hospitalizations) in patients with type 2 diabetes.130 Other hypoglycaemic agents have not been shown convincingly to reduce the risk of cardiovascular events and may increase the risk of HF. Intensification of hypoglycaemic therapy to drive down glycated haemoglobin (HbA1c) with agents other than empagliflozin does not reduce the risk of developing HF (for details see Section 11.6 on diabetes).
Although smoking cessation has not been shown to reduce the risk of developing HF, the epidemiological associations with the development of cardiovascular disease131 suggest that such advice, if followed, would be beneficial.
The association between alcohol intake and the risk of developing de novo HF is U-shaped, with the lowest risk with modest alcohol consumption (up to 7 drinks/week).132-134 Greater alcohol intake may trigger the development of toxic cardiomyopathy, and when present, complete abstention from alcohol is recommended.
An inverse relationship between physical activity and the risk of HF has been reported. A recent meta-analysis found that doses of physical activity in excess of the guideline recommended minimal levels may be required for more substantial reductions in HF risk.135
It has been shown that among subjects ≥40 years of age with either cardiovascular risk factors or cardiovascular disease (but neither asymptomatic LV dysfunction nor overt HF), BNP-driven collaborative care between the primary care physician and the specialist cardiovascular centre may reduce the combined rates of LV systolic dysfunction and overt HF.136
Statins reduce the rate of cardiovascular events and mortality; there is also reasonable evidence that they prevent or delay the onset of HF.137-140 Neither aspirin nor other antiplatelet agents, nor revascularization, have been shown to reduce the risk of developing HF or mortality in patients with stable CAD. Obesity is also a risk factor for HF,141 but the impact of treatments of obesity on the development of HF is unknown.
In patients with CAD, without LV systolic dysfunction or HF, ACEIs prevent or delay the onset of HF and reduce cardiovascular and all-cause mortality, although the benefit may be small in the contemporary setting, especially in patients receiving aspirin.142 Up-titration of renin–angiotensin system antagonists and beta-blockers to maximum tolerated dosages may improve outcomes, including HF, in patients with increased plasma concentrations of NPs.136, 143
A primary percutaneous coronary intervention (PCI) at the earliest phase of an ST segment elevation myocardial infarction (STEMI) to reduce infarct size decreases the risk of developing a substantial reduction in LVEF and subsequent development of HFrEF.112 Initiation of an ACEI, a beta-blocker and an MRA immediately after a myocardial infarction, especially when it is associated with LV systolic dysfunction, reduces the rate of hospitalization for HF and mortality,144-148 as do statins.137-139
In asymptomatic patients with chronically reduced LVEF, regardless of its aetiology, an ACEI can reduce the risk of HF requiring hospitalization.5, 144, 145 This has not yet been shown for beta-blockers or MRAs.
7 Pharmacological treatment of heart failure with reduced ejection fraction
7.1 Objectives in the management of heart failure
The goals of treatment in patients with HF are to improve their clinical status, functional capacity and quality of life, prevent hospital admission and reduce mortality. The fact that several drugs for HF have shown detrimental effects on long-term outcomes, despite showing beneficial effects on shorter-term surrogate markers, has led regulatory bodies and clinical practice guidelines to seek mortality/morbidity data for approving/recommending therapeutic interventions for HF. However, it is now recognized that preventing HF hospitalization and improving functional capacity are important benefits to be considered if a mortality excess is ruled out.159-161
Figure 7.1 shows a treatment strategy for the use of drugs (and devices) in patients with HFrEF. The recommendations for each treatment are summarized below.
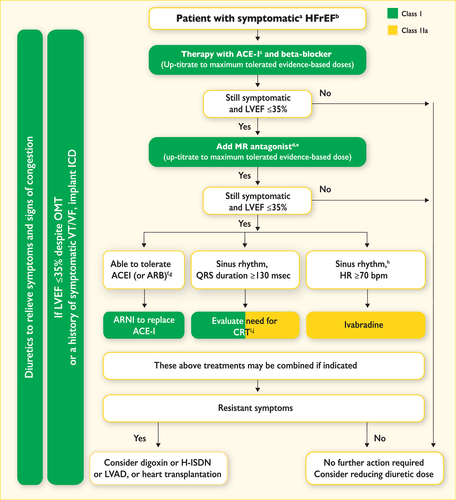
Therapeutic algorithm for a patient with symptomatic heart failure with reduced ejection fraction. Green indicates a class I recommendation; yellow indicates a class IIa recommendation. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; BNP = B-type natriuretic peptide; CRT = cardiac resynchronization therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; H-ISDN = hydralazine and isosorbide dinitrate; HR = heart rate; ICD = implantable cardioverter defibrillator; LBBB = left bundle branch block; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; MR = mineralocorticoid receptor; NT-proBNP = N-terminal pro-B type natriuretic peptide; NYHA = New York Heart Association; OMT = optimal medical therapy; VF = ventricular fibrillation; VT = ventricular tachycardia. aSymptomatic = NYHA Class II-IV. bHFrEF = LVEF <40%. cIf ACE inhibitor not tolerated/contra-indicated, use ARB. dIf MR antagonist not tolerated/contra-indicated, use ARB. eWith a hospital admission for HF within the last 6 months or with elevated natriuretic peptides (BNP > 250 pg/ml or NTproBNP > 500 pg/ml in men and 750 pg/ml in women). fWith an elevated plasma natriuretic peptide level (BNP ≥ 150 pg/mL or plasma NT-proBNP ≥ 600 pg/mL, or if HF hospitalization within recent 12 months plasma BNP ≥ 100 pg/mL or plasma NT-proBNP ≥ 400 pg/mL). gIn doses equivalent to enalapril 10 mg b.i.d. hWith a hospital admission for HF within the previous year. iCRT is recommended if QRS ≥ 130 msec and LBBB (in sinus rhythm). jCRT should/may be considered if QRS ≥ 130 msec with non-LBBB (in a sinus rhythm) or for patients in AF provided a strategy to ensure bi-ventricular capture in place (individualized decision). For further details, see Sections 7 and 8 and corresponding web pages.
Neuro-hormonal antagonists (ACEIs, MRAs and beta-blockers) have been shown to improve survival in patients with HFrEF and are recommended for the treatment of every patient with HFrEF, unless contraindicated or not tolerated. A new compound (LCZ696) that combines the moieties of an ARB (valsartan) and a neprilysin (NEP) inhibitor (sacubitril) has recently been shown to be superior to an ACEI (enalapril) in reducing the risk of death and of hospitalization for HF in a single trial with strict inclusion/exclusion criteria.162 Sacubitril/valsartan is therefore recommended to replace ACEIs in ambulatory HFrEF patients who remain symptomatic despite optimal therapy and who fit these trial criteria. ARBs have not been consistently proven to reduce mortality in patients with HFrEF and their use should be restricted to patients intolerant of an ACEI or those who take an ACEI but are unable to tolerate an MRA. Ivabradine reduces the elevated heart rate often seen in HFrEF and has also been shown to improve outcomes, and should be considered when appropriate.
The above medications should be used in conjunction with diuretics in patients with symptoms and/or signs of congestion. The use of diuretics should be modulated according to the patient's clinical status.
The key evidence supporting the recommendations in this section is given in Web Table 7.1. The recommended doses of these disease-modifying medications are given in Table 7.2. The recommendations given in Sections 7.5 and 7.6 summarize drugs that should be avoided or used with caution in patients with HFrEF.
7.2 Treatments recommended in all symptomatic patients with heart failure with reduced ejection fraction
7.2.1 Angiotensin-converting enzyme inhibitors
Practical guidance on how to use ACE inhibitors is given in Web Table 7.4.
7.2.2 Beta-blockers
Beta-blockers reduce mortality and morbidity in symptomatic patients with HFrEF, despite treatment with an ACEI and, in most cases, a diuretic,167, 168, 170, 172, 173 but have not been tested in congested or decompensated patients. There is consensus that beta-blockers and ACEIs are complementary, and can be started together as soon as the diagnosis of HFrEF is made. There is no evidence favouring the initiation of treatment with a beta-blocker before an ACEI has been started.176 Beta-blockers should be initiated in clinically stable patients at a low dose and gradually up-titrated to the maximum tolerated dose. In patients admitted due to acute HF (AHF) beta-blockers should be cautiously initiated in hospital, once the patient is stabilized.
An individual patient data meta-analysis of all the major beta-blocker trials in HFrEF has shown no benefit on hospital admissions and mortality in the subgroup of patients with HFrEF who are in AF.177 However, since this is a retrospective subgroup analysis, and because beta-blockers did not increase the risk, the guideline committee decided not to make a separate recommendation according to heart rhythm. Beta-blockers should be considered for rate control in patients with HFrEF and AF, especially in those with high heart rate (see Section 10.1 for details).
Beta-blockers are recommended in patients with a history of myocardial infarction and asymptomatic LV systolic dysfunction to reduce the risk of death (see Section 6).
Practical guidance on how to use beta-blockers is given in Web Table 7.5.
7.2.3 Mineralocorticoid/aldosterone receptor antagonists
MRAs (spironolactone and eplerenone) block receptors that bind aldosterone and, with different degrees of affinity, other steroid hormone (e.g. corticosteroids, androgens) receptors. Spironolactone or eplerenone are recommended in all symptomatic patients (despite treatment with an ACEI and a beta-blocker) with HFrEF and LVEF ≤35%, to reduce mortality and HF hospitalization.174, 175
Caution should be exercised when MRAs are used in patients with impaired renal function and in those with serum potassium levels >5.0 mmol/L. Regular checks of serum potassium levels and renal function should be performed according to clinical status.
Practical guidance on how to use MRAs is given in Web Table 7.6.
7.3 Other treatments recommended in selected symptomatic patients with heart failure with reduced ejection fraction
7.3.1 Diuretics
Diuretics are recommended to reduce the signs and symptoms of congestion in patients with HFrEF, but their effects on mortality and morbidity have not been studied in RCTs. A Cochrane meta-analysis has shown that in patients with chronic HF, loop and thiazide diuretics appear to reduce the risk of death and worsening HF compared with placebo, and compared with an active control, diuretics appear to improve exercise capacity.178, 179
Loop diuretics produce a more intense and shorter diuresis than thiazides, although they act synergistically and the combination may be used to treat resistant oedema. However, adverse effects are more likely and these combinations should only be used with care. The aim of diuretic therapy is to achieve and maintain euvolaemia with the lowest achievable dose. The dose of the diuretic must be adjusted according to the individual needs over time. In selected asymptomatic euvolaemic/hypovolaemic patients, the use of a diuretic drug might be (temporarily) discontinued. Patients can be trained to self-adjust their diuretic dose based on monitoring of symptoms/signs of congestion and daily weight measurements.
Doses of diuretics commonly used to treat HF are provided in Table 7.3. Practical guidance on how to use diuretics is given in Web Table 7.7.
7.3.2 Angiotensin receptor neprilysin inhibitor
A new therapeutic class of agents acting on the RAAS and the neutral endopeptidase system has been developed [angiotensin receptor neprilysin inhibitor (ARNI)]. The first in class is LCZ696, which is a molecule that combines the moieties of valsartan and sacubitril (neprilysin inhibitor) in a single substance. By inhibiting neprilysin, the degradation of NPs, bradykinin and other peptides is slowed. High circulating A-type natriuretic peptide (ANP) and BNP exert physiologic effects through binding to NP receptors and the augmented generation of cGMP, thereby enhancing diuresis, natriuresis and myocardial relaxation and anti-remodelling. ANP and BNP also inhibit renin and aldosterone secretion. Selective AT1-receptor blockade reduces vasoconstriction, sodium and water retention and myocardial hypertrophy.187, 188
A recent trial investigated the long-term effects of sacubitril/valsartan compared with an ACEI (enalapril) on morbidity and mortality in patients with ambulatory, symptomatic HFrEF with LVEF ≤40% (this was changed to ≤35% during the study), elevated plasma NP levels (BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL or, if they had been hospitalized for HF within the previous 12 months, BNP ≥100 pg/mL or NT-proBNP ≥400 pg/mL), and an estimated GFR (eGFR) ≥30 mL/min/1.73 m2 of body surface area, who were able to tolerate separate treatments periods with enalapril (10 mg b.i.d.) and sacubitril/valsartan (97/103 mg b.i.d.) during a run-in period.162 In this population, sacubitril/valsartan (97/103 mg b.i.d.) was superior to ACEI (enalapril 10 mg b.i.d.) in reducing hospitalizations for worsening HF, cardiovascular mortality and overall mortality.162 Sacubitril/valsartan is therefore recommended in patients with HFrEF who fit this profile.
Despite the superiority of sacubitril/valsartan over enalapril in the PARADIGM-HF trial, some relevant safety issues remain when initiating therapy with this drug in clinical practice. Symptomatic hypotension was more often present in the sacubitril/valsartan group (in those ≥75 years of age, it affected 18% in the sacubitril/valsartan group vs. 12% in the enalapril group), although there was no increase in the rate of discontinuation.162 The risk of angioedema in the trial was reduced by recruiting only those who tolerated therapy with enalapril 10 mg b.i.d. and an sacubitril/valsartan during an active run-in phase of 5–9 weeks (it resulted in a 0.4% rate of angioedema in sacubitril/valsartan group vs. 0.2% in an enalapril group). Also, the number of African American patients, who are at a higher risk of angioedema, was relatively small in this study. To minimize the risk of angioedema caused by overlapping ACE and neprilysin inhibition, the ACEI should be withheld for at least 36 h before initiating sacubitril/valsartan. Combined treatment with an ACEI (or ARB) and sacubitril/valsartan is contraindicated. There are additional concerns about its effects on the degradation of beta-amyloid peptide in the brain, which could theoretically accelerate amyloid deposition.189-191 However, a recent small 14-day study with healthy subjects showed elevation of the beta-amyloid protein in the soluble rather than the aggregable form, which if confirmed over longer time periods in patients with HFrEF may indicate the cerebral safety of sacubitril/valsartan.192 Long-term safety needs to be addressed.
7.3.3 If-channel inhibitor
Ivabradine slows the heart rate through inhibition of the If channel in the sinus node and therefore should only be used for patients in sinus rhythm. Ivabradine reduced the combined endpoint of mortality and hospitalization for HF in patients with symptomatic HFrEF and LVEF ≤35%, in sinus rhythm and with a heart rate ≥70 beats per minute (bpm) who had been hospitalized for HF within the previous 12 months, receiving treatment with an evidence-based dose of beta-blocker (or maximum tolerated dose), an ACEI (or ARB) and an MRA.180 The European Medicines Agency (EMA) approved ivabradine for use in Europe in patients with HFrEF with LVEF ≤35% and in sinus rhythm with a resting heart rate ≥75 bpm, because in this group ivabradine conferred a survival benefit193 based on a retrospective subgroup analysis requested by the EMA.
Practical guidance on how to use ivabradine is given in Web Table 7.8.
7.3.4 Angiotensin II type I receptor blockers
ARBs are recommended only as an alternative in patients intolerant of an ACEI.182 Candesartan has been shown to reduce cardiovascular mortality.182 Valsartan showed an effect on hospitalization for HF (but not on all-cause hospitalizations) in patients with HFrEF receiving background ACEIs.194
The combination of ACEI/ARB for HFrEF was reviewed by the EMA, which suggested that benefits are thought to outweigh risks only in a select group of patients with HFrEF in whom other treatments are unsuitable. Therefore, ARBs are indicated for the treatment of HFrEF only in patients who cannot tolerate an ACEI because of serious side effects. The combination of ACEI/ARB should be restricted to symptomatic HFrEF patients receiving a beta-blocker who are unable to tolerate an MRA, and must be used under strict supervision.
7.3.5 Combination of hydralazine and isosorbide dinitrate
There is no clear evidence to suggest the use of this fixed-dose combination therapy in all patients with HFrEF. Evidence on the clinical utility of this combination is scanty and comes from one relatively small RCT conducted exclusively in men and before ACEIs or beta-blockers were used to treat HF.184 A subsequent RCT conducted in self-identified black patients (defined as being of African descent) showed that addition of the combination of hydralazine and isosorbide dinitrate to conventional therapy (ACEI, beta-blocker and MRA) reduced mortality and HF hospitalizations in patients with HFrEF and NYHA Classes III–IV.183 The results of this study are difficult to translate to patients of other racial or ethnic origins.
Additionally, a combination of hydralazine and isosorbide dinitrate may be considered in symptomatic patients with HFrEF who can tolerate neither ACEI nor ARB (or they are contraindicated) to reduce mortality. However, this recommendation is based on the results of the Veterans Administration Cooperative Study, which recruited symptomatic HFrEF patients who received only digoxin and diuretics.184
7.4 Other treatments with less certain benefits in symptomatic patients with heart failure with reduced ejection fraction
This section describes treatments that have shown benefits in terms of symptomatic improvement, reduction in HF hospitalizations or both, and are useful additional treatments in patients with HFrEF.
7.4.1 Digoxin and other digitalis glycosides
Digoxin may be considered in patients in sinus rhythm with symptomatic HFrEF to reduce the risk of hospitalization (both all-cause and HF hospitalizations),185 although its effect on top of beta-blockers has never been tested. The effects of digoxin in patients with HFrEF and AF have not been studied in RCTs, and recent studies have suggested potentially higher risk of events (mortality and HF hospitalization) in patients with AF receiving digoxin.195, 196 However, this remains controversial, as another recent meta-analysis concluded on the basis of non-RCTs that digoxin has no deleterious effect on mortality in patients with AF and concomitant HF, most of whom had HFrEF.197
In patients with symptomatic HF and AF, digoxin may be useful to slow a rapid ventricular rate, but it is only recommended for the treatment of patients with HFrEF and AF with rapid ventricular rate when other therapeutic options cannot be pursued.196, 198-201 Of note, the optimal ventricular rate for patients with HF and AF has not been well established, but the prevailing evidence suggests that strict rate control might be deleterious. A resting ventricular rate in the range of 70–90 bpm is recommended based on current opinion, although one trial suggested that a resting ventricular rate of up to 110 bpm might still be acceptable.202 This should be tested and refined by further research.
Digitalis should always be prescribed under specialist supervision. Given its distribution and clearance, caution should be exerted in females, in the elderly and in patients with reduced renal function. In the latter patients, digitoxin should be preferred.
7.4.2 n-3 polyunsaturated fatty acids
n-3 polyunsaturated fatty acids (n-3 PUFAs) have shown a small treatment effect in a large RCT.186 n-3 PUFA preparations differ in composition and dose. Only preparations with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) as ethyl esters of at least 85% (850 mg/g) have shown an effect on the cumulative endpoint of cardiovascular death and hospitalization. No effect of n-3 PUFA preparations containing <850 mg/g has been shown in either HFrEF or post-myocardial infarction.203 n-3 PUFA preparations containing 850–882 mg of EPA and DHA as ethyl esters in the average ratio of 1 : 1.2 may be considered as an adjunctive therapy in patients with symptomatic HFrEF who are already receiving optimized recommended therapy with an ACEI (or ARB), a beta-blocker and an MRA.
7.5 Treatments not recommended (unproven benefit) in symptomatic patients with heart failure with reduced ejection fraction
7.5.1 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (‘statins’)
Although statins reduce mortality and morbidity in patients with atherosclerotic disease, statins are not effective in improving the prognosis in patients with HFrEF. Most statin trials excluded patients with HF (because it was uncertain that they would benefit).204 The two major trials that studied the effect of statin treatment in patients with chronic HF did not demonstrate any evidence of benefit.205 Therefore, evidence does not support the initiation of statins in most patients with chronic HF. However, in patients who already receive a statin because of underlying CAD or/and hyperlipidaemia, a continuation of this therapy should be considered.
7.5.2 Oral anticoagulants and antiplatelet therapy
Other than in patients with AF (both HFrEF and HFpEF), there is no evidence that an oral anticoagulant reduces mortality/morbidity compared with placebo or aspirin.206, 207 Studies testing the non-vitamin K antagonist oral anticoagulants (NOACs) in patients with HFrEF are currently ongoing. Patients with HFrEF receiving oral anticoagulation because of concurrent AF or risk of venous thromboembolism should continue anticoagulation. Detailed information is provided in Section 10.1.
Similarly, there is no evidence on the benefits of antiplatelet drugs (including acetylsalicylic acid) in patients with HF without accompanying CAD, whereas there is a substantial risk of gastrointestinal bleeding, particularly in elderly subjects, related with this treatment.
7.5.3 Renin inhibitors
7.6 Treatments not recommended (believed to cause harm) in symptomatic patients with heart failure with reduced ejection fraction
7.6.1 Calcium-channel blockers
Non-dihydropyridine calcium-channel blockers (CCBs) are not indicated for the treatment of patients with HFrEF. Diltiazem and verapamil have been shown to be unsafe in patients with HFrEF.214
There is a variety of dihydropyridine CCBs; some are known to increase sympathetic tone and they may have a negative safety profile in HFrEF. There is only evidence on safety for amlodipine215 and felodipine216 in patients with HFrEF, and they can be used only if there is a compelling indication in patients with HFrEF.
8 Non-surgical device treatment of heart failure with reduced ejection fraction
This section provides recommendations on the use of ICDs and CRT. Currently, the evidence is considered insufficient to support specific guideline recommendations for other therapeutic technologies, including baroreflex activation therapy,217 vagal stimulation,218 diaphragmatic pacing219, 220 and cardiac contractility modulation;221, 222 further research is required. Implantable devices to monitor arrhythmias or haemodynamics are discussed elsewhere in these guidelines.
8.1 Implantable cardioverter-defibrillator
8.1.1 Secondary prevention of sudden cardiac death
Compared with amiodarone treatment, ICDs reduce mortality in survivors of cardiac arrest and in patients who have experienced sustained symptomatic ventricular arrhythmias. An ICD is recommended in such patients when the intent is to increase survival; the decision to implant should take into account the patient's view and their quality of life, the LVEF (survival benefit is uncertain when the LVEF is >35%) and the absence of other diseases likely to cause death within the following year.223-225
8.1.2 Primary prevention of sudden cardiac death
Although amiodarone may have reduced mortality in older trials of HF,242, 243 contemporary studies conducted since the widespread introduction of beta-blockers suggest that it does not reduce mortality in patients with HFrEF.227, 244, 245 Dronedarone246, 247 and class I antiarrhythmic agents246, 248 should not be used for prevention of arrhythmias in this population.
Some guideline-recommended therapies, including beta-blockers, MRAs, sacubitril/valsartan and pacemakers with CRT (CRT-Ps), reduce the risk of sudden death (see Section 7).
An ICD reduces the rate of sudden arrhythmic death in patients with HFrEF.249, 250 In patients with moderate or severe HF, a reduction in sudden death may be partially or wholly offset by an increase in death due to worsening HF.227 In patients with mild HF (NYHA II), an ICD will prevent about two deaths per year for every 100 devices implanted.227 On average, patients with IHD are at greater risk of sudden death than patients with DCM and therefore, although the relative benefits are similar, the absolute benefit is greater in patients with IHD.249 Patients with longer QRS durations may also receive greater benefit from an ICD, but these patients should often receive a CRT device.227, 251
Two RCTs showed no benefit in patients who had an ICD implanted within 40 days after a myocardial infarction.158, 228 Although sudden arrhythmic deaths were reduced, this was balanced by an increase in non-arrhythmic deaths. Accordingly, an ICD is contraindicated in this time period. A wearable defibrillator may be considered if the patient is deemed to be at high risk of ventricular fibrillation, although evidence from randomized trials is lacking.239-241
ICD implantation is recommended only after a sufficient trial (minimum 3 months) of optimal medical therapy (OMT) has failed to increase the LVEF to >35%. However, one of the two landmark papers on which these recommendations are based included patients with an LVEF >30%. Fewer than 400 patients with an LVEF of 30–35% were included in the landmark studies, and although there was no statistical interaction between treatment effect and LVEF, the evidence of benefit is less robust in this group of patients.
Conservative programming with long delays252 between detection and the ICD delivering therapy dramatically reduces the risk of both inappropriate (due to artefacts or AF) and appropriate but unnecessary [due to self-terminating ventricular tachycardia (VT)] shocks.252-254
Patients with a QRS duration ≥130 ms should be considered for a defibrillator with CRT (CRT-D) rather than ICD. See the guideline on CRT for further details (Section 8.2).
ICD therapy is not recommended in patients in NYHA Class IV with severe symptoms refractory to pharmacological therapy who are not candidates for CRT, a ventricular assist device or cardiac transplantation, because such patients have a very limited life expectancy and are likely to die from pump failure.
Patients with serious co-morbidities who are unlikely to survive substantially more than 1 year are unlikely to obtain substantial benefit from an ICD.229-233
Patients should be counselled as to the purpose of an ICD, complications related to implantation and device activation (predominantly inappropriate shocks) and under what circumstances it might be deactivated (terminal disease) or explanted (infection, recovery of LV function).255
If HF deteriorates, deactivation of a patient's ICD may be considered after appropriate discussion with the patient and caregiver(s).
If the ICD generator reaches its end of life or requires explantation, it should not automatically be replaced.234-238 Patients should be carefully evaluated by an experienced cardiologist before generator replacement. Treatment goals may have changed and the risk of fatal arrhythmia may be lower or the risk of non-arrhythmic death higher. It is a matter of some controversy whether patients whose LVEF has greatly improved and who have not required device therapy during the lifetime of the ICD should have another device implanted.234-238
Subcutaneous defibrillators may be as effective as conventional ICDs with a lower risk from the implantation procedure.256, 257 They may be the preferred option for patients with difficult access or who require ICD explantation due to infection. Patients must be carefully selected, as they have limited capacity to treat serious bradyarrhythmia and can deliver neither antitachycardia pacing nor CRT. Substantial RCTs with these devices and more data on safety and efficacy are awaited.258, 259
A wearable ICD (an external defibrillator with leads and electrode pads attached to a wearable vest) that is able to recognize and interrupt VT/ventricular fibrillation may be considered for a limited period of time in selected patients with HF who are at high risk for sudden death but otherwise are not suitable for ICD implantation (e.g. those with poor LVEF after acute myocardial damage until LV function recovers, patients scheduled for heart transplantation).239-241, 260 However, no prospective RCTs evaluating this device have been reported.
For detailed recommendations on the use/indications of ICD we refer the reader to the ESC/European Heart Rhythm Association (EHRA) guidelines on ventricular tachyarrhythmias and sudden cardiac death.260
8.2 Cardiac resynchronization therapy
CRT improves cardiac performance in appropriately selected patients and improves symptoms286 and well-being286 and reduces morbidity and mortality.266 Of the improvement in quality-adjusted life-years (QALYs) with CRT among patients with moderate to severe HF, two-thirds may be attributed to improved quality of life and one-third to increased longevity.287
Only the COMPANION265 and CARE-HF trials262, 263 compared the effect of CRT to guideline-advised medical therapy. Most other trials have compared CRT-D to ICD, and a few have compared CRT-P to backup pacing. The prevention of lethal bradycardia might be an important mechanism of benefit shared by all pacing devices. In CARE-HF, at baseline, 25% of patients had a resting heart rate of ≤60 bpm.262-264 If prevention of bradycardia is important, the effect of CRT will appear greater in trials where there is no device in the control group.
Most studies of CRT have specified that the LVEF should be <35%, but RAFT267 and MADIT-CRT268, 269 specified an LVEF <30%, while REVERSE270-272 specified <40% and BLOCK-HF274 <50%. Relatively few patients with an LVEF of 35–40% have been randomized, but an individual participant data (IPD) meta-analysis suggests no diminution of the effect of CRT in this group.266
Not all patients respond favourably to CRT.286 Several characteristics predict improvement in morbidity and mortality, and the extent of reverse remodelling is one of the most important mechanisms of action of CRT. Patients with ischaemic aetiology will have less improvement in LV function due to myocardial scar tissue, which is less likely to undergo favourable remodelling.288 Conversely, women may be more likely to respond than men, possibly due to smaller body and heart size.273, 285, 289 QRS width predicts CRT response and was the inclusion criterion in all randomized trials. But QRS morphology has also been related to a beneficial response to CRT. Several studies have shown that patients with left bundle branch block (LBBB) morphology are more likely to respond favourably to CRT, whereas there is less certainty about patients with non-LBBB morphology. However, patients with LBBB morphology often have wider QRS duration, and there is a current debate about whether QRS duration or QRS morphology is the main predictor of a beneficial response to CRT. Evidence from two IPD meta-analyses indicates that after accounting for QRS duration, there is little evidence to suggest that QRS morphology or aetiology of disease influence the effect of CRT on morbidity or mortality.266, 273 In addition, none of the landmark trials selected patients for inclusion according to QRS morphology, sex or ischaemic aetiology, nor were they powered for subgroup analyses.
The Echo-CRT283, 284 trial and an IPD meta-analysis266 suggest possible harm from CRT when QRS duration is <130 ms, thus implantation of CRT is not recommended if QRS duration is <130 ms.266, 283, 284
If a patient is scheduled to receive an ICD and is in sinus rhythm with a QRS duration ≥130 ms, CRT-D should be considered if QRS is between 130 and 149 ms and is recommended if QRS is ≥150 ms. However, if the primary reason for implanting a CRT is for the relief of symptoms, then the clinician should choose CRT-P or CRT-D, whichever they consider appropriate. Clinical practice varies widely among countries. The only randomized trial to compare CRT-P and CRT-D265 failed to demonstrate a difference in morbidity or mortality between these technologies.288 If the primary reason for implanting CRT is to improve prognosis, then the majority of evidence lies with CRT-D for patients in NYHA Class II and with CRT-P for patients in NYHA Classes III–IV. It is unclear whether CRT reduces the need for an ICD (by reducing the arrhythmia burden) or increases the benefit from an ICD (by reducing mortality rates from worsening HF, leading to longer exposure to the risk of arrhythmia).
When LVEF is reduced, RV pacing may exacerbate cardiac dyssynchrony. This can be prevented by CRT, which might improve patient outcomes.274, 275, 277, 290 However, a difference in outcome was not observed between CRT and RV pacing in a subgroup analysis of RAFT267 or in patients without HFrEF in BioPACE.291 On balance, CRT rather than RV pacing is recommended for patients with HFrEF regardless of NYHA class who have an indication for ventricular pacing in order to reduce morbidity, although no clear effect on mortality was observed. Patients with HFrEF who have received a conventional pacemaker or an ICD and subsequently develop worsening HF with a high proportion of RV pacing, despite OMT, should be considered for upgrading to CRT.
Only two small trials have compared pharmacological therapy alone vs. CRT in patients with AF, with conflicting results. Several studies have indicated that CRT is superior to RV pacing in patients undergoing atrio-ventricular (AV) node ablation.275, 277, 290 However, CRT is not an indication to carry out AV node ablation except in rare cases when ventricular rate remains persistently high (>110 bpm) despite attempts at pharmacological rate control. A subgroup analysis of patients with AF from the RAFT study found no benefit from CRT-D compared with ICD, although less than half of patients had >90% biventricular capture.276 Observational studies report that when biventricular capture is <98%, the prognosis of patients with CRT declines.277 Whether this association reflects a loss of resynchronization (which might be remedied by device programming), poor placing of the LV lead (which might be avoided at implantation) or greater difficulty in pacing severely diseased myocardium (which might not be amenable to the above) is uncertain. This observation has not been confirmed in a randomized trial.
Imaging tests for dyssynchrony have not yet been shown to be of value in selecting patients for CRT.292 Patients with extensive myocardial scar will have less improvement in LV function with CRT, but this is true of any treatment for HFrEF and does not reliably predict less clinical benefit.293 Pacing thresholds are higher in scarred myocardium and, if possible, lead placement should avoid such regions.294, 295 Although patients with extensive scarring have an intrinsically worse prognosis, there is little evidence that they obtain less prognostic benefit from CRT.266
The reader is directed to guidelines on pacing and CRT for recommendations on device implantation procedures. The value of trying to optimize AV or VV intervals after implantation using echo- or electrocardiographic criteria or blood pressure response is uncertain, but may be considered for patients who have had a disappointing response to CRT.296, 297
8.3 Other implantable electrical devices
For patients with HFrEF who remain symptomatic despite OMT and do not have an indication for CRT, new device therapies have been proposed and in some cases are approved for clinical use in several European Union (EU) countries but remain under trial evaluation.
Cardiac contractility modulation (CCM) is similar in its mode of insertion to CRT, but it involves non-excitatory electrical stimulation of the ventricle during the absolute refractory period to enhance contractile performance without activating extra systolic contractions. CCM has been evaluated in patients with HFrEF in NYHA Classes II–III with normal QRS duration (<120 ms).221, 222 An individual patient data meta-analysis demonstrated an improvement in exercise tolerance (peak VO2) and quality of life (Minnesota Living with Heart Failure questionnaire). Thus CCM may be considered in selected patients with HF. The effect of CCM on HF morbidity and mortality remains to be established.
Most other devices under evaluation involve some modification of the activity of the autonomic nervous system (ANS) by targeted electrical stimulation.298, 299 These include vagal nerve stimulation, spinal cord stimulation, carotid body ablation and renal denervation, but so far none of the devices has improved symptoms or outcomes in RCTs.
Devices for remote monitoring are discussed in Section 14.
9 Treatment of heart failure with preserved ejection fraction
While there is clear agreement that the diagnosis of HFrEF requires an LVEF <40%, the exact definition of HFpEF is less clear. According to the definition provided in this document (see Section 3), the diagnosis of HFpEF requires an LVEF ≥50%, whereas patients with LVEF between 40 and 49% are considered to have HFmrEF (for details, please refer to Section 3). Patients with HFmrEF have generally been included in trials of HFpEF. Accordingly, the guidance in this section applies to patients with both HFmrEF and HFpEF. As new data and analyses become available, it might be possible to make recommendations for each phenotype separately.
In clinical practice and clinical trials, compared with HFrEF patients, only slightly fewer patients with HFpEF and HFmrEF currently appear to receive diuretics, beta-blockers, MRAs and ACEIs or ARBs.166, 300-302 This may reflect treatment of cardiovascular co-morbidities, such as hypertension, CAD and AF, or extrapolation of results from trials conducted for these conditions showing a reduction in new-onset HF,127 or failure to distinguish between guideline recommendations for HFrEF and HFmrEF/HFpEF or a belief that existing clinical trials provide some evidence of benefit with these agents.
A summary of phase II and III clinical trials of patients with HFpEF and HFmrEF is presented in Web Table 9.1.
The pathophysiology underlying HFpEF and HFmrEF is heterogeneous, and they are associated with different phenotypes including diverse concomitant cardiovascular diseases (e.g. AF, arterial hypertension, CAD, pulmonary hypertension) and non-cardiovascular diseases [diabetes, chronic kidney disease (CKD), anaemia, iron deficiency, COPD and obesity].303, 304 Compared with HFrEF patients, hospitalizations and deaths in patients with HFmrEF/HFpEF are more likely to be non-cardiovascular.305, 306 Therefore patients should be screened for cardiovascular and non-cardiovascular co-morbidities, which if present should be managed with interventions that have been shown to improve symptoms, well-being or outcome related to that co-morbidity and not to exacerbate HF (see Section 11).
No treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF or HFmrEF. However, since these patients are often elderly and highly symptomatic, and often have a poor quality of life,307 an important aim of therapy may be to alleviate symptoms and improve well-being.308
9.1 Effect of treatment on symptoms in heart failure with preserved ejection fraction
Diuretics will usually improve congestion, if present, thereby improving symptoms and signs of HF. The evidence that diuretics improve symptoms is similar across the spectrum of LVEF.178, 179
Evidence that beta-blockers and MRAs improve symptoms in these patients is lacking. There is inconsistent evidence for an improvement in symptoms in those treated with ARBs (only for candesartan was there an improvement in NYHA class)309, 310 and ACEIs.311
9.2 Effect of treatment on hospitalization for heart failure in heart failure with preserved ejection fraction
For patients in sinus rhythm, there is some evidence that nebivolol,173, 312, 313 digoxin,314 spironolactone301 and candesartan310 might reduce HF hospitalizations. For patients in AF, beta-blockers do not appear to be effective and digoxin has not been studied. The evidence in support of either ARBs315 or ACEIs311 is inconclusive.
9.3 Effect of treatment on mortality in heart failure with preserved ejection fraction
Trials of ACEIs, ARBs, beta-blockers and MRAs have all failed to reduce mortality in patients with HFpEF or HFmrEF. However, in older patients with HFrEF, HFpEF or HFmrEF, nebivolol reduced the combined endpoint of death or cardiovascular hospitalization,173, 312 with no significant interaction between treatment effect and baseline LVEF.313
9.4 Other considerations
Patients in AF should receive an anticoagulant to reduce the risk of thromboembolic events (for details, see the ESC guidelines of AF316]. Antiplatelet agents are ineffective for this purpose. Renal dysfunction, which is common in this population, may contraindicate or increase the risk of haemorrhage with NOACs.
The optimal ventricular rate in patients with HFmrEF/HFpEF and AF is uncertain, and aggressive rate control might be deleterious. Whether digoxin, beta-blockers or rate-limiting CCBs, or a combination of these, should be preferred is unknown. Verapamil or diltiazem should not be combined with a beta-blocker. There are insufficient data to recommend ablation strategies (either pulmonary venous or AV node) for HFpEF and HFmrEF.
Circumstantial evidence suggests that treating hypertension, often predominantly systolic, is important in HFmrEF/HFpEF.127, 317 Diuretics, ACEIs, ARBs and MRAs all appear appropriate agents, but beta-blockers may be less effective in reducing SBP. A recent study suggests that patients with hypertension and HFpEF or HFmrEF should not receive an ARB (olmesartan) if they are receiving ACEIs and beta-blockers.318
The first-line oral hypoglycaemic drug for patients with HFpEF and HFmrEF should be metformin319 (see also Section 11.6). Recently, a trial of empagliflozin showed a reduction in blood pressure and body weight, probably by inducing glycosuria and osmotic diuresis. Its use was associated with a reduction in hospitalization for HF and in cardiovascular mortality.130 However, aggressive management of dysglycaemia may be harmful.153, 320
Myocardial ischaemia may contribute to symptoms, morbidity and mortality and should be considered when assessing patients. However, there is only anecdotal evidence that revascularization improves symptoms or outcome. Patients with angina should follow the same management route as patients with HFrEF.112
10 Arrhythmias and conductance disturbances
Ambulatory electrocardiographic monitoring can be used to investigate symptoms that may be due to arrhythmias,322-324 but evidence is lacking to support routine, systematic monitoring for all patients with HF to identify tachy- and bradyarrhythmias. There is no evidence that clinical decisions based on routine ambulatory electrocardiographic monitoring improve outcomes for patients with HF.
Ambulatory electrocardiographic recording detects premature ventricular complexes in virtually all patients with HF. Episodes of asymptomatic, non-sustained VT are common, increasing in frequency with the severity of HF and ventricular dysfunction and indicating a poor prognosis in patients with HF, but provide little discrimination between sudden death or death due to progressive HF.316, 325 Bradycardia and pauses are also commonly observed, especially at night when sympathetic activity is often lower and parasympathetic activity higher; sleep apnoea may be a trigger.326-328 Pauses are associated with a poor prognosis in patients with CAD and left ventricular dysfunction.329 Bradyarrhythmias may make an important contribution to sudden death in HF.330
10.1 Atrial fibrillation
AF is the most common arrhythmia in HF irrespective of concomitant LVEF; it increases the risk of thromboembolic complications (particularly stroke) and may impair cardiac function, leading to worsening symptoms of HF.316 Incident HF precipitated by AF is associated with a more benign prognosis,331 but new-onset AF in a patient with established HF is associated with a worse outcome, probably because it is both a marker of a sicker patient and because it impairs cardiac function.332, 333 Patients with chronic HF and permanent AF have a worse outcome than those in sinus rhythm, although this is largely explained by more advanced age and HF severity.332, 333 Persistent ventricular rates >150 bpm may cause HFrEF that resolves with rate control or rhythm correction (‘tachycardiomyopathy’).334, 335 AF should be classified and managed according to the current AF guidelines (i.e. first diagnosed episode, paroxysmal, persistent, long-standing persistent or permanent), recognizing the uncertainty about the actual duration of the episode and about previous undetected episodes.316
-
identification of potentially correctable causes (e.g. hypothyroidism or hyperthyroidism, electrolyte disorders, uncontrolled hypertension, mitral valve disease) and precipitating factors (e.g. recent surgery, chest infection or exacerbation of COPD/asthma, acute myocardial ischaemia, alcohol binge), as this may determine management strategy;
-
assessment of stroke risk and need for anticoagulation;
-
assessment of ventricular rate and need for rate control;
-
evaluation of symptoms of HF and AF.
10.1.1 Prevention of atrial fibrillation in patients with heart failure
Many treatments for HF, including ACEIs,336 ARBs,337 beta-blockers177, 338 and MRAs,339, 340 will reduce the incidence of AF, but ivabradine may increase it.341 CRT has little effect on the incidence of AF.342
Amiodarone will reduce the incidence of AF, induce pharmacological cardioversion, maintain more patients in sinus rhythm after cardioversion and may be used to control symptoms in patients with paroxysmal AF if beta-blockers fail to do so.343-346 Amiodarone should generally be restricted to short-term (<6 months) use in patients with paroxysmal or persistent AF to help attain sinus rhythm and to reduce the high rate of recurrent AF immediately after cardioversion. Dronedarone is contraindicated in patients with HF and AF.246, 247, 347
10.1.2 Management of new-onset, rapid atrial fibrillation in patients with heart failure
10.1.3 Rate control
Assessment of ventricular rate control from the radial pulse is not ideal, especially in patients with HF, as ventricular activation may not always generate a palpable pulse. Rate control should be documented electrocardiographically. A wearable device enables ventricular rate to be assessed during rest, exercise and sleep, but the value of routine monitoring has not yet been established. Implanted devices such as pacemakers, CRT or ICDs can also be used to measure ventricular rate.
The optimal resting ventricular rate in patients with AF and HF is uncertain but may be between 60–100 bpm.350, 352-354 One trial suggested that a resting ventricular rate of up to 110 bpm might still be acceptable,198, 202 and 2016 ESC AF guidelines recommend this threshold as the target for rate control therapy.316 However, this Task Force believes that a lower rate for patients with HF may be preferable (60–100 bpm). Ventricular rates <70 bpm are associated with a worse outcome.351 This may explain why beta-blockers titrated to guideline-target doses failed to reduce morbidity or mortality in patients with HFrEF and AF,177 and might also explain the association between digoxin and adverse outcomes reported in some observational studies of AF.355-357 The optimal ventricular rate during exercise is also uncertain, but may be <110 bpm during light exercise.354 Beta-blockers, digoxin and their combination may be used to control ventricular rate.358 It is uncertain which approach is optimal, but beta-blockers appear safe as the first-line agent even if it is not clear that they reduce morbidity and mortality in patients with AF. Beta-blockers reduce ventricular rate during periods of activity, while digoxin exerts a greater effect at night.358 Persistently high ventricular rates may indicate thyrotoxicosis or excessive sympathetic activity due to congestion, which might respond to diuresis. Although amiodarone and non-dihydropyridine CCBs can reduce ventricular rate, they have more adverse effects and should generally be avoided in patients with HFrEF and, with less certainty, in patients with HFpEF and HFmrEF. Rarely, ventricular rate cannot be reduced below 100–110 bpm by pharmacological means alone and AV node ablation with ventricular pacing may be considered; in this situation, for patients with HFrEF, CRT should be considered instead of conventional RV pacing. There is little evidence, other than from registries, to support a strategy of AV node ablation and CRT compared with pharmacological therapy alone in patients with AF and a resting ventricular rate <100–110 bpm (see Section 8.2).281 However, in patients with a fast ventricular rate and intractable symptoms, AV node ablation may be considered. Also, if the patient is indicated for an ICD, AV node ablation with implantation of CRT-D may be a preferred option, especially if the patient has moderate to severe symptoms.
10.1.4 Rhythm control
In patients with chronic HF, a rhythm control strategy (including pharmacological or electrical cardioversion) has not been shown to be superior to a rate control strategy in reducing mortality or morbidity.359 Urgent cardioversion is indicated only if the AF is life threatening, otherwise both HF and ventricular rate should be controlled prior to cardioversion. A rhythm control strategy is probably best reserved for patients with a reversible secondary cause of AF (e.g. hyperthyroidism) or an obvious precipitant (e.g. recent pneumonia) and in patients with troublesome symptoms due to AF after optimization of rate control and HF therapy. The use of class I antiarrhythmic agents and dronedarone increases morbidity and mortality in patients with HF and AF and should be avoided.246, 247, 347 Amiodarone will cause some patients with chronic AF to revert to sinus rhythm, may reduce symptomatic paroxysms of AF and will help maintain patients in sinus rhythm after spontaneous or electrical cardioversion.343-346 When used, the need for continued administration of amiodarone should be regularly reviewed and justified.
10.1.5 Thromboembolism prophylaxis
Patients with HF and AF should generally be anticoagulated and the balance of benefit and risk of bleeding (using CHA2DS2-VASc and HAS-BLED scores; for details, please see Web Tables 10.1 and 10.2.) should be evaluated as recommended in the ESC guidelines for AF.316 A substantial proportion of patients with HF will have both benefit and risk scores ≥3, indicating that careful consideration should be given before prescribing an oral anticoagulant and that regular review is subsequently needed (and correctable risk factors for bleeding addressed) if an oral anticoagulant is given.
NOACs are preferred for patients with HF with non-valvular AF, as NOACs compared with vitamin K antagonists seem to be at least similarly effective and even safer (less intracranial haemorrhage) in patients with HF than in subjects without HF,316, 366, 367 although concerns exist about their safety in older patients with HF and poor renal function368, 369 [for a detailed description of the interaction between NOAC and renal function, see Heidbuchel et al.370]. In patients with HF and AF who have mechanical heart valves or at least moderate mitral stenosis, only oral vitamin K antagonists should be used for prevention of thromboembolic stroke.370
The dabigatran dose should be reduced to 110 mg b.i.d. when creatinine clearance is 30–49 mL/min, rivaroxaban to 15 mg daily and edoxaban to 30 mg daily when creatinine clearance is 30–50 mL/min and apixaban to 2.5 mg twice daily if a patient has two or more of the following: age ≥80 years, serum creatinine ≥1.5 mg/dL or body weight ≤60 kg.370-375 The summary of the recommendations for the prevention of thromboembolism in patients with symptomatic HF and paroxysmal or persistent/permanent AF is presented in the recommendations table. For further details, please refer to the recent ESC guidelines on AF.316
10.2 Ventricular arrhythmias
The initial management of asymptomatic ventricular arrhythmias is correction of electrolyte abnormalities, particularly low serum potassium and magnesium, withdrawal of agents that might provoke arrhythmias and, in patients with HFrEF, optimization of pharmacological therapy with ACEIs, beta-blockers and MRAs and sacubitril/valsartan, which all reduce the risk of sudden death.174, 177, 383, 384
The clinical relevance of myocardial ischaemia for the provocation of ventricular arrhythmias is uncertain, although anecdotal cases of ischaemia-induced arrhythmias exist. Randomized trials of revascularization for patients with HFrEF have not reduced overall mortality,107, 385 even in subgroups of patients with angina or myocardial ischaemia,115, 386 but further analysis did suggest a reduction in sudden deaths.387
10.3 Symptomatic bradycardia, pauses and atrio-ventricular block
11 Co-morbidities
11.1 Heart failure and co-morbidities
Co-morbidities are of great importance in HF (Table 11.1) and may affect the use of treatments for HF (e.g. it may not be possible to use renin–angiotensin system inhibitors is some patients with severe renal dysfunction) (see Section 7). The drugs used to treat co-morbidities may cause worsening of HF (e.g. NSAIDs given for arthritis, some anti-cancer drugs) (see Section 7). Management of co-morbidities is a key component of the holistic care of patients with HF (see Section 14). Many co-morbidities are actively managed by specialists in the field of the co-morbidity, and these physicians will follow their own specialist guidelines. The current guidelines will identify where the presence of HF should change the way a co-morbidity would normally be treated. This may be because either safety or efficacy may be different in the presence of HF (or may simply be unknown) or because of evidence of particular effects in an HF population, either beneficial or detrimental. HFpEF has an even higher prevalence of co-morbidities compared with HFrEF, and many of these may be instrumental in the progression of this syndrome.398
11.2 Angina and coronary artery disease
11.2.1 Pharmacological management
Beta-blockers, and in selected patients ivabradine,180 are effective agents for angina control, as well as an essential component of HFrEF therapy. In HFpEF patients, they may also be used for angina relief, although this has never been formally tested. In the SIGNIFY trial in patients with activity-limiting angina without HF, ivabradine increased the risk of death from cardiovascular causes or non-fatal myocardial infarction and therefore is not recommended in this setting.399
Trimetazidine has been shown to exert some beneficial effect as an add-on to beta-blockers in patients with HF and angina.400-406 There are data suggesting that it may improve NYHA functional capacity, exercise duration and LV function in patients with HFrEF.402-406 Certain other effective anti-anginal drugs have been studied in sizeable numbers of HFrEF/LV dysfunction patients and shown to be safe [e.g. amlodipine,215, 407 nicorandil408 and nitrates183, 184, 409]. The safety of other anti-anginal agents in HFrEF, such as ranolazine, is uncertain, while other drugs, specifically diltiazem and verapamil, are thought to be unsafe in patients with HFrEF (although they may be used in HFpEF).214 Dihydropyridine CCBs may all increase sympathetic tone, and their safety in HFrEF [except amlodipine215 and felodipine216] and HFpEF is uncertain.
11.2.2 Myocardial revascularization
For indications for invasive coronary angiography in patients with HF, please refer to Section 5.8.
Percutaneous and surgical revascularization are complementary approaches for symptomatic relief of angina in HFpEF, but whether these interventions improve outcomes is not entirely clear. Recent ESC guidelines on myocardial revascularization recommended coronary artery bypass grafting (CABG) for patients with significant left main stenosis and left main equivalent (proximal stenosis of both the left anterior descending and left circumflex arteries) to improve prognosis.112, 113 However, one needs to be aware of a lack of studies including patients who have well-defined HF, therefore this recommendation is solely based on expert opinion. On the basis of the results of the STICH trial [which excluded patients with left main disease and Canadian Cardiovascular Society (CCS) angina classes III–IV], CABG is also recommended in patients with HFrEF, significant CAD (left anterior descending artery or multivessel disease) and LVEF ≤35% to reduce death and hospitalization for cardiovascular causes.385 Patients with >10% dysfunctional but viable LV myocardium may be more likely to benefit from myocardial revascularization (and those with ≤10% are less likely to benefit), although this approach to patient selection for revascularization is unproven. In the STICH trial, neither the presence of viability nor the severity of LV remodelling identified those who benefited from CABG in terms of a reduction in mortality.118 For the assessment of techniques to assess myocardial viability, please refer to Section 5. Post hoc analyses from the STICH trial revealed that the presence of inducible myocardial ischaemia (either on radionuclide stress test or dobutamine stress echocardiogram) or angina does not identify those with worse prognosis and greater benefit from CABG over OMT.115, 386 However, CABG does improve angina to a greater extent than medical therapy alone.
11.3 Cachexia and sarcopenia (for frailty, please refer to Section 14)
Cachexia is a generalized wasting process affecting all body compartments [i.e. lean tissue (skeletal muscle), fat tissue (energy reserves) and bone tissue (osteoporosis)]. It may occur in 5–15% of patients with HF, especially those with HFrEF, and more advanced disease status.414-416 This serious complication is associated with more severe symptoms and reduced functional capacity, more frequent hospitalization and decreased survival. Cachexia in HF can be diagnosed and defined as involuntary non-oedematous weight loss ≥6% of total body weight within the previous 6–12 months.414-417
The causes are multifactorial, and in individual patients they are difficult to determine. These may include pro-inflammatory immune activation, neurohormonal derangements, poor nutrition and malabsorption, impaired calorie and protein balance, anabolic hormone resistance, reduced anabolic drive, prolonged immobilization and physical deconditioning, together characterized by catabolic/anabolic imbalance.418 Skeletal muscle wasting, when associated with impaired mobility and symptoms (termed sarcopenia or myopenia), occurs in 30–50% of patients with HFrEF.419 In its most severe form it is associated with frailty and poor morbidity and mortality.420
Potential treatments may include appetite stimulants, exercise training120 and anabolic agents, including testosterone, in combination with the application of nutritional supplements and anti-catabolic interventions, although none is of proven benefit and their safety is unknown.421
11.4 Cancer
Certain chemotherapeutic agents can cause (or aggravate) LV systolic dysfunction and HF. The best recognized of these are the anthracyclines (e.g. doxorubicin), trastuzumab and tyrosine kinase inhibitors.397, 422 A recent Cochrane review found that dexrazoxane may confer some cardioprotection in patients receiving anthracyclines.423 Pre- and post-evaluation of LVEF, if available with myocardial strain imaging, is essential in patients receiving cardiotoxic chemotherapy, as detailed elsewhere.397, 422 A risk score for identifying women with breast cancer at risk of developing HF during trastuzumab therapy has been developed based on age, chemotherapy details, baseline cardiovascular status and other co-morbidities, and may be helpful.424 Chemotherapy should be discontinued and HFrEF therapy commenced in patients developing moderate to severe LV systolic dysfunction. If LV function improves, the risks and benefits of further chemotherapy need to be reconsidered.397, 425, 426 Mediastinal irradiation can also lead to a variety of long-term cardiac complications. Cardiac biomarkers (NPs and troponins) can be used to identify patients at higher risk of cardiotoxicity and may be helpful in monitoring the use and dosing of cardiotoxic cytotoxics.397, 425, 426
11.5 Central nervous system (including depression, stroke and autonomic dysfunction)
Stroke and HF commonly coexist because of an overlap of shared risk factors. Both contribute to a worse prognosis. Stroke may make self-care more difficult for the HF patient. Management of high-risk stroke patients may require balancing the risk of anticoagulant and antiplatelet therapies.
Autonomic dysfunction is common in HFrEF, especially when severe.427 Combined with low blood pressure, it can make fainting and injuries more likely and can interfere with optimal dosing of beta-blockers, ACEIs, ARBs and MRAs. Diuretic dosage may be reduced to reduce the severity of postural hypotension.
Depression is common and is associated with worse clinical status and a poor prognosis in HF.428-430 It may also contribute to poor adherence and social isolation. A high index of suspicion is needed to make the diagnosis, especially in the elderly. Routine screening using a validated questionnaire is good practice. Until now, the Beck Depression Inventory (BDI) and Cardiac Depression Scale have been formally validated as reliable tools for the assessment of depressive mood in patients with HF,431, 432 but other questionnaires have been broadly used in this group of patients (e.g. Geriatric Depression Scale, Hamilton Depression Scale, Hospital Anxiety and Depression Scale).
Psychosocial intervention and pharmacological treatment are helpful, as well as exercise training, in patients with HFrEF and depression.433 Cognitive behavioural therapy delivered in patients with HF and major depression beyond standard care and a structured education programme were able to reduce depression severity, anxiety and fatigue symptoms, as well as improve social functioning and mental and HF-related quality of life.434
Selective serotonin reuptake inhibitors are thought to be safe, although the Sertraline Antidepressant Heart Attack Randomized Trial did not confirm that sertraline provides a greater reduction in depressive symptoms or improvement in cardiovascular status compared with placebo in HFrEF patients, but this trial was not powered enough to prove the latter.435 Similarly, escitalopram had no effect on either depression or clinical outcomes during the 24-month follow-up as compared with placebo in patients with HFrEF and depression. Importantly, tricyclic antidepressants should be avoided, because they may cause hypotension, worsening HF and arrhythmias.429, 435
11.6 Diabetes
Dysglycaemia and diabetes are very common in HF, and diabetes is associated with poorer functional status and worse prognosis. In patients with HFrEF, interventions that reduce morbidity and mortality confer similar benefit in the presence or absence of diabetes.320 For instance, beta-blockers improve outcome similarly, whether or not the patient has diabetes, although different beta-blockers may vary in their effects on glycaemic indices.436
Whether strict glycaemic control alters the risk of cardiovascular events in patients with HF is uncertain.437 Among patients with HF who have not been treated for diabetes, higher HbA1c is associated with greater risk of cardiovascular events,438, 439 but this may not be the case once treatment for diabetes has been commenced.439
In patients with diabetes and HF, glycaemic control should be implemented gradually and moderately, giving preference to those drugs, such as metformin, that have been shown to be safe and effective. In contrast to what was previously believed, metformin is safe to use in patients with HFrEF, and it should be the treatment of choice in patients with HF440, 441 but is contraindicated in patients with severe renal or hepatic impairment, because of the risk of lactic acidosis.
Insulin is required for patients with type 1 diabetes and to treat symptomatic hyperglycaemia in patients with type 2 diabetes and pancreatic islet β cell exhaustion. However, insulin is a powerful sodium-retaining hormone, and when combined with a reduction in glycosuria, may exacerbate fluid retention, leading to HF worsening. Sulphonylurea derivatives have also been associated with an increased risk of worsening HF and should be used with caution.
Thiazolidinediones (glitazones) cause sodium and water retention and increased risk of worsening HF and hospitalization and are not recommended in patients with HF.209, 210 Dipeptidylpeptidase-4 inhibitors (DPP4is; gliptins), which increase incretin secretion, thereby stimulating insulin release, and long-acting glucagon-like peptide 1 (GLP-1) receptor agonists, which act as incretin mimetics, improve glycaemic indices but do not reduce and may increase the risk of cardiovascular events and worsening HF.320, 442, 443 Importantly, there are no data on the safety of gliptins and GLP-1 analogues in patients with HF.
Recently, empagliflozin, an inhibitor of sodium-glucose co-transporter 2, reduced hospitalization for HF and mortality, but not myocardial infarction or stroke, in patients with diabetes at high cardiovascular risk, some of whom had HF.130 In the absence of other studies with drugs from this group, the results obtained with empaglifozin cannot be considered as a proof of a class effect.
As glycaemic derangement progresses, the judgement on glycaemic control should be made according to cardiac conditions, and if the new anti-diabetic drugs are to be prescribed, they have to be closely monitored by an HF team.
11.7 Erectile dysfunction
Erectile dysfunction is a common and important component of quality of life in men with HF.444, 445 Its treatment should include optimal therapies for underlying cardiovascular diseases and other interfering co-morbidities (e.g. diabetes) and amelioration of anxiety and depressive symptoms. Some drugs applied for HF therapy (e.g. thiazide diuretics, spironolactone and beta-blockers) may augment erectile dysfunction.444, 445 Phosphodiesterase type 5 inhibitors (PDE5Is) have been shown to have favourable haemodynamic and anti-remodelling effects and to improve exercise capacity and quality of life in patients with HFrEF,446, 447 but they are contraindicated in patients taking nitrates.
11.8 Gout and arthritis
Hyperuricaemia and gout are common in HF and may be caused or aggravated by diuretic treatment. Hyperuricaemia is associated with a worse prognosis in HFrEF.448 The current European League Against Rheumatism (EULAR) guideline for the management of gout recommends that urate-lowering therapy (ULT) is indicated in patients with recurrent acute flares, arthropathy, tophi or radiographic changes of gout, aiming to maintain a serum urate level below the saturation point for monosodium urate [<357 μmol/L (<6 mg/dL)].449
Xanthine oxidase inhibitors (allopurinol, oxypurinol) may be used to prevent gout, although their safety in HFrEF is uncertain.450 Gout attacks are better treated with colchicine rather than with NSAIDs (although colchicine should not be used in patients with very severe renal dysfunction and may cause diarrhoea). Intra-articular corticosteroids are an alternative for monoarticular gout, but systemic corticosteroids cause sodium and water retention.
Arthritis is a common co-morbidity and is a common cause of both self-taken and prescribed drugs that can worsen renal function and HF, especially NSAIDs. Rheumatoid arthritis is associated with an increased risk of HFpEF. The safety of disease-modifying drugs commonly given to patients with rheumatoid arthritis has not been established in HF.
11.9 Hypokalaemia and hyperkalaemia
Both hypokalaemia and hyperkalaemia are associated with HF and with many drugs used for HF treatment.451 Both can aggravate ventricular arrhythmias.
Loop and thiazide diuretics reduce serum potassium, while ACEIs, ARBs and MRAs can all increase serum potassium. Amiloride and triamterene are sometimes used as adjunct diuretics in resistant oedema and to assist in preventing hypokalaemia. The treatment of hypokalaemia can involve recommending high potassium foods or prescribing potassium supplements.
The management of acute hyperkalaemia (>6.0 mmol/L) may require a short-term cessation of potassium-retaining agents and RAAS inhibitors, but this should be minimized and RAAS inhibitors should be carefully reintroduced as soon as possible while monitoring potassium levels. A Cochrane review452 found no trial evidence of major outcome benefits for any emergency therapy regimen for hyperkalaemia. Two new potassium binders (patiromer and sodium zirconium cyclosilicate) are currently under consideration for regulatory approval.453, 454 Initial results from patients with HF are available and confirm the efficacy of these therapies in reducing serum potassium455 and preventing recurrent hyperkalaemia in patients with HF and CKD in the context of treatment with RAAS inhibitors.456
11.10 Hyperlipidaemia
Elevated low-density lipoprotein cholesterol is uncommon in HFrEF; patients with advanced HFrEF often have low concentrations of low-density lipoprotein, which is associated with a worse prognosis. Rosuvastatin did not reduce the primary composite mortality/morbidity endpoints in two large RCTs in patients with HF with or without IHD, but it also did not increase risk, and may have reduced, hospitalizations.205, 457 Therefore there is no evidence to recommend the initiation of statins in most patients with HF. However, in patients who are already receiving a statin for CAD, a continuation of this therapy may be considered.
11.11 Hypertension
Hypertension is associated with an increased risk of developing HF; antihypertensive therapy markedly reduces the incidence of HF (with an exception of α-adrenoceptor blockers, which are less effective than other antihypertensives in preventing HF).458 A recent prospective cohort study documented that in a population with incident HF, higher baseline systolic, diastolic and pulse pressure levels were associated with a higher rate of adverse events, which further supports the importance for optimized blood pressure control in this population.459 Blood pressure control is an element of the holistic management of patients with HF.
11.12 Iron deficiency and anaemia
Anaemia (defined as a haemoglobin concentration <13.0 g/dL in men and <12.0 g/dL in women) is common in HF, particularly in hospitalized patients. It is more common in women, the elderly and in patients with renal impairment and is associated with advanced myocardial remodelling, inflammation and volume overload.474 Anaemia is associated with advanced symptoms, worse functional status, greater risk of HF hospitalization and reduced survival. A diagnostic workup to seek a cause for any finding of anaemia is indicated (e.g. occult blood loss, iron deficiency, B12/folate deficiency, blood dyscrasias), although in many patients no specific cause is found. The erythropoietin-stimulating agent darbepoetin alfa did not improve clinical outcomes in HFrEF patients with mild to moderate anaemia, but led to an excess of thromboembolic events and is therefore not recommended.475
11.13 Kidney dysfunction (including chronic kidney disease, acute kidney injury, cardio-renal syndrome and prostatic obstruction)
HF and CKD frequently coexist, share many risk factors (diabetes, hypertension, hyperlipidaemia) and interact to worsen prognosis.476, 477 CKD is generally defined as an eGFR <60 mL/min/1.73 m2 and/or the presence of albuminuria (high 30–300 or very high >300 mg albumin/1 g of urine creatinine). Patients with severe renal dysfunction (eGFR <30 mL/min/1.73m2) have systematically been excluded from randomized clinical trials and therefore there is lack of evidence-based therapies in these patients.
A further deterioration in renal function, termed worsening renal function (WRF), is used to indicate an increase in serum creatinine, usually by >26.5 µmol/L (0.3 mg/dL) and/or a 25% increase or a 20% drop in GFR. The importance of these apparently small changes is that they are frequent, they promote the development and progression of CKD478 and, as a consequence, can worsen the prognosis of HF. Increases in creatinine during an AHF hospitalization are not always clinically relevant, especially when they are accompanied by appropriate decongestion, diuresis and haemoconcentration.479
Large increases in serum creatinine, termed acute kidney injury (AKI), are relatively rare in HF and are probably associated with the combination of diuretic therapy with other potentially nephrotoxic drugs such as some antibiotics (gentamicin and trimethoprim), contrast media, ACEIs, ARBs, NSAIDs, etc. Of relevance, some of these drugs may accumulate if they are renally excreted. In HF, WRF is relatively common, especially during initiation and up-titration of RAAS inhibitor therapy. Despite the fact that RAAS blockers can frequently cause a decrease in GFR in patients with HF, this reduction is usually small and should not lead to treatment discontinuation unless there is a marked decrease, as the treatment benefit in these patients is probably largely maintained.480 When large increases in serum creatinine occur, care should be taken to evaluate the patient thoroughly and should include assessment of a possible renal artery stenosis, excessive hyper- or hypovolaemia, concomitant medication and hyperkalaemia, which frequently coincides with WRF.
Diuretics, especially thiazides, but also loop diuretics, may be less effective in patients with a very low GFR, and if used, should be dosed appropriately (higher doses to achieve similar effects). Renally excreted drugs (e.g. digoxin, insulin and low molecular weight heparin) may accumulate in patients with renal impairment and may need dose adjustment if renal function deteriorates. Patients with HF and coronary or peripheral vascular disease are at risk of acute renal dysfunction when they undergo contrast media enhanced angiography [contrast-induced acute kidney injury (CI-AKI)]. Renal dysfunction and worsening renal function is further discussed in the section about AHF (see Section 12).
Prostatic obstruction is common in older men and can interfere with renal function; it should therefore be ruled out in men with HF with deteriorating renal function. α-adrenoceptor blockers cause hypotension and sodium and water retention, and may not be safe in HFrEF.458, 464, 465 For these reasons, 5-α-reductase inhibitors are generally preferred in the medical treatment of prostatic obstruction in patients with HF.
11.14 Lung disease (including asthma and chronic obstructive pulmonary disease)
The diagnosis of COPD and asthma may be difficult in patients with HF, due to overlap in symptoms and signs, but also problems in the interpretation of spirometry, especially in HFpEF.48, 49, 391 COPD (and asthma) in patients with HF may be overdiagnosed.481 Spirometry should be performed when patients have been stable and euvolaemic for at least 3 months, to avoid the confounding effect of pulmonary congestion causing external obstruction of alveoli and bronchioles.482 Both correctly and incorrectly labelled COPD are associated with worse functional status and a worse prognosis in HFrEF.
Beta-blockers are only relatively contraindicated in asthma, but not in COPD, although a more selective β1-adrenoceptor antagonist (i.e. bisoprolol, metoprolol succinate, or nebivolol) is preferred.48, 49, 391 The contraindication to beta-blockers in asthma, as mentioned on pharmacy leaflets, is based on small case series published in the 1980s and late 1990s with very high initial dosages in young patients with severe asthma. In clinical practice, starting with low doses of cardioselective beta-blockers combined with close monitoring for signs of airway obstruction (wheezing, shortness of breath with lengthening of the expiration) may allow the use of profoundly effective beta-blockers in HFrEF, especially in older people where true severe asthma is uncommon. Therefore, according to the 2015 GINA global strategy report,395, 396 asthma is not an absolute contraindication, but these medications should only be used under close medical supervision by a specialist, with consideration of the risks for and against their use. The long-term safety of cardioactive inhaled pulmonary drugs is uncertain and the need for their use should be reconsidered in patients with HFrEF, especially as their benefit in asthma and COPD may be symptomatic only without a clear effect on mortality. Oral corticosteroids can cause sodium and water retention, potentially leading to worsening of HF, but this is not believed to be a problem with inhaled corticosteroids. Pulmonary hypertension can complicate severe long-standing COPD, which, as a result, makes right-sided HF and congestion more likely. Non-invasive ventilation, added to conventional therapy, improves the outcome of patients with acute respiratory failure due to hypercapnic exacerbation of COPD or HF in situations of acute pulmonary oedema.
11.15 Obesity
Obesity is a risk factor for HF141 and complicates its diagnosis, because it can cause dyspnoea, exercise intolerance and ankle swelling and may result in poor-quality echocardiographic images. Obese individuals also have reduced NP levels.62 Obesity is more common in HFpEF than in HFrEF, although it is possible that misdiagnosis may explain at least some of this difference in prevalence. Although obesity is an independent risk factor for developing HF, once HF is diagnosed, it is well established that obesity is associated with lower mortality across a wide range of body mass indexes (BMIs) (see also cachexia in Section 11.3)—the so-called obesity paradox also seen in other chronic illnesses.414, 416 Obesity should be managed as recommended in the ESC guidelines on cardiovascular disease prevention,483 if the aim is to prevent future development of HF. However, these guidelines do not refer to the HF patient in whom higher BMI is not adverse, and, although often recommended for symptom benefit and risk factor control, weight loss as an intervention has never been prospectively shown to be either beneficial or safe in HFrEF. When weight loss is occurring in HF, it is associated with high mortality and morbidity, worse symptom status and poor quality of life. In patients with HF with moderate degrees of obesity (BMI <35 kg/m2), weight loss cannot be recommended. In more advanced obesity (BMI 35–45 kg/m2), weight loss may be considered to manage symptoms and exercise capacity.
11.16 Sleep disturbance and sleep-disordered breathing
Sleep-disordered breathing (SDB) occurs in more than one-third of patients with HF,484 being even more prevalent in patients with AHF.485 The most common types are: central sleep apnoea (CSA, similar to Cheyne Stokes respiration, CSR), obstructive sleep apnoea (OSA), and a mixed pattern of the two. Other causes of sleep disturbance include anxiety, depression, decubitus or paroxysmal pulmonary congestion (orthopnoea and paroxysmal nocturnal dyspnoea) and diuretic therapy causing nocturnal diuresis. Reviewing sleep history (including asking a partner) is part of the holistic care of patients with HF (see Section 14). CSA and OSA have been shown to be associated with a worse prognosis in HF.485, 486 OSA is associated with an increased risk of incident HF in men.487 CSA is the most common form of SDB in HFrEF, and HFrEF is the most common cause of CSA, so they are closely linked. Screening for, and the diagnosis and treatment of, sleep apnoea is discussed in detail elsewhere.484, 488 Diagnosis used to require overnight polysomnography, although advanced home testing equipment which can distinguish the type of sleep apnoea has been developed.
Nocturnal oxygen supplementation, continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP), and adaptive servo-ventilation (ASV) may be considered to treat nocturnal hypoxaemia in OSA as recommended in other guidelines.489, 490 An apnoea/hypopnoea index (AHI) of above 30 per hour can be treated using any of CPAP, BiPAP, ASV and nocturnal oxygen supplementation, which have all been shown to be effective in this regard. It should be noted, however, that none of these interventions has been prospectively shown to be beneficial on major outcomes in HFrEF.
CPAP in HF related CSA has been shown to reduce the frequency of episodes of apnoea and hypopnoea, and improve LVEF and 6 minute walk test distance, but did not improve prognosis or the rate of HF related hospitalizations.491
The recently published SERVE-HF473 trial has shown that ASV used in patients with HFrEF and a predominantly CSA was neutral regarding the composite primary endpoint (all-cause death, lifesaving cardiovascular intervention, i.e. cardiac transplantation, implantation of a ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock, or unplanned hospitalization for HF worsening), but more importantly led to an increase in both all-cause and cardiovascular mortality. Therefore ASV is not recommended in patients with HFrEF and predominantly CSA.
The safety and efficacy of alternative approaches to treating CSA in HFrEF patients, such as implantable phrenic nerve stimulation,219, 220, 492 are presently undergoing clinical investigation and may require additional long term study.
11.17 Valvular heart disease
Valvular heart disease may cause or aggravate HF. This section briefly addresses problems particularly relevant to HF, and the reader is referred to the recent guidelines on valvular disease for more information.493, 494
Patients with HF and concomitant valvular heart disease constitute a high-risk population. Thus, the whole process of decision-making through a comprehensive evaluation of the risk–benefit ratio of different treatment strategies should be made by a multidisciplinary ‘heart team’ with a particular expertise in valvular heart disease, including cardiologists with expertise in HF, cardiac surgeons, a structural valve interventionist if a catheter-based therapy is being considered, imaging specialists, anaesthetists and, if needed, general practitioners, geriatricians, or intensive care specialists. This may be particularly beneficial in patients with HF being considered for surgery, transcatheter aortic valve implantation or transcatheter mitral valve intervention.
All patients should receive OMT. In those with HFrEF pharmacological therapy should be planned according to a previously described algorithm (see Section 7 for details). Care must be taken using vasodilators (ACEI, ARBs, CCBs, hydralazine, and nitrates) in patients with severe aortic stenosis in order not to cause hypotension.
11.17.1 Aortic stenosis
The main concern in patients with severe aortic stenosis and reduced LVEF is the entity of ‘low-flow, low-gradient’ aortic stenosis (valve area < 1 cm2, LVEF < 40%, mean pressure gradient < 40 mmHg). In such individuals, low-dose dobutamine stress echocardiography should be considered to differentiate between patients with moderate aortic stenosis, and those with severe stenosis and low flow across the valve due to low stroke volume, and to evaluate for contractile or flow reserve.
If the mean gradient is > 40 mmHg, there is theoretically no lower LVEF limit for aortic valve replacement in symptomatic patients with severe aortic stenosis.
Transaortic valve implantation (TAVI) is recommended in patients with severe aortic stenosis who are not suitable for surgery as assessed by a ‘heart team’ and have predicted post-TAVI survival > 1 year. TAVI should be also considered in high-risk patients with severe aortic stenosis who may still be suitable for surgery, but in whom TAVI is favoured by a ‘heart team’ based on the individual risk profile and anatomic suitability.495, 496 In a recent trial in patients with severe aortic stenosis, TAVI with a self-expanding transcatheter aortic valve bioprosthesis was associated with a significantly higher rate of survival at 1 year which was sustained at 2 years.497, 498
11.17.2 Aortic regurgitation
In patients with severe aortic regurgitation, aortic valve repair or replacement is recommended in all symptomatic patients and in asymptomatic patients with resting LVEF ≤ 50%, who are otherwise fit for surgery.499, 500
11.17.3 Mitral regurgitation
This section refers to chronic settings while acute settings are discussed in Section 12.
Primary (organic) mitral regurgitation
Surgery is indicated in symptomatic patients with severe organic mitral regurgitation with no contra-indications to surgery. The decision of whether to replace or repair depends mostly on valve anatomy, surgical expertise available, and the patient's condition.
When the LVEF is < 30%, a durable surgical repair may improve symptoms, although its effect on survival is unknown. In this situation, the decision to operate should take account of response to medical therapy, co-morbidities, and the likelihood that the valve can be repaired (rather than replaced).
Secondary mitral regurgitation
This occurs because LV enlargement and remodelling lead to reduced leaflet closing. Effective medical therapy (including CRT in suitable patients) leading to reverse remodelling of the LV may reduce functional mitral regurgitation, and every effort should be made to optimize medical treatment in these patients.
Combined valve and coronary surgery should be considered in symptomatic patients with LV systolic dysfunction (LVEF < 30%), coronary arteries suitable for revascularization, and evidence of viability. Surgery is also recommended in patients with severe mitral regurgitation undergoing CABG with LVEF > 30%.
However, a recent study in patients with moderate, secondary ischaemic mitral regurgitation did not prove that the addition of mitral valve repair to CABG would lead to a higher degree of LV reverse remodelling.501 Also, there is no evidence favouring mitral valve repair over replacement in the context of better outcomes and magnitude of LV remodelling.502 In the presence of AF, atrial ablation and LA appendage closure may be considered at the time of mitral valve surgery.
The role of isolated mitral valve surgery in patients with severe functional mitral regurgitation and severe LV systolic dysfunction (LVEF < 30%) who cannot be revascularized or have non-ischaemic cardiomyopathy is questionable, and in most patients conventional medical and device therapy are preferred. In selected cases, repair may be considered in order to avoid or postpone transplantation. The decision should be based on comprehensive evaluation (including strain echocardiography or magnetic resonance imaging499, 503 and discussed within the ‘heart team’.
In patients with HF with moderate-severe, secondary mitral regurgitation who are judged inoperable or at high surgical risk, percutaneous mitral valve intervention (percutaneous edge-to-edge repair) may be considered in order to improve symptoms and quality of life, although no RCT evidence of improvement has been published, only registry studies.504-506
11.17.4 Tricuspid regurgitation
12 Acute heart failure
12.1 Definition and classification
AHF refers to rapid onset or worsening of symptoms and/or signs of HF. It is a life-threatening medical condition requiring urgent evaluation and treatment, typically leading to urgent hospital admission.
AHF may present as a first occurrence (de novo) or, more frequently, as a consequence of acute decompensation of chronic HF, and may be caused by primary cardiac dysfunction or precipitated by extrinsic factors, often in patients with chronic HF. Acute myocardial dysfunction (ischaemic, inflammatory or toxic), acute valve insufficiency or pericardial tamponade are among the most frequent acute primary cardiac causes of AHF. Decompensation of chronic HF can occur without known precipitant factors, but more often with one or more factors, such as infection, uncontrolled hypertension, rhythm disturbances or non-adherence with drugs/diet (Table 12.1).
A large number of overlapping classifications of AHF based on different criteria have been proposed.510-513 In practice the most useful classifications are those based on clinical presentation at admission, allowing clinicians to identify patients at high risk of complications and to direct management at specific targets, which creates a pathway for personalized care in the AHF setting. In most cases, patients with AHF present with either preserved (90–140 mmHg) or elevated (>140 mmHg; hypertensive AHF) systolic blood pressure (SBP). Only 5–8% of all patients present with low SBP (i.e. <90 mmHg; hypotensive AHF), which is associated with poor prognosis, particularly when hypoperfusion is also present.514, 515
Another approach is to classify patients according to the presence of the following precipitants/causes leading to decompensation, which need to be treated/corrected urgently (see Section 12.3.1): ACS, hypertensive emergency, rapid arrhythmias or severe bradycardia/conduction disturbance, acute mechanical cause underlying AHF or acute pulmonary embolism.
Clinical classification can be based on bedside physical examination in order to detect the presence of clinical symptoms/signs of congestion (‘wet’ vs. ‘dry’ if present vs. absent) and/or peripheral hypoperfusion (‘cold’ vs. ‘warm’ if present vs. absent) (Figure 12.1).514, 515 The combination of these options identifies four groups: warm and wet (well perfused and congested) —most commonly present; cold and wet (hypoperfused and congested); cold and dry (hypoperfused without congestion); and warm and dry (compensated, well perfused without congestion). This classification may be helpful to guide therapy in the initial phase and carries prognostic information.510, 514, 515

Patients with HF complicating AMI can be classified according to Killip and Kimball13 into class I, no clinical signs of HF; class II, HF with rales and S3 gallop; class III, with frank acute pulmonary oedema; class IV, cardiogenic shock, hypotension (SBP <90 mmHg) and evidence of peripheral vasoconstriction such as oliguria, cyanosis and diaphoresis.
Definitions of the terms used in this section related to clinical presentation of patients with AHF are provided in Table 12.2.
12.2 Diagnosis and initial prognostic evaluation
The diagnostic workup needs to be started in the pre-hospital setting and continued in the emergency department (ED) in order to establish the diagnosis in a timely manner and initiate appropriate management. The greater benefit of early treatment is well established in ACS and now needs to be considered in the setting of AHF.516, 517 In parallel, coexisting life-threatening clinical conditions and/or precipitants that require urgent treatment/correction need to be immediately identified and managed (Figure 12.2). Typically, an initial step in the diagnostic workup of AHF is to rule out alternative causes for the patient's symptoms and signs (i.e. pulmonary infection, severe anaemia, acute renal failure).
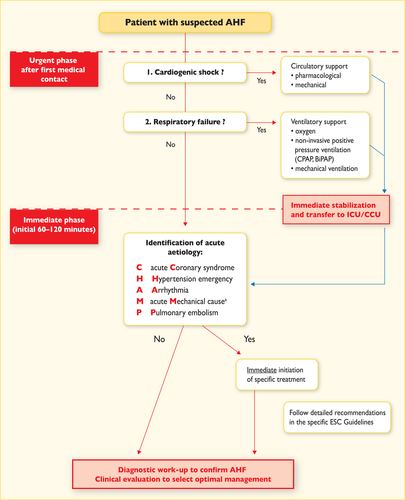
Initial management of a patient with acute heart failure. aAcute mechanical cause: myocardial rupture complicating acute coronary syndrome (free wall rupture, ventricular septal defect, acute mitral regurgitation), chest trauma or cardiac intervention, acute native or prosthetic valve incompetence secondary to endocarditis, aortic dissection or thrombosis, see above.
When AHF is confirmed clinical evaluation is mandatory to select further management.
It is recommended that initial diagnosis of AHF should be based on a thorough history assessing symptoms, prior cardiovascular history and potential cardiac and non-cardiac precipitants, as well as on the assessment of signs/symptoms of congestion and/or hypoperfusion by physical examination and further confirmed by appropriate additional investigations such as ECG, chest X-ray, laboratory assessment (with specific biomarkers) and echocardiography.
In patients presenting with AHF, early initiation of appropriate therapy (along with relevant investigations) is of key importance.516-518
-
Chest X-ray can be a useful test for the diagnosis of AHF. Pulmonary venous congestion, pleural effusion, interstitial or alveolar oedema and cardiomegaly are the most specific findings for AHF, although in up to 20% of patients with AHF, chest X-ray is nearly normal.519 Supine chest radiographs are of limited value in AHF. Chest X-ray is also useful to identify alternative non-cardiac diseases that may cause or contribute to the patient's symptoms (i.e. pneumonia, non-consolidative pulmonary infections).
-
ECG is rarely normal in AHF (high negative predictive value).520 It is also helpful in identifying underlying cardiac disease and potential precipitants (rapid AF, acute myocardial ischaemia).
-
Immediate echocardiography is mandatory only in patients with haemodynamic instability (particularly in cardiogenic shock) and in patients suspected of acute life-threatening structural or functional cardiac abnormalities (mechanical complications, acute valvular regurgitation, aortic dissection). Early echocardiography should be considered in all patients with de novo AHF and in those with unknown cardiac function; however, the optimal timing is unknown (preferably within 48 h from admission, if the expertise is available). Pocket-size echocardiography may be used as an extension of the clinical examination in the first instance where available. Repeated echocardiography is usually not needed unless there is relevant deterioration in clinical status. Bedside thoracic ultrasound for signs of interstitial oedema and pleural effusion may be useful in detecting AHF if the expertise is available.
-
Laboratory tests:
-
– Natriuretic peptides.
-
– Upon presentation to the ED or CCU/ICU, a plasma NP level (BNP, NT-proBNP or MR-proANP) should be measured in all patients with acute dyspnoea and suspected AHF to help in the differentiation of AHF from non-cardiac causes of acute dyspnoea. NPs have high sensitivity, and normal levels in patients with suspected AHF makes the diagnosis unlikely (thresholds: BNP <100 pg/mL, NT-proBNP <300 pg/mL, MR-proANP <120 pg/mL).57-61, 77, 78, 521 However, elevated levels of NPs do not automatically confirm the diagnosis of AHF, as they may also be associated with a wide variety of cardiac and non-cardiac causes (Table 12.3). Unexpectedly low levels of NPs can be detected in some patients with decompensated end-stage HF, flash pulmonary oedema or right sided AHF.
-
Other laboratory tests at presentation.
-
– The following laboratory assessments should be performed at admission on the blood of all patients with AHF: cardiac troponin, blood urea nitrogen (BUN) (or urea), creatinine, electrolytes (sodium, potassium), liver function tests, thyroid-stimulating hormone (TSH), glucose and complete blood count; D-dimer is indicated in patients with a suspicion of acute pulmonary embolism.
-
○ Routine arterial blood gas is not needed and should be restricted to patients in whom oxygenation cannot be readily assessed by pulse oximetry. However, arterial blood gas may be useful when a precise measurement of O2 and CO2 partial pressures is needed. A venous sample might acceptably indicate pH and CO2.
-
○ Of note, measurement of cardiac troponins is useful for detection of ACS as the underlying cause of AHF. However, elevated concentrations of circulating cardiac troponins are detected in the vast majority of patients with AHF, often without obvious myocardial ischaemia or an acute coronary event, suggesting ongoing myocyte injury or necrosis in these patients.525 Also in patients with acute pulmonary embolism as the underlying cause of acute decompensation, elevated troponins are useful for risk stratification and decision-making.526
-
○ It is recommended to measure creatinine, BUN and electrolytes every 1–2 days while in the hospital and before discharge from the hospital. Of note, more frequent testing might be justified according to the severity of the case. Pre-discharge assessment of NPs may be considered for prognostic evaluation.
-
○ Assessment of procalcitonin levels may be considered in patients with AHF with suspected coexisting infection, particularly for the differential diagnosis of pneumonia and to guide antibiotic therapy,527 if considered.
-
○ Liver function tests are often impaired in patients with AHF due to haemodynamic derangements (both reduced output and increased venous congestion). Abnormal liver function tests identify patients at risk of poor prognosis and may be useful for optimal management.528-530
-
○ Since both hypothyroidism and hyperthyroidism may precipitate AHF, TSH should be assessed in newly diagnosed AHF.
-
○ Multiple other biomarkers, including those reflecting inflammation, oxidative stress, neurohormonal disarray and myocardial and matrix remodelling, have been investigated for their diagnostic and prognostic value in AHF; however, none has reached the stage of being recommended for routine clinical use.
-
-
-
Routine invasive haemodynamic evaluation with a pulmonary artery catheter is not indicated for the diagnosis of AHF. It may be helpful in selected cases of haemodynamically unstable patients with an unknown mechanism of deterioration. Also, routine use of an arterial line or central venous line for diagnostic purposes is not indicated.
12.3 Management
AHF is a life-threatening medical condition, thus rapid transfer to the nearest hospital should be pursued, preferably to a site with a cardiology department and/or a coronary care/intensive care unit (CCU/ICU).
Early diagnosis is important in AHF. Therefore, all patients with suspected AHF should have a diagnostic workup and appropriate pharmacological and non-pharmacological treatment should be started promptly and in parallel.
Initial evaluation and continued non-invasive monitoring of the patient's vital cardiorespiratory functions, including pulse oximetry, blood pressure, respiratory rate and a continuous ECG instituted within minutes, is essential to evaluate whether ventilation, peripheral perfusion, oxygenation, heart rate and blood pressure are adequate. Urine output should also be monitored, although routine urinary catheterization is not recommended.
Patients with respiratory distress/failure or haemodynamic compromise should be triaged to a location where immediate respiratory and cardiovascular support can be provided (Figure 12.2).
12.3.1 Identification of precipitants/causes leading to decompensation that needs urgent management
-
Acute coronary syndrome. Patients presenting with ACS should be managed according to the ESC guidelines on non-ST elevation ACS (NSTE-ACS) and STEMI.114, 535 Coexistence of these two clinical conditions (ACS and AHF) always identifies a very-high-risk group where an immediate (i.e. <2 h from hospital admission in patients with NSTEMI, analogous to STEMI management) invasive strategy with intent to perform revascularization is recommended, irrespective of ECG or biomarker findings.114, 535 See below for patients presenting with persistent haemodynamic instability due to mechanical ACS complication.
-
Hypertensive emergency. AHF precipitated by rapid and excessive increase in arterial blood pressure typically manifests as acute pulmonary oedema. A prompt reduction in blood pressure should be considered as a primary therapeutic target and initiated as soon as possible. Aggressive blood pressure reduction (in the range of 25% during the first few hours and cautiously thereafter) with i.v. vasodilators in combination with loop diuretics is recommended.317, 536, 537
-
Rapid arrhythmias or severe bradycardia/conduction disturbance. Severe rhythm disturbances in patients with AHF and unstable conditions should be corrected urgently with medical therapy, electrical cardioversion or temporary pacing260, 316, 389 (see also Section 10.1 for AF management).
Electrical cardioversion is recommended if an atrial or ventricular arrhythmia is thought to be contributing to the patient's haemodynamic compromise in order to restore sinus rhythm and improve the patient's clinical condition.
Patients with AHF and incessant ventricular arrhythmias present a challenging scenario, as arrhythmias and haemodynamic instability operate in a vicious circle, perpetuating each other. In selected cases, immediate angiography (with resultant revascularization, if needed) and electrophysiological testing with radiofrequency ablation may be considered.260
-
Acute mechanical cause underlying AHF. This may present as a mechanical complication of ACS (free wall rupture, ventricular septal defect, acute mitral regurgitation), chest trauma or cardiac intervention, or as acute native or prosthetic valve incompetence secondary to endocarditis, aortic dissection or thrombosis and comprise rare causes of obstruction (e.g. cardiac tumours). Echocardiography is essential for diagnosis, and treatment typically requires circulatory support with surgical or percutaneous intervention.
-
Acute pulmonary embolism. When acute pulmonary embolism is confirmed as the cause of shock or hypotension, immediate specific treatment is recommended with primary reperfusion either with thrombolysis, catheter-based approach or surgical embolectomy.526 Patients presenting with acute pulmonary embolism should be managed according to the appropriate guidelines.526
12.3.2 Criteria for hospitalization in ward vs. intensive care/coronary care unit
-
Patients with persistent, significant dyspnoea or haemodynamic instability should be triaged to a location where immediate resuscitative support can be provided if needed.
-
For high-risk patients (i.e. with persistent, significant dyspnoea, haemodynamic instability, recurrent arrhythmias, AHF and associated ACS), initial care should be provided in a high-dependency setting (ICU/CCU). Clinical risk algorithms developed to predict in-hospital mortality of patients with AHF can assist in determining which patients in the ED need the highest level of inpatient care.538, 539
-
The criteria for ICU/CCU admission include any of the following:
-
– need for intubation (or already intubated)
-
– signs/symptoms of hypoperfusion
-
– oxygen saturation (SpO2) <90% (despite supplemental oxygen)
-
– use of accessory muscles for breathing, respiratory rate >25/min
-
– heart rate <40 or >130 bpm, SBP <90 mmHg.540
-
-
The remaining patients with AHF usually need hospitalization on an ordinary ward. Only a few patients admitted to the ED with AHF (mainly as exacerbation of HF symptoms with subtle signs of congestion) after a small dose of diuretics and some adjustments of oral therapy can be discharged directly home from the ED with advice to be clinically followed in an outpatient clinic.
-
Step-down care from the ICU/CCU is dictated by clinical stabilization and resolution of morbid conditions. Further treatment will be continued with the involvement of a multidisciplinary team and discharge planning.
12.3.3 Management of the early phase
In AHF, oxygen should not be used routinely in non-hypoxaemic patients, as it causes vasoconstriction and a reduction in cardiac output.546, 547 In COPD, hyperoxygenation may increase ventilation–perfusion mismatch, suppressing ventilation and leading to hypercapnia. During oxygen therapy, acid–base balance and transcutaneous SpO2 should be monitored.
Non-invasive positive pressure ventilation includes both CPAP and bi-level positive pressure ventilation (PPV). Bi-level PPV also allows inspiratory pressure support that improves minute ventilation and is especially useful in patients with hypercapnia, most typically COPD patients.
Congestion affects lung function and increases intrapulmonary shunting, resulting in hypoxaemia. The fraction of inspired oxygen (FiO2) should be increased up to 100% if necessary, according to SpO2, unless contraindicated. Hyperoxia, however, should be avoided.546, 547 Non-invasive positive pressure ventilation reduces respiratory distress541-545 and may decrease intubation and mortality rates,543 although data regarding mortality are less conclusive. CPAP is a feasible technique in the pre-hospital setting, because it is simpler than pressure support positive end-expiratory pressure (PS-PEEP) and requires minimal training and equipment. On hospital arrival, patients who still show signs of respiratory distress should continue with non-invasive ventilation, preferably PS-PEEP, in case of acidosis and hypercapnia, particularly in those with a previous history of COPD or signs of fatigue.540
Caution should be exercised with regard to side effects of anaesthetic drugs, among which propofol can induce hypotension and have cardiodepressive side effects. In contrast, midazolam may have fewer cardiac side effects and thus is preferred in patients with AHF or cardiogenic shock.
A management algorithm for patients with AHF based on the clinical profile during an early phase is presented in Figure 12.3.

Management of patients with acute heart failure based on clinical profile during an early phaseaSymptoms/signs of congestion: orthopnoea, paroxysmal nocturnal dyspnoea, breathlessness, bi-basilar rales, an abnormal blood pressure response to the Valsalva maneuver (left-sided); symptoms of gut congestion, jugular venous distension, hepatojugular reflux, hepatomegaly, ascites, and peripheral oedema (right-sided).
Diuretics
Diuretics are a cornerstone in the treatment of patients with AHF and signs of fluid overload and congestion. Diuretics increase renal salt and water excretion and have some vasodilatory effect. In patients with AHF and signs of hypoperfusion, diuretics should be avoided before adequate perfusion is attained.
The initial approach to congestion management involves i.v. diuretics with the addition of vasodilators for dyspnoea relief if blood pressure allows. To enhance diuresis or overcome diuretic resistance, options include dual nephron blockade by loop diuretics (i.e. furosemide or torasemide) with thiazide diuretics or natriuretic doses of MRAs.570, 571 However, this combination requires careful monitoring to avoid hypokalaemia, renal dysfunction and hypovolaemia.
Data defining optimal dosing, timing and method of delivery are incomplete. In the ‘high-dose’ arm of the DOSE study, administration of furosemide at 2.5 times the previous oral dose resulted in greater improvement in dyspnoea, larger weight change and fluid loss at the cost of transient worsening in renal function.548 In AHF, i.v. furosemide is the most commonly used first-line diuretic. The dose should be limited to the smallest amount to provide adequate clinical effect and modified according to previous renal function and previous dose of diuretics. The initial i.v. dose should be at least equal to the pre-existing oral dose used at home. Consequently, patients with new-onset AHF or those with chronic HF without a history of renal failure and previous use of diuretics may respond to i.v. boluses of 20–40 mg, whereas those with previous use of diuretics usually require higher doses. A bolus of 10–20 mg i.v. torasemide may be considered as an alternative.
Vasodilators
Intravenous vasodilators (Table 12.4) are the second most often used agents in AHF for symptomatic relief; however, there is no robust evidence confirming their beneficial effects.
They have dual benefit by decreasing venous tone (to optimize preload) and arterial tone (decrease afterload). Consequently, they may also increase stroke volume. Vasodilators are especially useful in patients with hypertensive AHF, whereas in those with SBP <90 mmHg (or with symptomatic hypotension) they should be avoided. Dosing should be carefully controlled to avoid excessive decreases in blood pressure, which is related to poor outcome. Vasodilators should be used with caution in patients with significant mitral or aortic stenosis.
Use of an inotrope (Table 12.5) should be reserved for patients with a severe reduction in cardiac output resulting in compromised vital organ perfusion, which occurs most often in hypotensive AHF. Inotropic agents are not recommended in cases of hypotensive AHF where the underlying cause is hypovolaemia or other potentially correctable factors before elimination of these causes. Levosimendan is preferable over dobutamine to reverse the effect of beta-blockade if beta-blockade is thought to be contributing to hypoperfusion.572 However, levosimendan is a vasodilator, thus it is not suitable for treatment of patients with hypotension (SBP <85 mmHg) or cardiogenic shock unless in combination with other inotropes or vasopressors.559, 573, 574 Inotropes, especially those with adrenergic mechanisms, can cause sinus tachycardia and may induce myocardial ischaemia and arrhythmias, thus ECG monitoring is required. There is long-standing concern that they may increase mortality, which derives from studies in which intermittent or continuous infusions of inotropes were given.559-563, 575 In any case, inotropes have to be used with caution starting from rather low doses and up-titrating with close monitoring.
Vasopressors
Drugs with prominent peripheral arterial vasoconstrictor action such as norepinephrine or dopamine in higher doses (>5 μg/kg/min) are given to patients with marked hypotension. These agents are given to raise blood pressure and redistribute blood to the vital organs. However, this is at the expense of an increase in LV afterload.
Dopamine was compared with norepinephrine in the treatment of various shock patients. A subgroup analysis suggested that norepinephrine would have fewer side effects and lower mortality.558 Epinephrine (adrenaline) should be restricted to patients with persistent hypotension despite adequate cardiac filling pressures and the use of other vasoactive agents, as well as for resuscitation protocols.576
Thromboembolism prophylaxis
Thromboembolism prophylaxis with heparin or another anticoagulant is recommended unless contraindicated or unnecessary (because of existing treatment with oral anticoagulants).
Digoxin
Digoxin is mostly indicated in patients with AF and rapid ventricular rate (>110 bpm) and given in boluses of 0.25–0.5 mg i.v. if not used previously (0.0625–0.125 mg may be an adequate dose in patients with moderate to severe renal dysfunction). However, in patients with co-morbidities or other factors affecting digoxin metabolism (including other drugs) and/or the elderly, the maintenance dose may be difficult to estimate theoretically, and in this situation it should be established empirically, based on the measurements of digoxin concentration in peripheral blood.
Vasopressin antagonists
Vasopressin antagonists such as tolvaptan block the action of arginine vasopressin (AVP) at the V2 receptor in renal tubules and promote aquaresis. Tolvaptan may be used to treat patients with volume overload and resistant hyponatraemia (thirst and dehydration are recognized adverse effects).577
Opiates
Opiates relieve dyspnoea and anxiety. In AHF, routine use of opiates is not recommended and they may only be cautiously considered in patients with severe dyspnoea, mostly with pulmonary oedema. Dose-dependent side effects include nausea, hypotension, bradycardia and respiratory depression (potentially increasing the need for invasive ventilation). There are controversies regarding the potentially elevated mortality risk in patients receiving morphine.568, 569
Anxiolytics and sedatives
Anxiolytics or sedatives may be needed in a patient with agitation or delirium. Cautious use of benzodiazepines (diazepam or lorazepam) may be the safest approach.
Renal replacement therapy
Ultrafiltration involves the removal of plasma water across a semipermeable membrane in response to a transmembrane pressure gradient. There is no evidence favouring ultrafiltration over loop diuretics as first-line therapy in patients with AHF.571, 578 At the present time, routine use of ultrafiltration is not recommended and should be confined to patients who fail to respond to diuretic-based strategies.
The following criteria may indicate the need for initiation of renal replacement therapy in patients with refractory volume overload: oliguria unresponsive to fluid resuscitation measures, severe hyperkalaemia (K+ >6.5 mmol/L), severe acidaemia (pH <7.2), serum urea level >25 mmol/L (150 mg/dL) and serum creatinine >300 µmol/L (>3.4 mg/dL).
Mechanical assist devices
Intra-aortic balloon pump
The conventional indications for an intra-aortic balloon pump (IABP) are to support the circulation before surgical correction of specific acute mechanical problems (e.g. interventricular septal rupture and acute mitral regurgitation), during severe acute myocarditis and in selected patients with acute myocardial ischaemia or infarction before, during and after percutaneous or surgical revascularization. There is no good evidence that an IABP is of benefit in other causes of cardiogenic shock (for details see below).
Ventricular assist devices
Ventricular assist devices and other forms of mechanical circulatory support (MCS) may be used as a ‘bridge to decision’ or longer term in selected patients (see Section 13).
Other interventions
In patients with AHF and pleural effusion, pleurocentesis with fluid evacuation may be considered if feasible in order to alleviate dyspnoea.
In patients with ascites, ascitic paracentesis with fluid evacuation may be considered in order to alleviate symptoms. This procedure, through reduction in intra-abdominal pressure, may also partially normalize the transrenal pressure gradient, thus improving renal filtration.581
12.3.4 Management of patients with cardiogenic shock
Cardiogenic shock is defined as hypotension (SBP <90 mmHg) despite adequate filling status with signs of hypoperfusion (Table 12.1). The pathogenetic scenarios of cardiogenic shock range from low-output advanced end-stage chronic HF to acute-onset de novo cardiogenic shock most often caused by STEMI, but also by various aetiologies other than ACS. A patient in cardiogenic shock should undergo immediate comprehensive assessment. ECG and echocardiography are required immediately in all patients with suspected cardiogenic shock. In patients with cardiogenic shock complicating ACS, an immediate coronary angiography is recommended (within 2 h from hospital admission) with an intent to perform coronary revascularization.114, 535 Invasive monitoring with an arterial line should be also considered.
There is no agreement on the optimal method of haemodynamic monitoring in assessing and treating patients in cardiogenic shock, including pulmonary artery catheterization.
Pharmacologic therapy aims to improve organ perfusion by increasing cardiac output and blood pressure. After fluid challenge, pharmacologic management consists of an inotropic agent and a vasopressor as needed. Treatment is guided by the continuous monitoring of organ perfusion and haemodynamics. Pulmonary artery catheterization may be considered. As a vasopressor, norepinephrine is recommended when mean arterial pressure needs pharmacologic support. Dobutamine is the most commonly used adrenergic inotrope. Levosimendan may also be used in combination with a vasopressor.582, 583 Levosimendan infusion in cardiogenic shock following AMI on top of dobutamine and norepinephrine improved cardiovascular haemodynamics without leading to hypotension.582, 583 PDE3 inhibitors may be another option, especially in non-ischaemic patients.561, 584
12.4 Management of evidence-based oral therapies
Oral disease-modifying HF therapy should be continued on admission with AHF, except in the presence of haemodynamic instability (symptomatic hypotension, hypoperfusion, bradycardia), hyperkalaemia or severely impaired renal function. In these cases, the daily dosage of oral therapy may be reduced or stopped temporarily until the patient is stabilized. In particular, beta-blockers can be safely continued during AHF presentations except in cardiogenic shock. A recent meta-analysis demonstrated that discontinuation of beta-blockers in patients hospitalized with AHF was associated with significantly increased in-hospital mortality, short-term mortality and the combined endpoint of short-term rehospitalization or mortality.587
12.5 Monitoring of clinical status of patients hospitalized due to acute heart failure
Patients should be weighed daily and an accurate fluid balance chart should be maintained. Renal function should preferably be monitored with daily measurement of BUN/urea, creatinine and electrolytes. Routine use of a urinary catheter is not recommended.
Renal function is commonly impaired at admission, but may improve or deteriorate with diuresis. Routine monitoring of pulse, respiratory rate and blood pressure should continue. There is no study showing the usefulness of invasive haemodynamic monitoring in patients with AHF excluding those with cardiogenic shock. There is evidence that measuring NPs during the hospital admission may help with discharge planning. Patients whose NP concentrations fall during admission have lower cardiovascular mortality and readmission rates at 6 months.588-590
12.6 Criteria for discharge from hospital and follow-up in high-risk period
-
when haemodynamically stable, euvolaemic, established on evidence-based oral medication and with stable renal function for at least 24 hours before discharge;
-
once provided with tailored education and advice about self-care.
-
enrolled in a disease management programme; follow-up plans must be in place prior to discharge and clearly communicated to the primary care team;
-
reviewed by their general practitioner within 1 week of discharge;
-
seen by the hospital cardiology team within 2 weeks of discharge if feasible.
12.7 Goals of treatment during the different stages of management of acute heart failure
During the treatment of patients with AHF, one can distinguish subsequent stages that require different therapeutic approaches (described in the previous sections of this chapter). Importantly, the goals of treatment during the different stages of management of patients with AHF also differ, and are summarized in Table 12.6.
13 Mechanical circulatory support and heart transplantation
13.1 Mechanical circulatory support
For patients with either chronic or acute HF who cannot be stabilized with medical therapy, MCS systems can be used to unload the failing ventricle and maintain sufficient end-organ perfusion. Patients in acute cardiogenic shock are initially treated with short-term assistance using extracorporeal, non-durable life support systems so that more definitive therapy may be planned. Patients with chronic, refractory HF despite medical therapy can be treated with a permanent implantable left ventricular assist device (LVAD). Table 13.1 lists the current indications for the use of mechanical circulatory assist devices.593
13.1.1 Mechanical circulatory support in acute heart failure
To manage patients with AHF or cardiogenic shock (INTERMACS level 1), short-term mechanical support systems, including percutaneous cardiac support devices, extracorporeal life support (ECLS) and extracorporeal membrane oxygenation (ECMO) may be used to support patients with left or biventricular failure until cardiac and other organ function have recovered. Typically the use of these devices is restricted to a few days to weeks. The Survival After Veno-arterial ECMO (SAVE) score can help to predict survival for patients receiving ECMO for refractory cardiogenic shock (online calculator at http://www.save-score.com).594
In addition, MCS systems, particularly ECLS and ECMO, can be used as a ‘bridge to decision’ (BTD) in patients with acute and rapidly deteriorating HF or cardiogenic shock to stabilize haemodynamics, recover end-organ function and allow for a full clinical evaluation for the possibility of either heart transplant or a more durable MCS device.595
Evidence regarding the benefits of temporary percutaneous MCS in patients not responding to standard therapy, including inotropes, is limited. In a meta-analysis of three randomized clinical trials comparing a percutaneous MCS vs. IABP in a total of 100 patients in cardiogenic shock, percutaneous MCS appeared safe and demonstrated better haemodynamics, but did not improve 30-day mortality and was associated with more bleeding complications.596 In a randomized trial on high-risk PCI in patients with impaired LV function (PROTECT II trial), the 30-day incidence of major adverse events was not different for patients with IABP or a haemodynamic support device.597 Based on these results, temporary percutaneous MCS cannot be recommended as a proven or efficacious treatment for acute cardiogenic shock. In selected patients it may serve as a bridge to definite therapy. A difficult decision to withdraw MCS may need to be made when the patient has no potential for cardiac recovery and is not eligible for longer-term MCS support or heart transplant.
13.1.2 Mechanical circulatory support in end-stage chronic heart failure
Heart transplantation has always been a limited therapeutic option for patients with end-stage chronic HF. The increasing number of patients with refractory, chronic HF and the declining willingness for organ donation have resulted in expanded waiting lists and prolonged waiting times for patients listed for heart transplantation (median 16 months in the region covered by Eurotransplant).598 More than 60% of patients are transplanted in high-urgency status, leaving little chance for patients listed for less urgent transplantation. Three times more patients are listed for heart transplantation annually than are actually transplanted, and the mortality rate on the Eurotransplant waiting list in 2013 was 21.7%.598
More recent data suggest that patients with ongoing LVAD support may have an improved survival on the transplant waiting list.599 Accordingly, MCS devices, particularly continuous-flow LVADs, are increasingly seen as an alternative to heart transplantation. Initially LVADs were developed for use as a short-term BTT approach (Table 12.6),600 but they are now being used for months to years in patients who will either face a long-term wait on the transplant list (currently only 10% of patients with an MCS device implanted with a BTT indication will receive an organ within 1 year of listing) or in patients who are not eligible for transplantation as permanent therapy or destination therapy. The number of patients with a permanent LVAD who are considered neither too old nor ineligible for transplantation is constantly growing. For a majority of these patients, lifelong LVAD therapy, despite eligibility for transplantation, has become a clinical reality. Current 2–3-year survival rates in carefully selected patients receiving the latest continuous flow devices are excellent, and comparable to early survival after heart transplantation.595 However, fewer data are available for longer-term outcomes. Among patients with continuous-flow LVADs, actuarial survival is 80% at 1 year and 70% at 2 years in predominantly non-transplant-eligible patients. Notably, survival of 85% at 2 years was recorded for patients up to 70 years of age without diabetes, renal impairment or cardiogenic shock.601, 602 Patients receiving LVAD devices as BTT have a post-transplant survival rate similar or better than those not requiring or receiving bridging.599 Despite technological improvements, bleeding, thromboembolism (both of which can cause stroke), pump thrombosis, driveline infections and device failure remain significant problems and affect the long-term outcome of patients on MCS.599, 603-606 It is recommended that such devices should only be implanted and managed at centres with appropriately trained specialist HF physicians and surgeons and an outpatient LVAD clinic with trained nursing staff.607
In some patients, LV reverse remodelling and functional improvement during MCS may permit removal of the LVAD [‘bridge to recovery’ (BTR)]. This outcome is more likely in younger patients with an acute fulminant but reversible cause of HF, such as acute myocarditis or peripartum cardiomyopathy.608, 609 LVADs may also be used as a ‘bridge to candidacy’ (BTC) in order to permit recovery of end-organ dysfunction, improve RV function and relieve pulmonary hypertension, which may allow initially ineligible patients to become eligible for heart transplantation.
Earlier ventricular assist device (VAD) implantation in less severely ill patients, e.g. those not yet on inotropic support, was tested in a recent trial that revealed better outcomes than in those patients continuing on medical therapy.605 The INTERMACS registry likewise shows better outcomes in patients implanted with a higher INTERMACS class, although the majority of VAD implants are done at INTERMACS levels 1–3.604, 610 Additionally, it needs to be remembered that no RCTs exist comparing medical therapy vs. MCS devices in these transplant-eligible patients (Table 13.2).
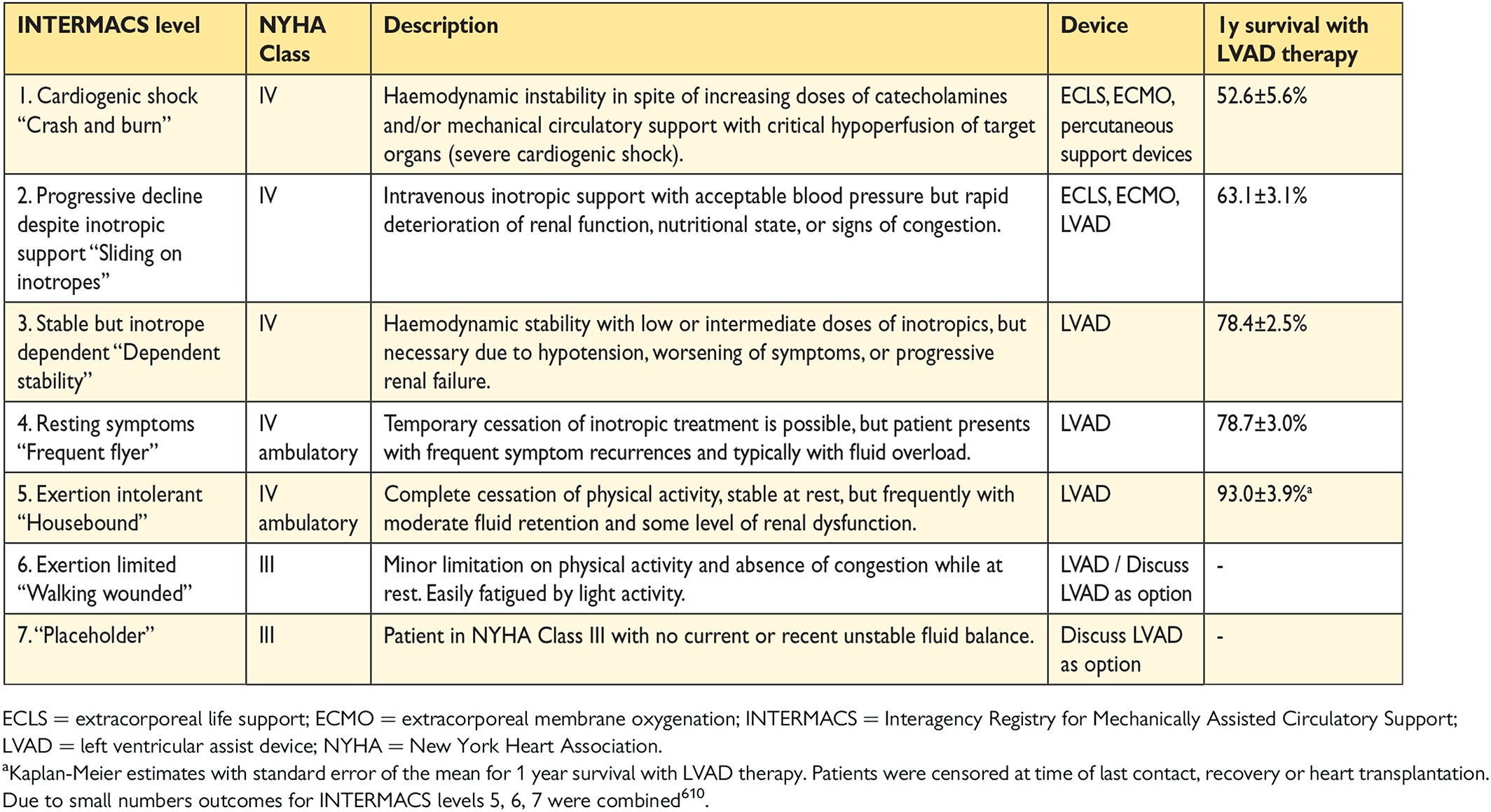
Typically, patients with end-stage HF considered for MCS exhibit many clinical hallmarks of declining cardiovascular function593 and may already be on continuous inotropic support or manifest a decline in end-organ function. Markers of liver and renal dysfunction, haematologic and coagulation abnormalities and lower serum albumin levels are associated with worse outcome.611, 612
Evaluation of RV function is crucial since postoperative RV failure greatly increases perioperative mortality and reduces survival to, and after, transplantation. There are, however, multiple approaches to assessment of the RV (see Section 5.2.3). If RV failure is expected to be potentially reversible, temporary (days to weeks) extracorporeal right ventricular assist device (RVAD) support using a centrifugal pump in addition to LVAD implantation may be considered. For patients with chronic biventricular failure or a high risk for persisting RV failure after LVAD implantation, implantation of a biventricular assist device (BiVAD) may be necessary. Patients requiring long-term BiVAD support must be transplant-eligible, as BiVAD therapy is not suitable for destination therapy. The outcomes of BiVAD therapy are inferior to those for LVAD therapy and therefore the indication for VAD therapy should be discussed before RV function deteriorates. The implantation of a total artificial heart with removal of the native heart should be restricted to selected patients who cannot be treated with an LVAD (unrepairable ventricular septal defect, cardiac rupture).
Patients with active infection, severe renal, pulmonary or hepatic dysfunction or uncertain neurological status after cardiac arrest or due to cardiogenic shock are not usually candidates for BTT or DT but may be candidates for BTC (Table 13.3).
13.2 Heart transplantation
Heart transplantation is an accepted treatment for end-stage HF.614, 615 Although controlled trials have never been conducted, there is a consensus that transplantation—provided that proper selection criteria are applied—significantly increases survival, exercise capacity, quality of life and return to work compared with conventional treatment.
Apart from the shortage of donor hearts, the main challenges in transplantation are the consequences of the limited effectiveness and complications of immunosuppressive therapy in the long term (i.e. antibody-mediated rejection, infection, hypertension, renal failure, malignancy and coronary artery vasculopathy). The indications for and contraindications to heart transplantation have recently been updated and are summarized in Table 13.4.616 It needs to be considered that some contraindications are transient and treatable. While an active infection remains a relative contraindication to heart transplantation, patients with HIV, hepatitis, Chagas disease and tuberculosis can be considered as suitable candidates provided certain strict management principles are adhered to by the teams. In patients with cancer requiring heart transplantation, a close collaboration with oncology specialists should occur to stratify each patient as to their risk of tumour recurrence.616
The use of mechanical circulatory support, particularly LVAD, should be considered for patients with potentially reversible or treatable co-morbidities, such as cancer, obesity, renal failure, tobacco use and pharmacologically irreversible pulmonary hypertension, with a subsequent re-evaluation to establish candidacy.
14 Multidisciplinary team management
Non-pharmacological non-device/surgical interventions used in the management of HF (both HFrEF and HFpEF) are summarized in Tables 14.1 and 14.2 and detailed practical recommendations on their use have been published by the HFA of the ESC.591, 592 There is no evidence that these on their own improve mortality, morbidity or quality of life. For this reason, these interventions have not been given a recommendation with an evidence level. The exceptions are implementation of care in a multidisciplinary framework, monitoring and exercise training (see recommendations table), all of which are discussed below.
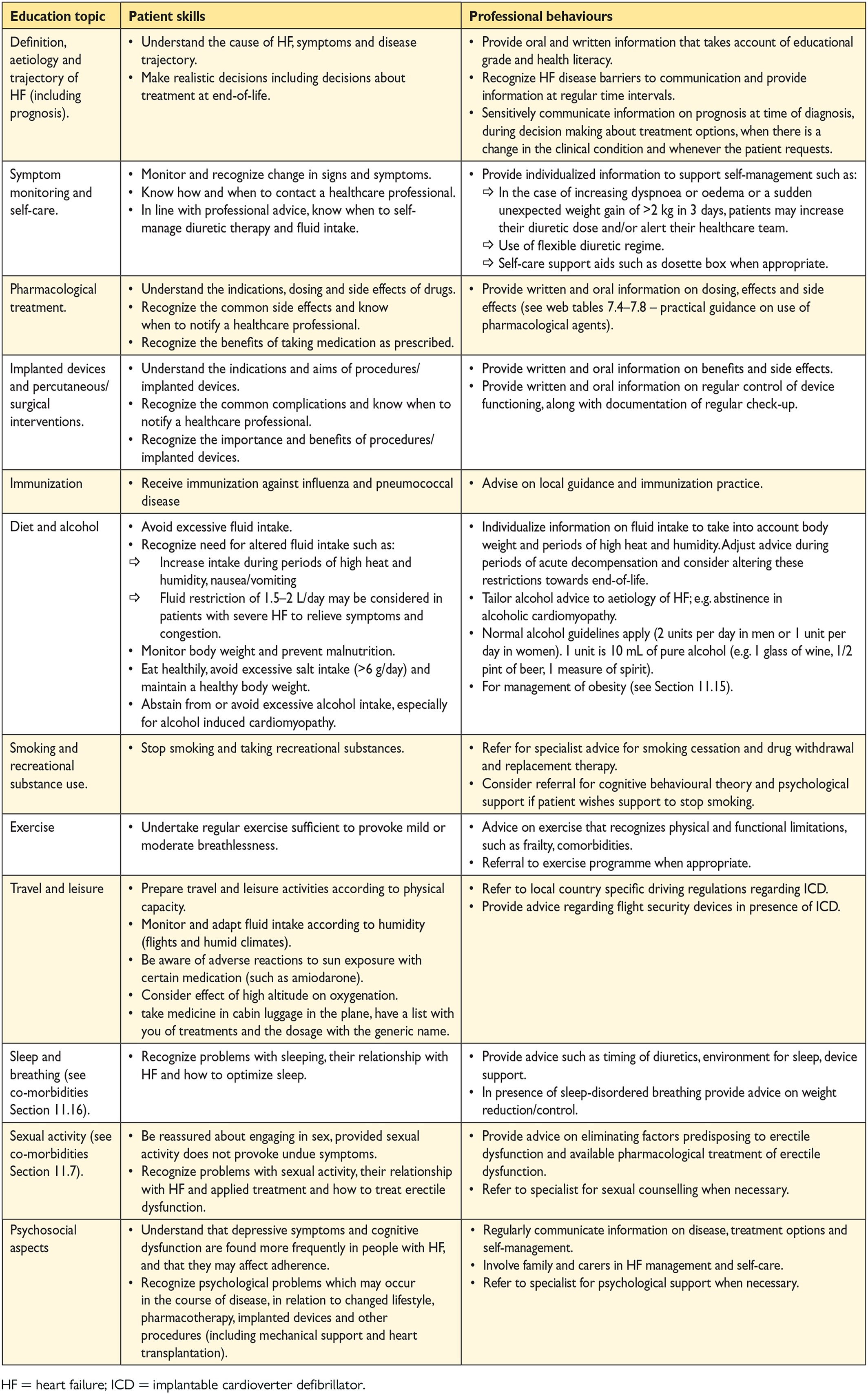
14.1 Organization of care
The goal of management of HF is to provide a ‘seamless’ system of care that embraces both the community and hospital throughout the health care journey. The standards of care that patients with HF should expect have been published by the ESC HFA.591 To achieve this goal, other services, such as cardiac rehabilitation and palliative care, must be integrated into the overall provision for patients with HF. Fundamental to the delivery of this complete package of care are multidisciplinary management programmes designed to improve outcomes through structured follow-up with patient education, optimization of medical treatment, psychosocial support and improved access to care (Table 14.1). Such strategies reduce HF hospitalization and mortality in patients discharged from the hospital.624, 625
Key to the success of these programmes is coordination of care along the continuum of HF and throughout the chain of care delivered by the various services within the health care system. This necessitates close collaboration between HF practitioners (primarily cardiologists, HF nurses and general practitioners) and other experts, including pharmacists, dieticians, physiotherapists, psychologists, palliative care providers and social workers. The content and structure of HF management programmes may vary in different countries and health care settings. The components shown in Table 14.1 are recommended. HF services should be easily accessible to the patient and his/her family and care providers. A telephone helpline may facilitate access to professional advice.
The website http://ww.heartfailurematters.org is an option for professional information for those patients and families with Internet access.
14.2 Discharge planning
Early readmission after hospital discharge is common and may be addressed through coordinated discharge planning. The standards of care that patients should expect have been published by the HFA and the Acute Cardiac Care Association.540, 631 Discharge planning should commence as soon as the patient's condition is stable. During hospital admission, providing patients with information and education for self-care improves outcome. Discharge should be arranged for when the patient is euvolaemic and any precipitants of the admission have been treated. Hospitals with early physician follow-up after discharge show reduced 30-day readmission, and those that initiated programmes to discharge patients with an outpatient follow-up appointment already scheduled experienced a greater reduction in readmissions than those not taking up this strategy.632
14.3 Lifestyle advice
There is little evidence that specific lifestyle advice improves quality of life or prognosis; however, providing this information has become a key component of education for self-care. Patients should be provided with sufficient up-to-date information to make decisions on lifestyle adjustment and self-care. Ideally for those patients admitted to the hospital, lifestyle advice should begin prior to discharge. Information should be individually tailored to need and take into account relevant co-morbidities that may influence retention of information (such as cognitive impairment and depression). Practical recommendations have been published by the HFA.591 Key topics to include are recommended in Table 14.2.
14.4 Exercise training
Several systematic reviews and meta-analyses of small studies have shown that physical conditioning by exercise training improves exercise tolerance, health-related quality of life and HF hospitalization rates in patients with HF. A single large RCT618 showed a modest and non-significant reduction in the primary composite outcome of all-cause mortality or all-cause hospitalization. There was no reduction in mortality and no safety concerns were raised.618, 633 The most recent Cochrane review of exercise training619 included 33 trials with 4740 patients with HF (predominantly HFrEF). There was a trend towards a reduction in mortality with exercise in trials with >1 year of follow-up. Compared with the control group, exercise training reduced the rate of overall and HF-specific hospitalization and improved quality of life. Practical recommendations on exercise training have been published by the HFA.120
There is evidence that in patients with HFpEF, exercise training has several benefits, including improvements in exercise capacity, as measured objectively using peak oxygen consumption, quality of life and diastolic function, assessed by echocardiography.321, 620, 621, 634
Patients with HF, regardless of LVEF, are recommended to perform properly designed exercise training (see the recommendations table).
14.5 Follow-up and monitoring
Patients with HF benefit from regular follow-up and monitoring of biomedical parameters to ensure the safety and optimal dosing of medicines and detect the development of complications or disease progression that may require a change in management (e.g. the onset of AF or development of anaemia). Monitoring may be undertaken by the patients themselves during home visits, in community or hospital clinics, by remote monitoring with or without implanted devices or by structured telephone support (STS). The optimal method of monitoring will depend on local organizations and resources and will vary among patients. For example, more frequent monitoring will be required during periods of instability or optimization of medication. Older adults may also benefit from more frequent monitoring. Some patients will be keen and able to participate in self-monitoring.
High circulating NPs predict unfavourable outcomes in patients with HF, and a decrease in NP levels during recovery from circulatory decompensation is associated with a better prognosis.588-590 Although it is plausible to monitor clinical status and tailor treatment based on changes in circulating NPs in patients with HF, published studies have provided differing results.635-638 This does not enable us to recommend a broad application of such an approach.
Telemedicine in HF, which is also termed remote patient management, has variable clinical trial results.639 Several meta-analyses suggest clinical benefits, but numerous prospectively initiated clinical trials including >3700 patients have not confirmed this. These clinical trials include Tele-HF,640 TIM-HF,641 INH,642 WISH643 and TEHAF.644 It is clear that there is not just one type of telemedicine, and each approach needs to be assessed on its individual merit.
Recently, two individual approaches were shown to be successful in improving clinical outcome when used in patients with HFpEF or HFrEF. These approaches include the CardioMems system (tested in 550 patients with both HFrEF and HFpEF)628 and the IN-TIME approach (tested in 664 HFrEF patients)630, which may be considered for use in selected patients with HF (see the recommendations table).
14.6 The older adult, frailty and cognitive impairment
HF management and self-care behaviour are complicated by ageing, co-morbid conditions, cognitive impairment, frailty and limited social support. HF is also a leading cause of hospital admission in the older adult, where it is associated with increased hospital length of stay and risk of mortality.645
Frailty is common in older adults with HF, with a recent study suggesting it may be present in >70% of patients with HF and >80 years of age.645 Frailty scoring systems provide an objective assessment and identify the presence of or change in the level of frailty. Patients with a high frailty score will benefit from closer contact with the HF specialist team, more frequent follow-up and monitoring and individualized self-care support.
Frailty scores include646 walking speed (gait speed test), timed up-and-go test, PRISMA 7 questionnaire, Frail Score,647 Fried Score647, 648 and Short Physical Performance Battery (SPPB).
Cognitive impairment and HF frequently coexist. Acute delirium is also associated with decompensated HF and may be present on hospital admission. Cognitive function can be assessed using the Mini-Mental State Examination649 or the Montreal cognitive assessment.650 The presence of delirium and HF is found more commonly in older adults and is associated with increased mortality and poorer self-care ability and increases any hospital length of stay.651 There is currently no clinical evidence that HF medication worsens or improves cognitive function. However, its effect on HF outcome suggests such medication should be used. Support from a multidisciplinary HF team in collaboration with specialist dementia support teams, alongside medication compliance aids, tailored self-care advice and involvement of family and caregivers, may improve adherence with complex HF medication and self-care regimens (see Table 14.2 on patient education) (Table 14.3).
14.7 Palliative and end-of-life care
Palliative care approaches include a focus on symptom management, emotional support and communication between the patient and his/her family. Ideally this should be introduced early in the disease trajectory and increased as the disease progresses. A decision to alter the focus of care from modifying disease progression to optimising quality of life should be made in discussion with the patient, cardiologist, nurse and general practitioner. The patient's family should be involved in such discussions if requested by the patient652, 653 (Table 14.4).
Key components of a palliative care service are recommended in Table 14.5. Palliative care has been discussed in detail in a position paper from the ESC HFA.654
Liaison between the specialist palliative care services and the HF team and/or the primary care physician, using a shared care approach, is required in order to address and optimally coordinate the patient's care. Recent pilot studies have suggested an improvement in symptom burden and quality of life,653, 655 but these data are too limited to provide a recommendation.
-
Morphine (with an antiemetic when high doses are needed) can be used to reduce breathlessness, pain and anxiety.656
-
Increasing the inspired oxygen concentration may provide relief of dyspnoea.
-
Diuretic management can be used to relieve severe congestion or optimize symptom control (congestion and thirst).
-
Reduce HF drugs that reduce blood pressure to maintain sufficient oxygenation and reduce the risk of falls.
-
A discussion about stopping medication that does not have an immediate effect on symptom management or health-related quality of life, such as agents to lower cholesterol or treat osteoporosis
-
Documentation of the patient's decision regarding resuscitation attempts
-
Deactivation of an ICD at end-of-life (according to local legal regulations)
-
Preferred place for care and death
-
Emotional support to the patient and family/caregiver with appropriate referral for psychological or spiritual support
Palliative outcome scores include the Palliative Care Outcome Scale,657 Karnofsky Performance Status658 and Functional Assessment of Chronic Illness Therapy – Palliative Care (FACIT-Pal).659
15 Gaps in evidence
Clinicians responsible for managing patients with HF must frequently make treatment decisions without adequate evidence or a consensus of expert opinion. The following is a short list of selected, common issues that deserve to be addressed in future clinical research.
-
• For HFmrEF/HFpEF, research into the underlying characteristics, pathophysiology and diagnosis (with new modalities)
-
• Updated epidemiology on HF incidence and prevalence including patients from all continents
-
• For imaging and biomarkers, studies on effects of specific imaging modalities and biomarkers to improve clinical outcome (e.g. biomarker-guided therapies, detection of CAD/myocardial ischaemia, late-gadolinium enhancement CMR, echocardiographic strain measurements, stress echocardiography, etc.)
-
• Increasing awareness of HF in the medical community, lay public and among policy makers.
-
• Evaluate the comparative clinical effectiveness and cost-effectiveness of different strategies to screen for HF.
-
• Identification of non-responders to current guideline-advised medical treatment
-
• Targeted therapies for specific aetiologies of HFrEF (e.g. myocarditis, peripartum cardiomyopathy)
-
• Therapies directly improving cardiomyocyte function (e.g. acto-myosin cross-bridge activation, sarco/endoplasmic reticulum Ca2+-ATPase activation, ryanodine receptor stabilization, energetic modulation) or targeting non-myocytic compartment (e.g. anti-fibrosis/matrix remodelling)
-
• Therapies for HFmrEF/HFpEF (ARNIs, beta-blockers, soluble guanyl cyclase inhibitors, i.v. iron)
-
• Indications for ICDs in specific subgroups (e.g. ARVC and HFmrEF/HFpEF) and optimal selection of ICD candidates
-
• QRS morphology or duration as a predictor of response to CRT
-
• CRT in patients with AF
-
• Efficacy of PV ablation as a rhythm-control strategy in patients with AF
-
• Interventional approach to recurrent, life-threatening ventricular tachyarrhythmias
-
• The role of remote monitoring strategies in HF
-
• Non-surgical (percutaneous) correction of functional mitral and tricuspid regurgitations
-
• Identification of indications for coronary angiography/revascularization in patients with HF and chronic stable CAD
-
• Effects of novel LVADs as destination therapy and bridge to transplantation
-
• A better understanding of pathophysiology and potential treatments in specific HF populations, including the
-
• very elderly,
-
• young patients,
-
• eGFR <30 mL/min,
-
• diabetic patients,
-
• cardiotoxic chemotherapy-induced HF,
-
• muscular dystrophies,
-
• cachexia and depression.
-
-
• Therapies for HF-related sleep-disordered breathing in HFrEF/HFpEF/HFmrEF.
-
• Prospective evaluation of the ‘time-to-treatment’ concept in AHF
-
• Evaluation of whether inadequate phenotyping is responsible for the failure of treatments to improve outcome in AHF
-
• Better definition and treatment of diuretic resistance
-
• Role of nitrates in the management of AHF
-
• Treatments improving mortality and morbidity
-
• Strategies and therapies to prevent early rehospitalization after discharge for a hospital admission for AHF.
-
• Treatment algorithms for patients with HF excluded by pivotal clinical trials
-
• Palliative and end-of-life care management and assessment of outcome
-
• Optimal integration of multidisciplinary care, self-management of patients and their adherence.
16 To do and not to do messages from the Guidelines
17 Web Addenda
All Web figures and Web tables are available in the Web addenda, available at European Heart Journal online and also via the ESC website.
18 Appendix
ESC Committee for Practice Guidelines (CPG): Jose Luis Zamorano (Chairperson) (Spain), Victor Aboyans (France), Stephan Achenbach (Germany), Stefan Agewall (Norway), Lina Badimon (Spain), Gonzalo Barón-Esquivias (Spain), Helmut Baumgartner (Germany), Jeroen J. Bax (The Netherlands), Héctor Bueno (Spain), Scipione Carerj (Italy), Veronica Dean (France), Çetin Erol (Turkey), Donna Fitzsimons (UK), Oliver Gaemperli (Switzerland), Paulus Kirchhof (UK/Germany), Philippe Kolh (Belgium), Patrizio Lancellotti (Belgium), Gregory Y. H. Lip (UK), Petros Nihoyannopoulos (UK), Massimo F. Piepoli (Italy), Piotr Ponikowski (Poland), Marco Roffi (Switzerland), Adam Torbicki (Poland), António Vaz Carneiro (Portugal), Stephan Windecker (Switzerland).
ESC National Cardiac Societies actively involved in the review process of the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
Armenia: Armenian Cardiologists Association, Hamayak S. Sisakian; Azerbaijan: Azerbaijan Society of Cardiology, Elnur Isayev; Belarus: Belorussian Scientific Society of Cardiologists, Alena Kurlianskaya; Belgium: Belgian Society of Cardiology, Wilfried Mullens; Bulgaria: Bulgarian Society of Cardiology, Mariya Tokmakova; Cyprus: Cyprus Society of Cardiology, Petros Agathangelou; Czech Republic: Czech Society of Cardiology, Vojtech Melenovsky; Denmark: Danish Society of Cardiology, Henrik Wiggers; Egypt: Egyptian Society of Cardiology, Mahmoud Hassanein; Estonia: Estonian Society of Cardiology, Tiina Uuetoa; Finland: Finnish Cardiac Society, Jyri Lommi; Former Yugoslav Republic of Macedonia: Macedonian FYR Society of Cardiology, Elizabeta Srbinovska Kostovska; France: French Society of Cardiology, Yves Juillière; Georgia: Georgian Society of Cardiology, Alexander Aladashvili; Germany: German Cardiac Society, Andreas Luchner; Greece: Hellenic Cardiological Society, Christina Chrysohoou; Hungary: Hungarian Society of Cardiology, Noémi Nyolczas; Iceland: Icelandic Society of Cardiology, Gestur Thorgeirsson; Israel: Israel Heart Society, Jean Marc Weinstein; Italy: Italian Federation of Cardiology, Andrea Di Lenarda; Kazakhstan: Association of Cardiologists of Kazakhstan, Nazipa Aidargaliyeva; Kosovo: Kosovo Society of Cardiology, Gani Bajraktari; Kyrgyzstan: Kyrgyz Society of Cardiology, Medet Beishenkulov; Latvia: Latvian Society of Cardiology, Ginta Kamzola; Lebanon: Lebanese Society of Cardiology, Tony Abdel-Massih; Lithuania: Lithuanian Society of Cardiology, Jelena Čelutkienė; Luxembourg: Luxembourg Society of Cardiology, Stéphanie Noppe; Malta: Maltese Cardiac Society, Andrew Cassar; Moldova: Moldavian Society of Cardiology, Eleonora Vataman; Morocco: Moroccan Society of Cardiology, Saadia Abir-Khalil; The Netherlands: Netherlands Society of Cardiology, Petra van Pol; Norway: Norwegian Society of Cardiology, Rune Mo; Poland: Polish Cardiac Society, Ewa Straburzyńska-Migaj; Portugal: Portuguese Society of Cardiology, Cândida Fonseca; Romania: Romanian Society of Cardiology, Ovidiu Chioncel; Russian Federation: Russian Society of Cardiology, Evgeny Shlyakhto; San Marino: San Marino Society of Cardiology, Marco Zavatta; Serbia: Cardiology Society of Serbia, Petar Otasevic; Slovakia: Slovak Society of Cardiology, Eva Goncalvesová; Slovenia: Slovenian Society of Cardiology, Mitja Lainscak; Spain: Spanish Society of Cardiology, Beatriz Díaz Molina; Sweden: Swedish Society of Cardiology, Maria Schaufelberger; Switzerland: Swiss Society of Cardiology, Thomas Suter; Turkey: Turkish Society of Cardiology, Mehmet Birhan Yılmaz; Ukraine: Ukrainian Association of Cardiology, Leonid Voronkov; United Kingdom: British Cardiovascular Society, Ceri Davies.