Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial
Abstract
Aims
This study sought to investigate regional variation in clinical characteristics, therapy utilization, and post-discharge outcomes among patients hospitalized for heart failure (HHF) enrolled in the multinational ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trial.
Methods and results
The ASTRONAUT trial randomized 1615 HHF patients with ejection fraction ≤40% to aliskiren or placebo. Enrolled patients were from Eastern Europe (n = 495, 30.7%), Asia/Pacific (n = 439, 27.2%), Western Europe (n = 395, 24.5%), Latin America (n = 163, 10.1%), and North America (n = 123, 7.6%). Marked differences were seen across geographic regions in terms of baseline demographics, vital signs, laboratory tests, co-morbidity burden, and use of guideline-recommended therapies. All-cause death at 12 months ranged from 7.3% in North America to 26.7% in Asia/Pacific, with differences largely driven by sudden cardiac death. Rates of repeat HHF at 12 months ranged from 22.7% in Latin America to 43.9% in North America. After adjustment for patient characteristics, region was an independent predictor of death at 12 months, with highest risk in Asia/Pacific (hazard ratio 3.04, 95% confidence interval 1.47–6.29, compared with North America) and lowest risk in North America and Western Europe. There was no association between region and the composite of cardiovascular mortality or HHF.
Conclusion
For patients enrolled in this HHF trial, baseline characteristics and risk of death differed markedly by geographic region. Rates of death and repeat HHF demonstrated a general inverse relationship. Global differences in patient characteristics and outcomes should be accounted for when designing future HHF clinical trials.
Introduction
Heart failure (HF) is a global public health problem afflicting >25 million people worldwide.1 Although advancements in drug- and device-based therapy have substantially improved clinical outcomes for ambulatory patients with chronic HF and reduced EF, no such parallel progress has been made with the hospitalized HF (HHF) population where post-discharge mortality and rehospitalization rates approach 15% and 30%, respectively, within 60–90 days of discharge.2, 3 With one possible exception,4 a decades' worth of large, costly, phase III international trials has consistently failed to reduce the post-discharge event rate.5-8 The heterogeneity of the HHF patient population has been postulated as a key reason underlying the failure of recent drug development programmes.9, 10 In this respect, there is growing recognition of geographic variation in HF patient profiles and the interplay with increasing globalization of clinical trials.11, 12 Despite comprehensive inclusion and exclusion criteria, wide variation in patient characteristics by geographic region has been well documented in previous studies.13-15 Likewise, region of origin has been shown to be an independent predictor of clinical outcomes following adjustment for patient risk factors and therapy.13, 15 However, the degree to which patient-level factors, disease management/treatment factors, and study execution/site performance factors mediate differential global event rates remains unclear.
Recently, the ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trial examined contemporary HHF patients from five global regions and found addition of aliskiren to standard therapy to have no significant effect on clinical outcomes.8 The study offers an ideal opportunity to gain added and unique perspectives on the influence of geographic variation on HHF patient profile, management, and post-discharge events. Specifically, unlike many previous multinational HHF trials, ASTRONAUT included a substantial proportion of patients from the Asia and Pacific regions, an area relatively less well characterized in the existing literature. Furthermore, ASTRONAUT represents a previously uncharacterized HHF trial population whereby patients were randomized a median 5 days after admission following substantial improvements in clinical symptoms and natriuretic peptide levels. We thus explore the regional differences in clinical, laboratory, and treatment profiles among HHF patients enrolled in ASTRONAUT and the impact of geographic region on post-discharge outcomes.
Methods
Study design
The study design and primary results of the ASTRONAUT trial have been previously reported.8, 16 Briefly, ASTRONAUT was a prospective, multicentre, multinational, randomized, placebo-controlled trial studying the effects of aliskiren, a direct renin inhibitor, on outcomes among stable HHF patients. Eligible patients were ≥18 years old with left ventricular ejection fraction (EF) ≤40%, elevated admission natriuretic peptide level (B-type natriuretic peptide [BNP] ≥400 pg/mL or N-terminal pro-B-type natriuretic peptide [NT-proBNP] ≥1,600 pg/mL), and signs and symptoms of fluid overload that required hospitalization. Informed consent was obtained from all patients. The trial was conducted in full accordance with the Declaration of Helsinki and with institutional review board and ethics committee approval at all sites.
Overall, the 1615 patients included in the full analysis set for efficacy analysis were enrolled from 289 sites (i.e., sites that enrolled at least 1 patient) between May 2009 and December 2011. The ASTRONAUT statistical analysis plan organized the study cohort into five geographic regions, defined as follows: North America—the USA and Canada; Latin America—Argentina, Brazil, and Colombia; Western Europe—Belgium, Finland, France, Germany, Italy, Spain, and Sweden; Eastern Europe—Czech Republic, Hungary, Poland, Romania, Russia, and Slovakia; and Asia/Pacific—India, Israel, Philippines, Singapore, Turkey, and Taiwan.
Study endpoints and definitions
The co-primary endpoints of the present study were (i) all-cause death within 12 months and (ii) the first occurrence of cardiovascular mortality (CVM) or HHF (CVM/HHF) within 12 months. These same outcomes at 6 months were evaluated as secondary endpoints. Median follow-up was 11.3 months post-randomization. All study endpoints were adjudicated by a blinded clinical event committee (Brigham and Women's Hospital, Harvard Medical School). The definition of HHF was presentation requiring overnight hospitalization with signs and symptoms of HF and treatment with i.v. medications (i.e. diuretics, vasodilators, or inotropes), mechanical fluid removal, an intra-aortic balloon pump, or initiation or intensification (i.e. doubling) of the maintenance diuretic dose. Sudden cardiac death (SCD) and presumed SCD were defined as unexpected death in an otherwise stable patient last seen alive <24 h previously and >24 h previously, respectively.
Statistical analysis
Patients were grouped by geographic region at the time of randomization. Baseline demographics, vital signs, and laboratory values, medical and medication history, and clinical events were compared across groups using χ2, analysis if variance (ANOVA), and Kruskal–Wallis tests where appropriate. All continuous variables were reported as mean ± standard deviation (SD) if normally distributed or median (interquartile range) if non-normally distributed.
The primary predictor of the present study was geographic region, with North America as the referent group. Kaplan–Meier curves were constructed for each geographic region and outcomes were compared using log-rank tests. Univariate and multivariable Cox proportional hazards models were constructed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary predictor. The proportional hazards assumption was confirmed by Kolmogorov-type supremum tests.
Multivariable models, with the exception of the 6-month all-cause death model, were adjusted for 23 pre-selected baseline covariates deemed clinically significant or known to influence clinical endpoints:3 age, gender, EF, ischaemic HF aetiology, New York Heart Association (NYHA) functional class, NTproBNP, serum sodium, blood urea nitrogen (BUN), QRS duration, systolic blood pressure, aliskiren randomization, medical history (prior HHF, hypertension, coronary artery disease (CAD), atrial fibrillation, diabetes, chronic obstructive pulmonary disease [COPD]), and background therapy (angiotensin-converting enzyme [ACE] inhibitor/angiotensin II receptor blocker [ARB], beta-blocker, mineralocorticoid receptor antagonist [MRA], digoxin, implantable cardioverter-defibrillator [ICD], cardiac resynchronization therapy [CRT]). To prevent overfitting with the lower number of events, the 6-month all-cause death model was adjusted for fewer covariates (gender, EF, ischaemic HF aetiology, NYHA functional class, NT-proBNP, serum sodium, BUN, QRS duration, systolic blood pressure, CAD, diabetes, COPD, ACE inhibitor/ARB, beta-blocker, CRT). None of the omitted control variables were independently associated with 6-month all-cause mortality. Since additional adjustment variables could be accommodated with more outcome events, race (i.e. white, black, Asian, other) was added as a covariate to composite endpoint models. Race was tested within all-cause mortality models and was not independently associated with outcome. The multiple imputation procedure [fully conditional specification methods as implemented in MI and MIANALYZE procedures in SAS (SAS Institute, Cary, NC, USA)] was used for missing covariate data (<5% for all variables). All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA), and two-tailed P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
Of the 1615 patients in the ASTRONAUT efficacy cohort, there were 495 (30.7%) patients from Eastern Europe, 439 (27.2%) patients from Asia/Pacific, 395 (24.5%) patients from Western Europe, 163 (10.1%) patients from Latin America, and 123 (7.6%) patients from North America (Table 1). The number of patients enrolled per site ranged from 2.9 in North America to 9.5 in Asia/Pacific.
| North America | Latin America | Western Europe | Eastern Europe | Asia/ Pacific | P-value | |
|---|---|---|---|---|---|---|
| Enrolment data | ||||||
| Number of patientsa,b | 123 (7.6) | 163 (10.1) | 395 (24.5) | 495 (30.7) | 439 (27.2) | – |
| Number of sitesa,c | 43 (14.9) | 33 (11.4) | 97 (33.6) | 70 (24.2) | 46 (15.9) | – |
| Number of patients per site | 2.9 ± 2.4 | 4.9 ± 4.8 | 4.1 ± 3.2 | 7.1 ± 6.2 | 9.5 ± 9.8 | – |
| Number of countries | 2 | 3 | 7 | 6 | 6 | – |
| Demographics | ||||||
| Age (years) | 59.2 ± 13.5 | 64.9 ± 11.3 | 69.1 ± 10.8 | 65.4 ± 10.9 | 61.1 ± 13.0 | <0.001 |
| Male | 93 (75.6) | 117 (71.8) | 316 (80.0) | 383 (77.4) | 338 (77.0) | 0.322 |
| Race | <0.001 | |||||
| Caucasian | 57 (46.3) | 101 (62.0) | 386 (97.7) | 493 (99.6) | 103 (23.5) | |
| Black | 62 (50.4) | 13 (8.0) | 2 (0.5) | 0 (0.0) | 1 (0.2) | |
| Asian | 1 (0.8) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 333 (75.9) | |
| Other | 3 (2.4) | 49 (30.1) | 5 (1.3) | 2 (0.4) | 2 (0.5) | |
| Time from admission to randomization (days) | 2 (2–4) | 4 (2–6) | 5 (3–8) | 6 (3–9) | 3 (2–6) | <0.001 |
| Hospital length of stay (days) | 5 (3–7) | 6 (4–9) | 10 (8–14) | 11 (8–17) | 5 (4–9) | <0.001 |
| Ejection fraction (%) | 23.6 ± 7.8 | 25.8 ± 7.1 | 27.9 ± 6.9 | 30.0 ± 6.6 | 27.3 ± 7.4 | <0.001 |
| Ischaemic HF aetiology | 59 (48.4) | 48 (29.4) | 219 (55.4) | 359 (72.5) | 342 (77.9) | <0.001 |
| NYHA class at admission | <0.001 | |||||
| III | 87 (70.7) | 62 (38.0) | 267 (67.6) | 269 (54.3) | 298 (67.9) | |
| IV | 36 (29.3) | 101 (62.0) | 128 (32.4) | 226 (45.7) | 141 (32.1) | |
| NYHA class at baseline | <0.001 | |||||
| I/II | 31 (25.2) | 86 (52.8) | 144 (36.5) | 102 (20.6) | 184 (41.9) | |
| III/IV | 90 (73.2) | 77 (47.2) | 239 (60.5) | 383 (77.4) | 253 (57.6) | |
| Missing | 2 (1.6) | 0 (0.0) | 12 (3.0) | 10 (2.0) | 2 (0.5) | |
| Atrial fibrillation on baseline ECG | 19 (17.0) | 42 (25.8) | 149 (38.4) | 204 (41.6) | 72 (16.6) | <0.001 |
| QRS duration on baseline ECG (ms) | 122 ± 34 | 127 ± 51 | 130 ± 44 | 115 ± 36 | 107 ± 33 | <0.001 |
| Vital sign and laboratory data | ||||||
| Systolic blood pressure (mmHg) | 128.7 ± 18.0 | 119.9 ± 10.1 | 122.8 ± 14.7 | 125.5 ± 11.7 | 121.1 ± 12.0 | <0.001 |
| Heart rate (b.p.m.) | 82.5 ± 15.1 | 75.1 ± 14.8 | 75.0 ± 14.4 | 76.7 ± 15.6 | 81.6 ± 17.4 | <0.001 |
| Weight (kg) | 98.5 ± 28.9 | 74.2 ± 18.6 | 80.6 ± 18.0 | 83.6 ± 18.5 | 64.7 ± 15.3 | <0.001 |
| BMI (kg/m2) | 33.0 ± 9.3 | 26.6 ± 5.6 | 27.6 ± 5.1 | 28.6 ± 5.4 | 23.9 ± 4.8 | <0.001 |
| Haemoglobin (g/dL) | 13.0 ± 1.9 | 14.3 ± 1.7 | 13.5 ± 2.0 | 14.1 ± 1.9 | 13.3 ± 2.0 | <0.001 |
| Serum sodium (mmol/L) | 139.9 ± 2.9 | 138.8 ± 3.8 | 139.0 ± 3.5 | 139.6 ± 3.0 | 137.3 ± 4.1 | <0.001 |
| Serum potassium (mmol/L) | 4.1 ± 0.5 | 4.3 ± 0.6 | 4.3 ± 0.5 | 4.4 ± 0.5 | 4.2 ± 0.7 | <0.001 |
| BUN (mmol/L) | 9.3 ± 4.1 | 8.7 ± 3.3 | 11.0 ± 4.2 | 8.8 ± 3.2 | 7.9 ± 3.5 | <0.001 |
| Creatinine (mmol/L) | 108.5 ± 29.5 | 99.0 ± 24.5 | 104.4 ± 27.1 | 97.1 ± 26.6 | 98.6 ± 26.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 68.3 ± 21.4 | 65.9 ± 15.8 | 62.6 ± 18.8 | 68.4 ± 20.2 | 68.4 ± 20.6 | <0.001 |
| NT-proBNP at admission (pg/mL)d | 5660 (3021–8600) | 4317 (2708–7057) | 4562 (2788–7900) | 3000 (2422–5185) | 5567 (3000–11300) | <0.001 |
| NT-proBNP at baseline (pg/mL)d | 2555 (1395–4900) | 3060 (1616–5500) | 2651 (1557–5261) | 2537 (1446–4400) | 3040 (1549–5949) | 0.042 |
| BNP at admission (pg/mL)d | 1110 (650–2038) | 1300 (646–2120) | 880 (585–1430) | 835 (558–1370) | 800 (482–1730) | 0.001 |
| BNP at baseline (pg/mL)d | 423 (219–871) | 494 (215–874) | 377 (225–818) | 428 (219–790) | 530 (262–1058) | 0.011 |
| Troponin I (ng/mL) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.108 |
| PRA (µIU/mL) | 0.9 (0.2–4.0) | 1.6 (0.4–8.4) | 4.4 (0.9–25.4) | 3.4 (0.6–19.2) | 3.2 (0.8–16.4) | <0.001 |
| Past medical history | ||||||
| Previous HF hospitalization | 93 (75.6) | 95 (58.0) | 270 (68.4) | 336 (67.9) | 290 (66.1) | 0.034 |
| Coronary artery disease | 64 (52.0) | 46 (28.2) | 219 (55.4) | 302 (61.0) | 250 (56.9) | <0.001 |
| Previous PCI | 36 (29.3) | 20 (12.3) | 118 (29.9) | 62 (12.5) | 87 (19.8) | <0.001 |
| Previous CABG | 29 (23.6) | 13 (8.0) | 92 (23.3) | 62 (12.5) | 83 (18.9) | <0.001 |
| Previous MI | 45 (36.6) | 31 (19.0) | 165 (41.8) | 245 (49.5) | 203 (46.2) | <0.001 |
| Previous stroke | 15 (12.2) | 11 (6.7) | 37 (9.4) | 53 (10.7) | 33 (7.5) | 0.255 |
| Previous TIA | 4 (3.3) | 2 (1.2) | 15 (3.8) | 20 (4.0) | 9 (2.1) | 0.229 |
| Hypertension | 116 (94.3) | 139 (85.3) | 307 (77.7) | 405 (81.8) | 258 (58.8) | <0.001 |
| Atrial fibrillation | 42 (34.1) | 51 (31.3) | 217 (54.9) | 269 (54.3) | 97 (22.1) | <0.001 |
| Diabetes | 65 (52.8) | 54 (33.1) | 178 (45.1) | 176 (35.6) | 189 (43.1) | <0.001 |
| COPD | 32 (26.0) | 12 (7.4) | 131 (33.2) | 86 (17.4) | 61 (13.9) | <0.001 |
| Smoking status | <0.001 | |||||
| Active smoker | 24 (19.5) | 9 (5.5) | 50 (12.7) | 80 (16.2) | 49 (11.2) | |
| Former smoker | 64 (52.0) | 82 (50.3) | 183 (46.3) | 178 (36.0) | 169 (38.5) | |
| Never smoker | 35 (28.5) | 72 (44.2) | 162 (41.0) | 237 (47.9) | 221 (50.3) | |
| Baseline therapies | ||||||
| Diuretic | 111 (90.2) | 156 (95.7) | 382 (96.7) | 479 (96.8) | 420 (95.7) | 0.021 |
| Beta-blocker | 113 (91.9) | 136 (83.4) | 346 (87.6) | 437 (88.3) | 301 (68.6) | <0.001 |
| ACE inhibitor/ARB | 107 (87.0) | 145 (89.0) | 346 (87.6) | 423 (85.5) | 332 (75.6) | <0.001 |
| MRA | 40 (32.5) | 110 (67.5) | 221 (55.9) | 299 (60.4) | 251 (57.2) | <0.001 |
| MRA + ACE inhibitor/ARB | 33 (26.8) | 97 (59.5) | 197 (49.9) | 256 (51.7) | 182 (41.5) | <0.001 |
| Digoxin | 29 (23.6) | 66 (40.5) | 121 (30.6) | 210 (42.4) | 202 (46.0) | <0.001 |
| ICD | 47 (38.2) | 6 (3.7) | 119 (30.1) | 56 (11.3) | 25 (5.7) | <0.001 |
| CRT | 14 (11.4 ) | 6 (3.7) | 48 (12.2) | 25 (5.1) | 16 (3.6) | <0.001 |
| Permanent pacemaker | 24 (19.5) | 14 (8.6) | 71 (18) | 50 (10.1) | 22 (5.0) | <0.001 |
- Data are expressed as mean ± standard deviation, median (interquartile range), or n (%).
- ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PRA, plasma renin activity; TIA, transient ischaemic attack.
- a North America: Canada (7 sites, n = 13); USA (36 sites, n = 110); Latin America: Argentina (17 sites, n = 92), Brazil (9 sites, n = 31), Colombia (7 sites, n = 40); Western Europe: Belgium (9 sites, n = 29), Finland (2 sites, n = 5), France (8 sites, n = 32), Germany (36 sites, n = 123), Italy (27 sites, n = 122), Spain (11 sites, n = 61), Sweden (4 sites, n = 23); Eastern Europe: Czech Republic (9 sites, n = 73), Hungary (8 sites, n = 28), Poland (13 sites, n = 91), Romania (4 sites, n = 36), Russia (25 sites, n = 168), Slovakia (11 sites, n = 99); Asia/Pacific: India (18 sites, n = 219), Israel (7 sites, n = 36), Philippines (4 sites, n = 69), Singapore (2 sites, n = 15), Turkey (9 sites, n = 67), Taiwan (6 sites, n = 33).
- b Data expressed as n (%) relative to the efficacy analysis cohort (n = 1615).
- c Data expressed as n (%) relative to 289 total sites that enrolled ≥1 patient.
- d NT-proBNP data available for 758 patients at admission and 1554 patients at baseline. BNP data available for 880 patients at admission and 1209 patients at baseline.
Overall, patient demographics, vital signs, laboratory findings, medical history, and baseline medications varied widely by geographic region. Despite having the lowest EF (23.6%), patients from North America had the highest systolic blood pressure (128.7 mmHg), serum sodium (139.9 mmol/L), and body mass index (33.0 kg/m2), and were least likely to have NYHA class IV symptoms at admission (29.3%). Moreover, despite having the highest median admission level NT-proBNP (5660 pg/mL) and the shortest median time from admission to randomization (2 days), North Americans had nearly the lowest NT-proBNP level at randomization (2555 pg/mL). Eastern Europe had the highest rate of atrial fibrillation on baseline ECG (41.6%) whereas Asia/Pacific had the lowest (16.6%).
Latin American patients had the lowest prevalence of several co-morbidities, including history of HHF (58.0%), CAD (28.2%), coronary revascularization, stroke (6.7%), transient ischaemic attack (1.2%), diabetes (33.1%), and COPD (7.4%). In contrast, Eastern European patients had the highest rates of CAD (61.0%) and previous myocardial infarction (49.5%), but had low rates of prior percutaneous coronary intervention (12.5%) and coronary bypass surgery (12.5%).
In terms of medical therapies, >80% of patients in all regions were receiving beta-blockers and an ACE inhibitor/ARB at baseline, with the exception of Asia/Pacific where utilization rates were 68.6% and 75.6%, respectively. Baseline receipt of an MRA was >50% in all regions except North America, where only 32.5% of patients were receiving therapy. Overall, rates of guideline-recommended device therapy in ASTRONAUT were low, but did vary widely by region. Rates of ICD implantation were 38.2% and 30.1% in North America and Western Europe, respectively, but <6% in Latin American and Asia Pacific. Similarly, rates of CRT were approximately 11–12% in North America and Western Europe, but 3–5% in other regions.
Clinical outcomes
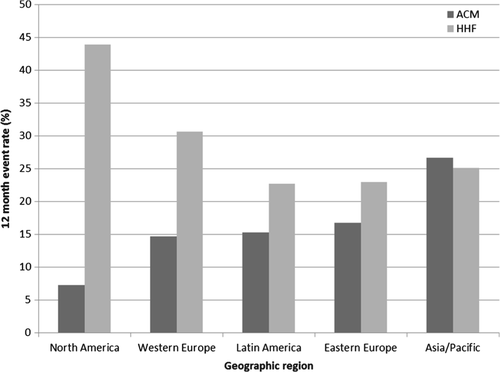
Event rates by geographic region are displayed in Table 2. Rates of death differed significantly by region. North America and Asia/Pacific had the lowest and highest rates of 12-month all-cause death at 7.3% and 26.7%, respectively, while other regions had intermediate mortality rates (P < 0.001) (Figure 1). Cardiovascular mortality rates followed a similar regional pattern (P < 0.001) and accounted for the vast majority of total deaths at 12 months (90.8%). Variation in CVM rates was largely driven by differences in SCD (P < 0.001). Overall, North America had the highest rates of 12-month HHF and all-cause rehospitalization, with rates of 43.9% and 65.9%, respectively. The next highest rate of 12-month HHF was Western Europe at 30.6%, with the remaining regions having rates of 22.7–25.1%. Similar trends in hospitalization rates were seen at 6 months and 30 days post-randomization.
| North America n = 123 | Latin America n = 163 | Western Europe n = 395 | Eastern Europe n = 495 | Asia/ Pacific n = 439 | P-value | |
|---|---|---|---|---|---|---|
| 12-month event rates | ||||||
| All-cause mortality | 9 (7.3) | 25 (15.3) | 58 (14.7) | 83 (16.8) | 117 (26.7) | <0.001 |
| CVM or HHF | 58 (47.2) | 49 (30.1) | 142 (35.9) | 159 (32.1) | 177 (40.3) | 0.003 |
| CVM | 8 (6.5) | 22 (13.5) | 50 (12.7) | 76 (15.4) | 109 (24.8) | <0.001 |
| Pump failure | 3 (2.4) | 11 (6.7) | 26 (6.6) | 28 (5.7) | 34 (7.7) | 0.277 |
| Sudden cardiac death | 2 (1.6) | 7 (4.3) | 13 (3.3) | 21 (4.2) | 45 (10.3) | <0.001 |
| Fatal MI | 1 (0.8) | 1 (0.6) | 3 (0.8) | 6 (1.2) | 5 (1.1) | 0.933 |
| Presumed sudden death | 0 (0.0) | 0 (0.0) | 2 (0.5) | 5 (1.0) | 2 (0.5) | 0.473 |
| Presumed CV death | 0 (0.0) | 2 (1.2) | 3 (0.8) | 7 (1.4) | 13 (3.0) | 0.050 |
| Other CV death | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.2) | 0 (0.0) | 0.795 |
| Fatal stroke | 1 (0.8) | 0 (0.0) | 1 (0.3) | 4 (0.8) | 7 (1.6) | 0.181 |
| CV procedural | 0 (0.0) | 1 (0.6) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0.287 |
| Unknown | 1 (0.8) | 0 (0.0) | 0 (0.0) | 4 (0.8) | 3 (0.7) | 0.364 |
| HHF | 54 (43.9) | 37 (22.7) | 121 (30.6) | 114 (23.0) | 110 (25.1) | <0.001 |
| All-cause rehospitalization | 81 (65.9) | 66 (40.5) | 231 (58.5) | 199 (40.2) | 188 (42.8) | <0.001 |
| CV event | 60 (48.8) | 53 (32.5) | 152 (38.5) | 164 (33.1) | 185 (42.1) | 0.003 |
| MI | 8 (6.5) | 2 (1.2) | 20 (5.1) | 15 (3.0) | 11 (2.5) | 0.035 |
| Stroke | 5 (4.1) | 5 (3.1) | 7 (1.8) | 12 (2.4) | 16 (3.6) | 0.445 |
| 6-month event rates | ||||||
| All-cause mortality | 4 (3.3) | 14 (8.6) | 34 (8.6) | 48 (9.7) | 74 (16.9) | <0.001 |
| CVM or HHF | 36 (29.3) | 34 (20.9) | 104 (26.3) | 110 (22.2) | 131 (29.8) | 0.041 |
| CVM | 4 (3.3) | 12 (7.4) | 30 (7.6) | 44 (8.9) | 72 (16.4) | <0.001 |
| HHF | 34 (27.6) | 27 (16.6) | 89 (22.5) | 83 (16.8) | 86 (19.6) | 0.032 |
| All-cause rehospitalization | 59 (48.0) | 52 (31.9) | 196 (49.6) | 157 (31.7) | 149 (33.9) | <0.001 |
| 30-day event rates | ||||||
| All-cause mortality | 0 (0.0) | 3 (1.8) | 7 (1.8) | 7 (1.4) | 12 (2.7) | 0.303 |
| HHF | 15 (12.2) | 1 (0.6) | 27 (6.8) | 19 (3.8) | 27 (6.2) | <0.001 |
| All-cause rehospitalization | 28 (22.8) | 9 (5.5) | 70 (17.7) | 55 (11.1) | 55 (12.5) | <0.001 |
- Data are expressed as n (%).
- CV, cardiovascular; CVM, cardiovascular mortality; HHF, hospitalization for heart failure; MI, myocardial infarction.
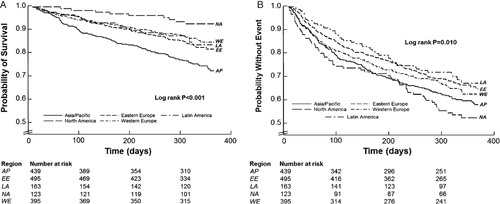
Unadjusted and adjusted outcome analyses for the primary and secondary endpoints are displayed in Table 3. Compared with North America, the unadjusted risk of 12-month death was significantly greater in all other regions, with patients from Asia/Pacific at greatest risk (HR 4.18, 95% CI 2.12–8.23). Unadjusted estimates of the composite endpoint at 12 months demonstrated lower risk in Latin America (HR 0.58, 95% CI 0.40–0.85) and Eastern Europe (HR 0.65, 95% CI 0.48–0.88) compared with North America, and similar risk in Western Europe and Asia/Pacific. Times to first event stratified by region were significantly different by the Kaplan–Meier method for death and CVM/HHF at both 12 (Figure 2) (log rank P < 0.001 and P = 0.010, respectively) and 6 months (log rank P < 0.001 and P = 0.028, respectively).
| Latin America | Western Europe | Eastern Europe | Asia/Pacific | |
|---|---|---|---|---|
| 6 months | ||||
| Unadjusted | ||||
| ACM | 2.76 (0.91–8.37) | 2.76 (0.98–7.78) | 3.11 (1.12–8.61) | 5.66 (2.07–15.47) |
| CVM/HHF | 0.64 (0.40–1.03) | 0.86 (0.59–1.26) | 0.70 (0.48–1.02) | 0.99 (0.68–1.43) |
| Adjusted | ||||
| ACMb | 2.77 (0.89–8.64) | 2.38 (0.83–6.78) | 3.19 (1.13–9.02) | 4.39 (1.55–12.43) |
| CVM/HHFc | 0.95 (0.53–1.70) | 1.19 (0.72–1.95) | 1.11 (0.67–1.84) | 1.50 (0.86–2.59) |
| 12 months | ||||
| Unadjusted | ||||
| ACM | 2.34 (1.09–5.00) | 2.18 (1.08–4.39) | 2.61 (1.32–5.19) | 4.18 (2.12–8.23) |
| CVM/HHF | 0.58 (0.40–0.85) | 0.92 (0.54–1.00) | 0.65 (0.48–0.87) | 0.83 (0.62–1.12) |
| Adjusted | ||||
| ACMd | 2.29 (1.03–5.10) | 1.70 (0.83–3.52) | 2.39 (1.17–4.91) | 3.04 (1.47–6.29) |
| CVM/HHFc | 0.92 (0.57–1.48) | 0.96 (0.65–1.43) | 1.00 (0.66–1.50) | 1.28 (0.82–2.01) |
- ACE, angiotensin-converting enzyme; ACM, all-cause mortality; ARB, angiotensin II receptor blocker; AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVM/HHF, cardiovascular mortality or hospitalization for heart failure; EF, ejection fraction; HF, heart failure; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
- a Data represent hazard ratios and associated 95% confidence intervals.
- b Adjusted for gender, EF, ischaemic HF aetiology, NYHA functional class, NT-proBNP, serum sodium, blood urea nitrogen, QRS duration, systolic blood pressure, CAD, diabetes, COPD, ACE inhibitor/ARB, beta-blocker, CRT.
- c Adjusted for race, age, gender, EF, ischaemic HF aetiology, NYHA functional class, NT-proBNP, serum sodium, blood urea nitrogen QRS duration, systolic blood pressure, aliskiren randomization, prior HHF, hypertension, CAD, AF, diabetes, COPD, ACE inhibitor/ARB, beta-blocker, MRA, digoxin, ICD, CRT.
- d Adjusted for age, gender, EF, ischaemic HF aetiology, NYHA functional class, NT-proBNP, serum sodium, blood urea nitrogen QRS duration, systolic blood pressure, aliskiren randomization, prior HHF, hypertension, CAD, AF, diabetes, COPD, ACE inhibitor/ARB, beta-blocker, MRA, digoxin, ICD, CRT.
After accounting for patient characteristics, risk of 12-month death remained higher in all other regions compared with North America, with the exception of Western Europe where adjusted mortality risk was similar (HR 1.70, 95% CI 0.83–3.52). In contrast, after adjustment for other risk factors, all regions shared similar risk for CVM/HHF at 6 and 12 months.
Discussion
In this large cohort of HHF patients with reduced EF, patient profile and rates of post-discharge mortality and rehospitalization differed markedly by geographic region of enrolment. Similarly, mode of death also varied significantly, with Asia/Pacific patients experiencing rates of SCD >5 times higher than North Americans. After adjustment for patient characteristics, North Americans and Western Europeans had a similar risk for 12-month death, while other regions carried a substantially greater independent risk for death.
Perhaps the most striking finding of the present data is the exceptionally low mortality rate seen in North America, standing in stark contrast to previous HHF experiences. For example, the estimated 12-month mortality rate for North Americans in the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) trial was 30.4%, >4 times greater than that seen in ASTRONAUT. Similarly, the 6-month mortality rate for North Americans in the PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function study) study was 20.1% compared with 3.3% in ASTRONAUT. This low mortality rate in ASTRONAUT is accompanied by overall wider heterogeneity in regional mortality rates than seen previously. In EVEREST, unadjusted 12-month mortality rates across regions fell within 20.5% in Eastern Europe and 30.4% in North America; 6-month regional mortality rates in PROTECT ranged from 12% to 20.4%. Taken together with the raw regional event rates seen among these three trials, the predictive value of region seen in ASTRONAUT appears mostly driven by exceedingly low rates of North American mortality rather than enhancement of mortality risk in non-North American regions.
There are multiple plausible explanations for the low mortality rate and high HHF rate seen in North America. This inverse relationship between mortality and rehospitalization risk has been previously reported.17, 18 This correlation did appear to be generally present in our study across regions, probably explaining the lack of independent association between region and the composite primary endpoint. One potential mechanism relates to ICD utilization. Despite being standard of care for SCD prevention, ICD use has been independently associated with heightened risk for HHF.19 Further, the largest driver of regional differences in mortality was SCD, and baseline rates of ICD implantation were highest in North America at 38.2%. Nevertheless, the particularly low rates of ICD use outside North America in ASTRONAUT are consistent with other recent HF trials, and baseline use was included in multivariable models, suggesting that this factor is less likely to be a dominant influence on regional mortality differences.13, 15, 20, 21
Alternatively, the mortality and HHF rates in North America could be explained by differences in patient selection. Despite strict and uniform inclusion and exclusion criteria, recent HF clinical trials have highlighted potential problems with quality control and the failure of study sites to enrol high numbers of representative patients.22, 23 The particularly low site enrolment rate in North America supports the possibility of these patients being highly selected for, although existing data had found poor site enrolment to correlate with heightened, not reduced, patient risk.24 Similarly, a regional bias for a lower threshold for hospitalizing patients with worsening HF in North America would be compatible with both the low rate of mortality following index hospitalization and the high rates of repeat HHF. Although regional differences in mortality risk persisted despite adjustment for numerous patient risk characteristics, it is possible that unmeasured factors are at play. Likewise, factors such as the treating physician culture, patient expectations, reimbursement structures, and medical malpractice environments may drive differences in rehospitalization. Consistent with North Americans carrying a lower mortality risk was the large in-hospital decrease in natriuretic peptide level, whereby median NT-proBNP at randomization was <50% of the median admission value. This large decrease in natriuretic peptide level occurred despite North America having the shortest time between admission and randomization. Although previous data contend that the single pre-discharge natriuretic peptide level carries greater prognostic value than the in-hospital trajectory,25 and that baseline NT-proBNP was included in the present multivariable models, it is conceivable that the tendency for robust and rapid improvement in natriuretic peptide levels with in-hospital therapy would be compatible with favourable long-term survival.
Lastly, the rates of mortality and HHF in North America could be secondary to a causal relationship, whereby the high rate of hospitalization translated into improved HF management and surveillance, and hence lower mortality. However, this causal relationship cannot be definitively proven, and recent evidence suggests that worsening HF, regardless of hospitalization status, is associated with similar subsequent mortality.26 In fact, reverse directionality of this relationship is perhaps equally likely, where the lower mortality rate in North America resulted in a larger relative pool of patients at risk for hospitalization.
The ASTRONAUT trial population was notable for inclusion of >25% of patients from Asian/Pacific countries. To our knowledge, we present the first data on post-discharge outcomes beyond 30 days for HHF patients from India, Phillipines, Singapore, Turkey, and Taiwan.14 Compared with North Americans, ASTRONAUT patients from these countries carried substantially greater risk of post-discharge death at 6- and 12-month follow-up. Specifically, the rate of SCD at 12 months (10.3%) was more than twice as high as that of the next highest region, and the prevalence of ICDs was low (5.7%). Previously, HHF data from these specific countries were mainly limited to inpatient data from the ADHERE-AP (Acute Decompensated Heart Failure Registry International–Asia Pacific) registry.27 Similar to our analysis, previous comparison of ADHERE-AP with the US-based ADHERE registry revealed higher discharge utilization of MRA and digoxin and lower utilization of beta-blockers. Co-morbidity burden was also remarkably similar in ASTRONAUT and ADHERE-AP for these countries with respect to prior HF hospitalization, hypertension, atrial fibrillation, and diabetes.
Future regulatory and clinical trial implications
Whether secondary to global differences in patient-level factors (i.e. HF biology, care practices, and therapy utilization) or study site factors (i.e. interpretation of selection criteria, enrolment rate, protocol adherence, data collection and accuracy), patients enrolled into recent HF ‘mega-trials’ differ markedly by geographic region.13, 15, 20, 23 While multinational trials have been favoured for purposes of adequate recruitment and cost containment, such wide regional variation likely influences overall study results. Moreover, global patient heterogeneity in HHF trials is problematic for regulators, guideline writers, third party payers, and practising clinicians in determining whether or not results from a international trial are applicable to their respective patients.12 For example, global variation conflicts strongly with the US regulatory mandate that data from international trials be relevant to US medical practice, thus constituting a hurdle for regulatory approval even if ‘positive’ study results are achieved. Indeed, when event rates within a trial vary several fold by geographic region, the validity of the trial population must be questioned.
The present analysis from ASTRONAUT represents a striking example of global heterogeneity in patient profiles and outcomes from a multinational clinical trial of HF with reduced EF. Additionally, these data reaffirm the existence of a potential inverse mortality–rehospitalization relationship, the character of which may be influenced by geographic region and presents an added complicating factor when using the standard CVM/HHF composite efficacy endpoint in future global HF trials. Although limiting future HF trial enrolment to specific geographic regions may come at the price of slower and less efficient enrolment unless accompanied by additional compensatory measures,28 the utility of continuing to execute global HF trials with the degree of regional population heterogeneity seen in ASTRONAUT should be re-evaluated.
Limitations
Despite rigorous multivariate modelling techniques, other measured and unmeasured factors may have influenced these results. In addition, patients included in this analysis were highly selected in the context of a randomized clinical trial, potentially limiting the generalizability of these findings. Moreover, our regional findings may not be generalizable to all countries assigned to that region in the ASTRONAUT protocol. This may apply most to the Asia/Pacific region where many of the included countries (e.g. India and Israel) may have significant socio-economic and cultural differences. Further, subgroup analysis by region of origin resulted in comparison with fewer patients in each group, thus limiting study power and possibly increasing the risk of chance findings. However, despite wide confidence intervals, adjusted mortality results did achieve standard definitions of statistical significance. Lastly, data regarding background medication dosing are not available in ASTRONAUT. Thus, the ability to determine potential influences of regional medication utilization on clinical outcomes is limited.
Conclusions
In this large multinational cohort of HHF patients, baseline patient characteristics and uptake of guideline-recommended medical and device therapies differed markedly according to geographic region of enrolment. Rates of death at 12 months were highly variable, ranging from 7.3% in North America to 26.7% in Asia/Pacific, and there was a general inverse relationship between regional rates of death and repeat HHF. After adjustment for patient characteristics, region of enrolment was an independent and strong predictor of death, but not CVM/HHF, at 6- and 12-month follow-up. ASTRONAUT presents a striking example of geographic heterogeneity within a global HF trial. Geographic differences in patient characteristics and outcomes should be accounted for when designing future HHF clinical trials.
Acknowledgements
We thank Drs Tsushung A. Hua, Anastasia Lesogor, and Bernard Reimund, and Mr Albert Kandra for assisting in the design of this analysis.
Funding
Financial and material support for the ASTRONAUT trial was provided by Novartis Pharma AG (Basel, Switzerland). H.S. conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Conflict of interest: G.C.F. reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Gambro, Medtronic, and Janssen; he holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is also supported by the Ahmanson Foundation (Los Angeles, CA). S.D.S. has received grant funding, consultant fees, and travel support from Novartis. A.P.M. has served on committees of clinical studies sponsored by Amgen, Bayer, Abbott Vascular, Cardiorentis, Johnson & Johnson, and Novartis Pharma AG. M.B. has served as a consultant for AstraZeneca, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, AWD Dresden, Berlin-Chemie, MSD, Novartis, Pfizer, Sanofi-Aventis, and Servier. F.Z. has received grant funding from Novartis, BG Medicine, and Roche Diagnostics; served on a board for Boston Scientific; and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm SA, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical, and Trevena Therapeutics. All other authors have no conflicts of interest to declare.




