Which heart failure patients profit from natriuretic peptide guided therapy? A meta-analysis from individual patient data of randomized trials
Abstract
Aims
Previous analyses suggest that heart failure (HF) therapy guided by (N-terminal pro-)brain natriuretic peptide (NT-proBNP) might be dependent on left ventricular ejection fraction, age and co-morbidities, but the reasons remain unclear.
Methods and results
To determine interactions between (NT-pro)BNP-guided therapy and HF with reduced [ejection fraction (EF) ≤45%; HF with reduced EF (HFrEF), n = 1731] vs. preserved EF [EF > 45%; HF with preserved EF (HFpEF), n = 301] and co-morbidities (hypertension, renal failure, chronic obstructive pulmonary disease, diabetes, cerebrovascular insult, peripheral vascular disease) on outcome, individual patient data (n = 2137) from eight NT-proBNP guidance trials were analysed using Cox-regression with multiplicative interaction terms. Endpoints were mortality and admission because of HF. Whereas in HFrEF patients (NT-pro)BNP-guided compared with symptom-guided therapy resulted in lower mortality [hazard ratio (HR) = 0.78, 95% confidence interval (CI) 0.62–0.97, P = 0.03] and fewer HF admissions (HR = 0.80, 95% CI 0.67–0.97, P = 0.02), no such effect was seen in HFpEF (mortality: HR = 1.22, 95% CI 0.76–1.96, P = 0.41; HF admissions HR = 1.01, 95% CI 0.67–1.53, P = 0.97; interactions P < 0.02). Age (74 ± 11 years) interacted with treatment strategy allocation independently of EF regarding mortality (P = 0.02), but not HF admission (P = 0.54). The interaction of age and mortality was explained by the interaction of treatment strategy allocation with co-morbidities. In HFpEF, renal failure provided strongest interaction (P < 0.01; increased risk of (NT-pro)BNP-guided therapy if renal failure present), whereas in HFrEF patients, the presence of at least two of the following co-morbidities provided strongest interaction (P < 0.01; (NT-pro)BNP-guided therapy beneficial only if none or one of chronic obstructive pulmonary disease, diabetes, cardiovascular insult, or peripheral vascular disease present). (NT-pro)BNP-guided therapy was harmful in HFpEF patients without hypertension (P = 0.02).
Conclusion
The benefits of therapy guided by (NT-pro)BNP were present in HFrEF only. Co-morbidities seem to influence the response to (NT-pro)BNP-guided therapy and may explain the lower efficacy of this approach in elderly patients.
Introduction
A recent individual patient data meta-analysis showed that (N-terminal pro-)brain natriuretic peptide (NT-proBNP)-guided therapy improves outcome in heart failure (HF), at least in those aged 75 years or younger;1 in line with other aggregate data meta-analyses of (NT-pro)BNP-guided therapy in HF.2, 3 Thus, treatment effects of (NT-pro)BNP-guided therapy may be dependent on age.1 One possible explanation of the apparent dependency of the efficacy of natriuretic peptide (NP)-guided treatment upon age is that co-morbidities, which are more common with increasing age, may limit HF therapy titration and/or reduce the benefits of treatment. This question has, however, not yet been appropriately addressed.
In HF, data on the elderly, those patients with significant co-morbidities and those with HFpEF are scant. Therefore, these questions may not only shed further light on the efficacy of biomarker-guided therapy in HF, but also on potential differences in treatment response dependent upon age and co-morbidities. As most patients included in the large randomized therapeutic trials that underpin clinical practice guidelines4 had HF with reduced left-ventricular ejection fraction (HFrEF), were not truly elderly and had few co-morbidities, the findings from these trials might be less applicable to the majority of patients seen in daily practice; thus, perceived shortcomings of biomarker-guided HF care may reflect limitations of therapeutic efficacy in patients with co-morbidity.
Moreover, no treatment interaction with left ventricular ejection fraction (LVEF) was seen in previous analysis, but less than 10% of the trial participants had preserved LVEF (HFpEF) >45%, precluding any firm conclusions in this patient group. As no randomized therapeutic trials in HFpEF have shown convincing benefit from medical therapy,5-7 it is uncertain whether (NT-pro)BNP-guidance is equally effective in HFpEF and HFrEF, i.e. LVEF ≤45%.
Therefore, we investigated (i) potential interactions between co-morbidities and (ii) age with treatment response, as well as (iii) potential differences in treatment response between HFrEF and HFpEF in patients included in randomized trials of (NT-pro)BNP-guided therapy in HF.
Methods
Criteria for inclusion of studies and patient data
For this analysis, we used the collaborative database formed for the recently published individual patient data meta–analysis on the effect of (NT-pro)BNP-guided treatment of chronic HF.1
As the current meta-analysis focuses on effects of (NT-pro)BNP-guided therapy on outcome in subgroups we have included only those studies that provided individual patient data8-15 and excluded aggregated data presented in the primary meta-analysis. In addition, data from STARBRITE16 were not included as we did not have access to perform additional analyses on this dataset. Conversely, we added the recently published results from the HFpEF subgroup in TIME-CHF to increase the number of HFpEF patients included,17 which increased the percentage of HFpEF from 8% to 15% of the total cohort. In 5% of cases LVEF was not known. These patients were excluded from endpoint analyses. The HF patients with no known LVEF did not differ significantly with respect to their baseline characteristics from those included except that chronic obstructive pulmonary disease (COPD) was more common in those without LVEF measured in BATTLESCARRED12 (43% vs. 18%, P = 0.004) and in PRIMA9 (34% vs. 15%, P = 0.01) and age was greater in BATTLESCARRED (77.3 ± 6.6 years vs. 73.6 ± 9.4 years, P = 0.04; other data not shown). The inclusion and exclusion criteria of each individual trial have been published previously.8-15, 17
Data extraction
Individual patient data from eligible studies were entered into the meta-analysis database. The data included patient age, sex, co-morbidities, baseline BNP or NT-proBNP level (pg/mL), baseline creatinine (µmol/L), baseline LVEF (%), treatment assignment (NP-guided or clinically guided) and randomization date. The presence or absence of co-morbidities was based on the medical history listed in the medical records of individual patients in all trials. No specific additional testing was done for the diagnosis of co-morbidities. Outcome data encompassed all-cause mortality, date of all-cause death or last follow-up, and first HF hospitalization along with the date of hospitalization. Only events occurring during the application of the treatment strategy were included in the analysis.
We used the same cut-off to distinguish HFrEF from HFpEF (i.e. LVEF of 45%), as in the previous analysis, which was based on the cut-off used in the majority of the trials.1 Using cut-offs of 40% or 50% did not change results significantly and are therefore not presented.
Statistical analysis
Data are presented as frequencies, mean ± standard deviation (SD) or median (interquartile range, IQR), as appropriate. Comparisons of baseline characteristics between the different studies and between HFpEF and HFrEF were performed using χ2-test for categorical data and one-way ANOVA or Kruskal–Wallis H-test for continuous data, as appropriate.
The pre-specified primary endpoint was all-cause mortality. Secondary endpoints encompassed time to first HF admission and the combined endpoint of HF hospitalization or death. We analysed time-to-event for assessing outcome by using unadjusted Cox proportional hazards regression models. Effect of treatment strategy allocation on outcome in HFpEF and HFrEF patients was visualized by Kaplan–Meier analysis. After analysis of potential differences in the treatment effect between HFrEF and HFpEF in all patients, further analyses were performed for HFpEF and HFrEF separately. Heterogeneity between studies was tested by treatment × study interaction effect across studies. Cox proportional hazard regression models were used to test the influence of co-morbidities on treatment response in both HFpEF and HFrEF. Furthermore, interaction between treatment strategy allocation and co-morbidities on outcome was assessed by incorporating co-morbidities × treatment as terms in the Cox regression model. All analyses were performed using IBM SPSS Statistics version 21.0 apart from aggregated hazard ratios (HR), which were calculated using Review Manager 5.2 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
The baseline characteristics of the patients participating in the eight trials included in this analysis are depicted in Table 1. The patients were on average elderly, one-third were female and co-morbidities were frequent.
| Overall(n = 2137) | BATTLESCARRED(n = 242)12 | Troughtonet al. (n = 69)15 | PRIMA(n = 345)9 | PROTECT(n = 151)10 | Signal-HF(n = 252)13 | TIME-CHF(n = 622)14,17 | UPSTEP(n = 268)11 | Bergeret al. (n = 188)8 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 73.5 ± 10.6 | 74.0 ± 9.2 | 70.0 ± 9.9 | 72.2 ± 11.9 | 63.3 ± 14.0 | 77.8 ± 7.6 | 76.9 ± 7.6 | 70.8 ± 9.8 | 71.1 ± 11.8 |
| Age ≥75 years | 1123 (53%) | 138 (57%) | 24 (35%) | 167 (48%) | 38 (25%) | 183 (73%) | 380 (61%) | 105 (39%) | 88 (47%) |
| Male gender | 1406 (66%) | 157 (65%) | 53 (77%) | 199 (58%) | 127 (n = 84%) | 180 (71%) | 369 (59%) | 196 (73%) | 125 (66%) |
| BMI (kg/m2) (n = 1591) | 26.5 ± 4.8 | 26.8 ± 4.9 | 27.4 ± 4.8 (n = 67) | 26.5 ± 5.0 (n = 238) | na | na | 25.6 ± 4.4 | 27.3 ± 4.8 | 27.1 ± 5.2 |
| Hypertension (n = 2129) | 1251 (59%) | 117 (48%) | 45 (66% of 68) | 167 (49% of 338) | 79 (52%) | 139 (55%) | 462 (74%) | 116 (43%) | 126 (67%) |
| Renal failure (n = 1985) | 524 (26%) | 63 (26%) | 16 (23%) | 47 (14% of 344) | na | 6 (2%) | 355 (57%) | 7 (3%) | 30 (16%) |
| CVI/TIA (n = 1986) | 325 (16%) | 52 (21%) | 19 (28%) | 66 (19%) | na | 30 (12%) | 98 (16%) | 39 (15%) | 21 (11%) |
| Diabetes | 668 (31%) | 52 (21%) | 16 (23%) | 91 (26%) | 63 (42%) | 53 (21%) | 222 (36%) | 85 (32%) | 86 (46%) |
| COPD | 364 (17%) | 52 (21%) | 23 (33%) | 59 (17%) | 31 (21%) | 28 (11%) | 124 (20%) | 17 (6%) | 30 (16%) |
| PVD (n = 1798) | 249 (14%) | 30 (12%) | 12 (17%) | 65 (19%) | na | 0 (0%) | 124 (20%) | 18 (15%) | na |
| Cancer (n = 1554) | 186 (12%) | na | 5 (7%) | 47 (14% of 343) | na | 24 (10%) | 86 (14%) | 24 (9%) | na |
| NYHA ≥ III | 1163 (54%) | 56 (23%) | 14 (20%) | 74 (21%) | 83 (55%) | 96 (38%) | 473 (76%) | 184 (69%) | 183 (93%) |
| LVEF ≤45%* | 1731 (85%) | 134 (63%) | 69 (100%) | 229 (73%) | 151 (100%) | 204 (98%) | 499 (80%) | 268 (100%) | 177 (94%) |
| LVEF >45%* | 301 (15%) | 78 (37%) | 0 | 84 (27%) | 0 | 5 (2%) | 123 (20%) | 0 | 11 (6%) |
| LVEF unknown | 105 (5%) | 30 (12%) | 0 | 32 (9%) | 0 | 43 (17%) | 0 | 0 | 0 |
| LVEF (%) (n = 1672) | 32 [25–40] | 40 [28–53] (n = 212) | 27 [21–34) | 34 [25–48] (n = 313) | 26 [20–34] | 34 (25–38] (n = 209) | 32 [25–42] | na | 30 [22–35] (n = 96) |
| Creatinine (µmol/L) (n = 2125) | 109 [88.2–140] | 110 [90–130] | 90 [80–110] | 121 [101–158] | 124 [97–150] | 95 [76–117] (n = 242) | 108 [87–140] | 100 [83–126] (n = 264) | 115 [89–149] |
| NT-proBNP or BNP (pg/mL) | 2645 [1434–5088] (excl UPSTEP) | 2001 [1236–2974] | 1467 [1077–2807] | 2950 [1319–5445] | 2118 [1122–3831] | 2363 [1373–4040] | 3836 [1916–6905] | 609 [356–947]† | 2280 [1256–5193] |
| ACE-inhibitor (n = 1976) | 1460 (74%) | 202 (83%) | 69 (100%) | 255 (74%) | na | 130 (54%) (n = 242) | 479 (77%) | 196 (73%) | 129 (69%) |
| ARB (n = 1976) | 401 (20%) | 47 (19%) | 0 (0%) | 63 (18%) | na | 55 (23%) (n = 242) | 102 (16%) | 95 (35%) | 39 (21%) |
| β-Blocker (n = 1976) | 1443 (73%) | 131 (54%) | 5 (7%) | 284 (82%) | na | 146 (60%) (n = 242) | 476 (77%) | 252 (94%) | 149 (79%) |
| Spironolactone (n = 1976) | 716 (36%) | 27 (11%) | 0 (0%) | 186 (54%) | na | 36 (15%) (n = 242) | 234 (38%) | 151 (56%) | 82 (44%) |
| Loop diuretic (n = 1976) | 1706 (86%) | 232 (96%) | 69 (100%) | 333 (97%) | na | 112 (46%) (n = 242) | 574 (92%) | 232 (87%) | 154 (82%) |
| (NT-pro)BNP-guided therapy | 1072 (50%) | 121 (50%) | 33 (48%) | 174 (50%) | 75 (50%) | 127 (50%) | 310 (50%) | 140 (52%) | 92 (49%) |
- Numbers are n (%) unless otherwise indicated.
- * Percentage of those with known left ventricular ejection fraction (LVEF).
- † BNP levels.
- BMI, body mass index; CVI, cerebrovascular insult; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; NYHA, New York heart association classification; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blocker; (NT-pro)BNP, (N-terminal pro-)brain natriuretic peptide; na, not available.
There were significant differences in patients' characteristics between the studies (P < 0.05 for all), as shown in detail in Table 1. In addition, not all studies collected all the information used for this analysis (Table 1). In three trials, patients with HFrEF only were included,10, 11, 15 whereas five also included patients with relatively preserved LVEF (HFpEF, i.e. LVEF >45%).8, 9, 12, 13, 17 One study included only five such patients, none of which experienced an event.13 Therefore, HFpEF patients of this study (i.e. Signal-HF) were not included in the endpoint analysis, leaving four studies, which included a total of 296 HFpEF patients.8, 9, 12, 17
Heart failure with preserved ejection fraction vs. heart failure with reduced ejection fraction
Patients with HFpEF were significantly older, more often female, with slightly higher body mass index (BMI) and lower NT-proBNP concentrations than patients with HFrEF (Table 2). Hypertension, renal disease and peripheral vascular disease (PVD) were more prevalent in HFpEF than in HFrEF. HFpEF was more often treated with loop diuretics, but less frequently with β-blockers. There was no difference between HFpEF and HFrEF in terms of mortality (HR = 1.15, 95% CI 0.89–1.50, P = 0.28 for HFpEF vs. HFrEF) or HF admission (HR = 1.20, 95% CI 0.95–1.51, P = 0.12), but the combined endpoint of HF hospitalization or death was more common in HFpEF patients (HR = 1.24, 95% CI 1.02–1.51, P = 0.03; 1- and 2-year event rate in HFpEF vs. HFrEF 36% and 50% vs. 30% and 44%, respectively). This increased hazard in HFpEF was not independent of age (bivariate Cox regression: HR = 1.14, P = 0.18 after adjustment for age).
| HFpEF (n = 296) | HFrEF (n = 1731) | P | Age <75 years (n = 977) | Age ≥75 years (n = 1050) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 77.2 ± 9.3 | 72.6 ± 10.7 | <0.001 | 64.7 ± 8.7 | 81.2 ± 4.0 | – |
| Age ≥75 years | 200 (68%) | 850 (49%) | <0.001 | 0 (0%) | 1050 (100%) | – |
| Male gender | 121 (41%) | 1215 (70%) | <0.001 | 713 (73%) | 623 (59%) | <0.001 |
| BMI (kg/m2) (n = 1546) | 27.2 ± 5.2 (n = 257) | 26.3 ± 4.6 (n = 1289) | 0.01 | 27.6 ± 5.0 (n = 747) | 25.3 ± 4.2 (n = 799) | <0.001 |
| Hypertension (n = 2019) | 216 (73%) of 295 | 982 (57%) of 1724 | <0.001 | 517 (53%) | 681 (65%) of 1042 | <0.001 |
| Renal failure (n = 1875) | 117 (40%) | 395 (25%) of 1579 | <0.001 | 182 (21%) of 863 | 330 (33%) of 1012 | <0.001 |
| CVI/TIA (n = 1876) | 58 (20%) | 253 (16%) of 1580 | 0.13 | 120 (14%) of 864 | 191 (19%) of 1012 | 0.004 |
| Diabetes | 89 (30%) | 549 (32%) | 0.57 | 331 (34%) | 307 (29%) | 0.03 |
| COPD | 44 (15%) | 289 (17%) | 0.43 | 170 (17%) | 163 (16%) | 0.26 |
| PVD (n = 1688) | 60 (21%) of 285 | 174 (12%) of 1403 | <0.001 | 95 (12%) of 764 | 139 (15%) of 924 | 0.12 |
| Cancer (n = 1474) | 32 (15%) of 207 | 143 (11%) of 1267 | 0.09 | 57 (9%) of 667 | 118 (15%) of 807 | <0.001 |
| CVI/TIA, COPD or diabetes (n = 1876) | 152 (51%) | 798 (51%) | 0.79 | 439 (51%) of 864 | 551 (50%) of 1012 | 0.89 |
| CVI/TIA, COPD, diabetes or PVD (n = 1688) | 163 (57%) | 745 (53%) | 0.21 | 401 (52%) of 764 | 507 (55%) of 924 | 0.33 |
| NYHA ≥ III | 149 (50%) | 980 (57%) | 0.04 | 504 (52%) | 625 (60%) | <0.001 |
| LVEF (%) (n = 1667) | 55 [50–60] (n = 289) | 30 [23–35] (n = 1378) | – | 30 [22–37] (n = 771) | 35 [25–45] (n = 896) | <0.001 |
| HFrEF | 0 (0%) | 1731 (100%) | – | 881 (90%) | 850 (81%) | <0.001 |
| Creatinine (µmol/L) (n = 2016) | 111 [87–142] | 108 [88–140] (n = 1720) | 0.35 | 103 [85–132] (n = 972) | 114 [90–146] (n = 1044) | <0.001 |
| NT-proBNP (pg/mL) (n = 1758)* | 2061 [1350–3933] | 2811 [1467–5481] (n = 1462)* | <0.001 | 2077 [1110–4026] (n = 813)* | 3256 [1818–6323] (n = 945)* | <0.001 |
| ACE-inhibitor (n = 1866) | 207 (70%) | 1179 (75%) of 1570 | 0.06 | 660 (76%) of 863 | 726 (72%) of 1003 | 0.04 |
| ARB (n = 1866) | 55 (19%) | 332 (21%) of 1570 | 0.32 | 191 (22%) of 863 | 196 (20%) of 1003 | 0.17 |
| β-Blocker (n = 1866) | 195 (66%) | 1184 (75%) of 1570 | 0.001 | 662 (77%) of 863 | 717 (71%) of 1003 | 0.01 |
| Spironolactone (n = 1866) | 81 (27%) | 407 (26%) of 1570 | 0.61 | 265 (31%) of 863 | 223 (22%) of 1003 | <0.001 |
| Loop diuretic (n = 1866) | 274 (93%) | 1349 (86%) of 1570 | 0.002 | 757 (88%) of 863 | 866 (86%) of 1003 | 0.38 |
| (NT-pro) BNP-guided therapy | 144 (49%) | 862 (50%) | 0.75 | 493 (50%) | 513 (49%) | 0.47 |
- Numbers are n (%) unless otherwise indicated.
- * Excluding patients from UPSTEP trial.
- HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; BMI, body mass index; CVI, cerebrovascular insult; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; NYHA, New York Heart Association classification; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blocker; (NT-pro)BNP, (N-terminal pro-)brain natriuretic peptide.
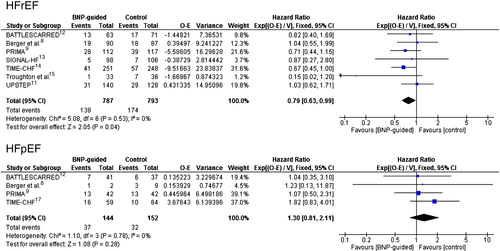
Figure 1 depicts the effects of (NT-pro)BNP-guided therapy on mortality based on the individual data of patients showing a significant beneficial effect in HFrEF (HR = 0.78, 95% CI 0.62–0.97), but not in HFpEF patients (HR = 1.22, 95% CI 0.76–1.96; interaction P = 0.016). Data from individual studies are depicted in Figure 2, showing no significant heterogeneity between studies according to LVEF subgroup.
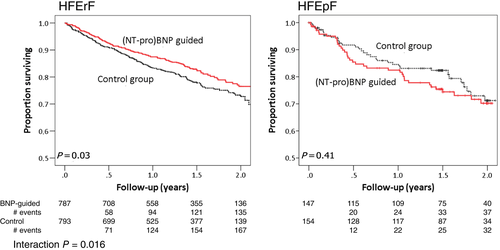
Time to first HF admission was significantly prolonged by (NT-pro)BNP-guided therapy in the HFrEF group (HR = 0.80, 95% CI 0.67–0.97, P = 0.02), but not in the HFpEF group (HR = 1.01, 95% CI 0.67–1.53, P = 0.97; interaction P = 0.007) with no significant heterogeneity between studies. The combined endpoint of HF admission or death was also reduced in HFrEF patients by the use of (NT-pro)BNP-guided therapy (HR = 0.78, 95% CI 0.66–0.92, P = 0.004), but not in the HFpEF group (HR = 1.08, 95% CI 0.76–1.53, P = 0.66; interaction P = 0.001). There was no significant heterogeneity between studies.
Influence of co-morbidities on (N-terminal pro-)brain natriuretic peptide-guided therapy
Co-morbidities influenced response to (NT-pro)BNP-guided therapy with respect to mortality in both HFrEF and HFpEF (Figure 3). In HFrEF, the response to (NT-pro)BNP-guided therapy was primarily seen in patients without COPD, diabetes, cardiovascular insult (CVI)/transient ischaemic attack (TIA) or PVD. Although any single co-morbidity interaction with treatment efficacy did not reach statistical significance, when co-morbidities were considered in combination, this interaction was significant. Thus, compared with symptom-guided therapy, (NT-pro)BNP-guided therapy reduced mortality (HR = 0.61, 95% CI 0.42–0.88, P = 0.008) in patients with no history of CVA/TIA, diabetes or COPD. Such benefit was absent in those with any one of these co-morbidities (HR = 0.94, 95% CI 0.71–1.24, P = 0.65). When also considering PVD, patients with none or only one of these four co-morbidities (i.e. COPD, diabetes, CVI/TIA, PVD) had a mortality benefit of 33% (HR = 0.67, 95% CI 0.51–0.89, P = 0.005), whereas those with two or more of them did not benefit (HR = 0.99, 95% CI 0.62–1.59, P = 0.97). Interestingly, a history of renal failure had no influence on treatment response (Figure 3). When using baseline estimated glomerular filtration rate (eGFR) to define renal failure (i.e. ≤60 mL/min.1.73m2 using simplified Modification of Diet in Renal Disease equation),18 treatment response was not influenced by either eGFR >60 (n = 776: HR = 0.78, 95% CI 0.51–1.20, P = 0.25) or eGFR ≤60 (n = 944: HR = 0.81, 95% CI 0.63–1.06, P = 0.12; interaction p > 0.2).
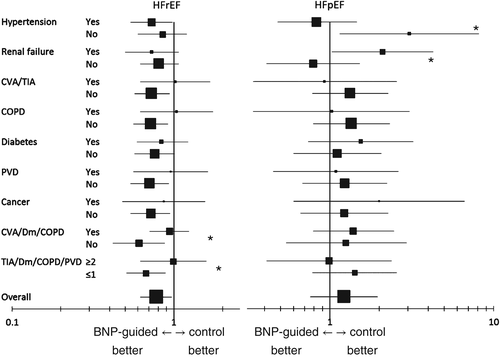
In HFpEF, co-morbidities also influenced treatment response, but the pattern differed from that in HFrEF. Patients without hypertension allocated to (NT-pro)BNP-guided therapy had worse outcome than those allocated to clinically guided therapy, whereas in those with hypertension no such harm was seen (interaction P = 0.02). Conversely, HFpEF patients with a history of renal failure fared worse on (NT-pro)BNP-guided therapy. This was also the case when using eGFR to define renal failure (eGFR >60 n = 98: HR = 0.71, 95% CI 0.27–1.86; eGFR ≤60 n = 203: HR = 1.47, 95% CI 0.85–2.55; interaction P = 0.05). In contrast to HFrEF, other co-morbidities or combinations thereof did not influence treatment response (Figure 3).
The influence of co-morbidities on the response to (NT-pro)BNP-guided therapy with respect to HF admissions was considerably less than for mortality. For this end-point, no statistically significant interaction between co-morbidities and the efficacy of (NT-pro)BNP-guided treatment was found in either HFrEF or HFpEF. The hazard ratios for HF admission on (NT-pro)BNP-guided therapy compared with clinically guided management according to co-morbidities are given in the Supplementary material online, Table S1; Table S2 gives an overview of the number of patients included in each subgroup and the number of events.
Influence of age on (N-terminal pro-)brain natriuretic peptide-guided therapy
The previously described interaction between age and treatment strategy allocation on mortality was confirmed in the current analysis. Thus in HFrEF patients, the beneficial effect was mainly seen in patients aged <75 years (HR = 0.68, 95% CI 0.48–0.96, P = 0.03; n = 881), but not in those aged ≥75 years (HR = 0.87, 95% CI 0.65–1.16, P = 0.35; n = 850; interaction P = 0.22). In HFpEF patients aged <75 years, NT-proBNP-guided therapy resulted in a HR of 0.76 (95% CI 0.29–1.96, P = 0.56; n = 96), whereas in those aged ≥75 years the HR was 1.56 (95% CI 0.90–2.70, P = 0.11; n = 200; interaction P = 0.02). The interaction between age and treatment efficacy disappeared when interactions between co-morbidities and treatment strategy allocation were considered (Table 3), whereas the interactions between co-morbidities and treatment efficacy were not influenced by age. Thus in patients with HFpEF, the effect of age on treatment response was no longer apparent when additional interactions with renal failure or the combination of CVI, diabetes mellitus, and COPD were considered. In patients with HFrEF, the presence of one of the following four co-morbidities explained the potential influence of age: CVI/TIA, diabetes, COPD, or PVD. In both HFpEF and HFrEF, the benefit of (NT-pro)BNP-guided therapy was greater in patients with hypertension, independently of the interaction with age.
| HFpEF | HFrEF | |
|---|---|---|
| Age ≥75 years | 0.08* | 0.05* |
| Hypertension | 0.005† | 0.05† |
| Age ≥75 years | 0.22 | 0.51 |
| Renal failure | 0.01* | 0.11 |
| Age ≥75 years | 0.19 | 0.61 |
| CVI/Dm/COPD | 0.03* | 0.06 * |
| Age ≥ 75 years | 0.03* | 0.52 |
| CVI/Dm/COPD/PVD ≥2 | 0.61 | 0.01* |
- Significant interactions indicate different response to treatment with as compared to without presence of indicated criterion in bivariate interaction Cox-regression model.
- * Less positive effect of (NT-pro)BNP-guided therapy in patients with this criterion.
- † More positive effect of (NT-pro)BNP-guided therapy in patients with this criterion.
- HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; CVI, cerebrovascular insult (also includes transient ischaemic attack); Dm, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease.
Age had little impact upon the effect of (NT-pro)BNP-guided therapy upon HF admissions in either patient group (both interactions P > 0.30).
Discussion
There is considerable uncertainty as to which patients will benefit from (NP-pro)BNP-guided therapy for HF. The present analysis based on individual patient data from eight randomized trials provides important insights, showing that positive effects were seen almost exclusively in patients with reduced LVEF. Importantly, co-morbidities strongly influenced the response to guided therapy and explained the lower efficacy of this approach in elderly patients. These findings better define the group of patients possibly benefiting from guided therapy and suggests lack of uniformity of response to evidence-based treatments among HF patients.
Influence of co-morbidities on biomarker-guided therapy
In addition to the interactions with age and LVEF outlined above, the efficacy of (NT-pro)BNP-guided therapy was significantly influenced by co-morbidities. In fact, the suggested effect of age on (NT-pro)BNP-guided therapy efficacy can be explained entirely by the presence of co-morbidities. This was true with respect to mortality but less to the combined endpoint and not to HF hospitalizations (data not shown), in line with the finding that the effect of age was only seen with respect to mortality, but not HF hospitalizations.1 Thus, HF hospitalization might be reduced by more intense HF-specific treatment irrespective of the presence or absence of co-morbidities.
Our results call into question the belief that (NT-pro)BNP-guided HFrEF care is limited simply by age. We hypothesize that co-morbidities rather than age per se globally affect HF care. Thus, it might be that co-morbidities influence the treatment response to HF medication. It is well known that co-morbidities negatively influence prognosis in HF patients. Moreover, there are numerous studies showing the potential risk of drug–drug interactions leading to adverse effects with the increasing number of co-morbidities and, as a consequence, increasing number of prescribed drugs.19 However, it is less clear if this may result in fewer beneficial effects of HF-specific medication. Unfortunately, full understanding of how multiple co-morbidities in ‘real world’ patients affect effectiveness of proven therapies for HF is lacking. Based on the large randomized treatment trials in HF, trends were seen towards less positive effects of active treatment in patients with co-morbidities (e.g. SENIORS, EMPHASIS-HF),20, 21 but a systematic evaluation of such potential interaction is, to the best of our knowledge, still lacking. Therefore, the potential reason(s) for lower effectiveness of more intensified therapy in HF patients with co-morbidities must remain speculative.
In HFrEF patients forming the majority of the study population, co-morbidities including diabetes, PVD, CVI/TIA and COPD influenced the effects of NT-proBNP-guided therapy upon mortality. Diabetes mellitus and COPD are frequent co-morbidities in HF.22 They are of independent prognostic importance23 and might, therefore, influence prognosis such that NT-proBNP-guided therapy has less effect. Interestingly, no interaction was seen with renal failure, which is in line with the recent finding that intensifying HFrEF therapy may reduce the negative effects of worsening renal failure on prognosis.24 This might be related to the fact that renal dysfunction is often an expression of poor cardiac function in patients with HFrEF, whereas this is not so much the case in HFpEF.
In addition to a better understanding of the effects of (NT-pro)BNP-guided therapy in HF, our results shed new light on HF treatment in general. Only a minority of real-life HF patients fulfil the enrolment criteria of landmark HF trials25 because patients with co-morbidities have often been excluded. In contrast, most of the (NT-pro)BNP-guided HF trials did not have similarly restrictive inclusion criteria, resulting in recruitment of more ‘real world’ patients. Our results on co-morbidities might explain why in daily practice, recommended therapies are often not used in adequate doses. It might be speculated that in elderly multi-morbid patients, use of biomarkers may help to identify patients in whom avoiding up-titration or down-titration may be superior to the current approach. The effect of HF medication in patients with combined co-morbidities and the feasibility and wisdom of titrating to currently recommended target doses in such patients remains to be assessed in future trials.
Heart failure with preserved vs. reduced left ventricular ejection fraction
Compared with patients with HFrEF, patients with HFpEF have substantially different demographics.26-28 Previous data suggest lower event rates among those with HFpEF compared with HFrEF even after correction for other significant predictors.7, 26 However, in our cohort, patients with HFpEF and HFrEF had comparable mortality rates and the combined endpoint of hospitalization because of HF or death was more often reached in HFpEF patients. This difference might be partly explained by the fact that the studies which included HFpEF (TIME-CHF, PRIMA, BATTLESCARRED and Vienna) included patients (recently) hospitalized for acutely decompensated HF.8, 9, 12, 17 As survival differences between HFrEF and HFpEF diminish with increasing age,26 inclusion of the generally more aged HFpEF group of TIME-CHF17 could also have contributed to this finding. Finally, guided therapy positively influenced mortality and combined endpoint rates in HFrEF only, where treatments that reduce mortality are available, in contrast to HFpEF.
The lack of benefit from (NT-pro)BNP-guided therapy in HFpEF was not detected in our previous meta-analysis, probably owing to the very limited power by the small number of HFpEF cases included.1 TIME-CHF contributes substantially to this lack of benefit in HFpEF and it might be argued that this is entirely caused by TIME-CHF. When excluding the TIME-CHF data the interaction between the treatment response on mortality and the two groups based on LVEF was no longer statistically significant (data not shown). However, the treatment effects were homogeneous between studies in both the HFpEF and the HFrEF group. In addition to the notion that HFrEF and HFpEF may be two distinct diseases,17 several other concepts may be relevant to these findings. Although a large cohort study found some positive results of renin–angiotensin system blockade in patients with LVEF of 40% or higher,29 such treatments failed to improve outcome in HFpEF in large prospective randomized therapeutic trials.5-7 Therefore, it is not surprising that delivering currently available HF therapies in an alternative manner does not affect outcome. Notably, HFpEF patients without hypertension and those with renal failure incurred worse outcomes when treated with guided therapy, whereas those with hypertension and/or without renal failure showed neutral results. In contrast, medical and device treatment has markedly improved prognosis in HFrEF over recent decades.4 Our findings support current treatment recommendations for HFpEF, which are restricted to treatment of co-morbidities and symptoms4 and the role of natriuretic peptides in HFpEF appears limited, at least at present, to diagnosis and prognosis.30, 31
Limitations
Our study has important limitations. We were able to include only those studies on NT-proBNP guided therapy that provided individual patient data, which might introduce a bias. Although based on individual patient data, our analyses could not address potentially important aspects of management because such information was not collected equally in the trials. In particular, we do not know if effects of co-morbidities on (NT-pro)BNP guided therapy were mainly driven by less uptitration in such patients compared with those with little or no co-morbidities. Uptitration was less in elderly patients irrespective of treatment if reported specifically (BATTLESCARRED, TIME-CHF),12, 14 but it was still significantly more in the NT-proBNP guided group than in the control group in TIME-CHF.14 Moreover, results on HF hospitalizations were not influenced by co-morbidities, arguing against lack of therapy intensification as main reason for this finding. In addition, diagnosis of co-morbidities was not based on specific testing, but rather on medical records. However, this is common practice and represents clinical care in patients with HF. Moreover, we do not have sufficient data in the majority of the studies to calculate an established co-morbidity index (e.g. Charlson index), although we included the most common co-morbidities in HF patients. In addition, this is a post-hoc analysis best regarded as hypothesis-generating. Finally, the number of patients was not sufficiently large to address all subgroup analyses with sufficient power. This is particularly true for HFpEF patients, in which the number of events was small. In addition, we used multiple testing and statistical tests for interactions are not powerful. This prevented us from doing in-depth analysis which factors may explain the different response between HFrEF and HFpEF. Finally, we do not have sufficient data to test if uptitration differed between these two groups. However, in the study including most HFpEF patients, uptitration of medication did not differ between the two groups, but reduction in NT-proBNP levels was less in HFpEF patients.17 Thus, interpretation of our findings must be done with caution, particularly in patients with HFpEF.
Conclusion
Our individual patient data meta-analysis indicates that NT-proBNP-guided therapy may be helpful in HFrEF but not in HFpEF. Our results support the notion that HFrEF and HFpEF are two distinct entities. Moreover, the effects of intensifying HF treatment seem to be strongly influenced by co-morbidities and not by age per se, but further prospective studies are required to test these hypotheses.
Funding
No funding was received specific to the meta-analysis. Funding related to the original studies included in the meta-analysis is provided elsewhere.1
Conflict of interest: H-P.B.LR. received unrestricted grant support and honoraria from Roche Diagnostics. L.E. received consultancy fees from Roche Diagnostics. M.R. received honoraria, travel grants, and research grants from Roche Diagnostics and Alere. J.J. received grant support from Siemens, Brahms, and Singulex. consultancy fees from Roche Diagnostics, Sphingotec, Critical Diagnostics. U.D. received consultancy fees from Vifor Pharma, Alere, and Novartis, honoraria from Roche Diagnostics and unrestricted grant support from Astra Zeneca. H.G. received honoraria or consultancy fees from Roche Diagnostics, Critical Diagnostics, and Luitpold Pharmaceuticals. C.O'C. received institutional grant support from Roche Diagnostics. R.T. received grant support and honoraria from Roche Diagnostics and St Jude Medical. The remaining authors report no conflicts of interest.




