The cancer patient and cardiology
Abstract
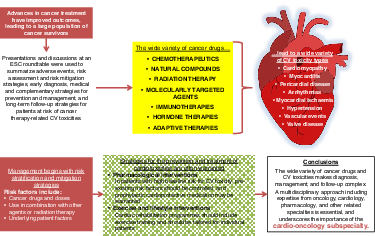
Advances in cancer treatments have improved clinical outcomes, leading to an increasing population of cancer survivors. However, this success is associated with high rates of short- and long-term cardiovascular (CV) toxicities. The number and variety of cancer drugs and CV toxicity types make long-term care a complex undertaking. This requires a multidisciplinary approach that includes expertise in oncology, cardiology and other related specialties, and has led to the development of the cardio-oncology subspecialty.
This paper aims to provide an overview of the main adverse events, risk assessment and risk mitigation strategies, early diagnosis, medical and complementary strategies for prevention and management, and long-term follow-up strategies for patients at risk of cancer therapy-related cardiotoxicities. Research to better define strategies for early identification, follow-up and management is highly necessary. Although the academic cardio-oncology community may be the best vehicle to foster awareness and research in this field, additional stakeholders (industry, government agencies and patient organizations) must be involved to facilitate cross-discipline interactions and help in the design and funding of cardio-oncology trials.
The overarching goals of cardio-oncology are to assist clinicians in providing optimal care for patients with cancer and cancer survivors, to provide insight into future areas of research and to search for collaborations with industry, funding bodies and patient advocates. However, many unmet needs remain. This document is the product of brainstorming presentations and active discussions held at the Cardiovascular Round Table workshop organized in January 2020 by the European Society of Cardiology.
Graphical Abstract
Cardio-oncology: an introduction
Advances in cancer treatments have improved patient survival,1 with the result that large numbers of patients are cured of or are able to live with cancer for much longer periods of time. In addition, the ageing population will develop more cancers, which will result in higher numbers of cancer survivors.2 This has led to increased rates of morbidity and mortality caused by the short- and long-term side effects of cancer therapy.3 Cardiovascular (CV) toxicity, which refers to the direct harmful effects of cancer treatments on the CV system and the acceleration of CV diseases (CVDs) related to traditional CV risk factors, has become of paramount concern.3, 4
The increasing number and variety of cancer drugs has increased the complexity of long-term care and in many instances cancer has become a chronic disease that requires long-term oncologic treatment, as well as the management of cancer therapy-related toxicity. Therefore, managing cardiotoxicity requires a multidisciplinary approach that involves specialists in oncology, cardiology, clinical pharmacology and other related specialties. The expanding field of cardio-oncology can help to prevent or minimize adverse events, and ensure that potentially life-saving cancer treatments are not interrupted or withheld.3 Furthermore, as many of the late CV side effects are initially asymptomatic but lead to progressive disease with an impaired prognosis, early detection is of the utmost importance.
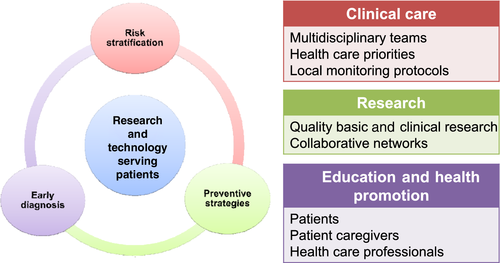
The goals of this article are to provide an overview of the main CV adverse events of cancer therapy. Using a few examples of old and newer cancer drugs that have been associated with CV events, we will focus on current issues in risk assessment and risk mitigation strategies, early diagnosis, medical and complementary strategies for prevention and management, and long-term follow-up strategies for patients at risk for cancer therapy-related cardiotoxicities. Future research and clinical directions, and the growing importance of education programmes and networking among different stakeholders are also highlighted. This article does not therefore aim to provide consensus statements, for which the underlying research and clinical evidence are felt to be preliminary or inconsistent at some points. Rather, it aims to summarize current knowledge and to provide a framework for the pillars of cardio-oncology care (Figure 1).
Overview of cardiotoxicities associated with cancer treatments
Cancer therapies have been associated with many short- and long-term CV risks but clinical phenotypes of CV toxicities have changed remarkably since conventional chemotherapeutics were replaced by, or began to be used in combination with, newer targeted drugs, also referred to as molecularly targeted agents (MTAs). Many authoritative reviews have illustrated how CV events (type and incidence rate) may vary according to cancer drugs, doses, use in combination with other agents or radiation therapy, and underlying patient risk factors. Table 1 shows examples of CV toxicities that have been associated with conventional chemotherapeutics, MTAs, radiation therapy and other oncologic therapies.3, 5-19 We will not discuss each cardiotoxic agent in detail, but emphasize that older drugs, newer drugs and radiation therapy, alone or in combination, may increase the risk for a number of diverse CV events. This highlights the biological and clinical complexity of cardio-oncology.
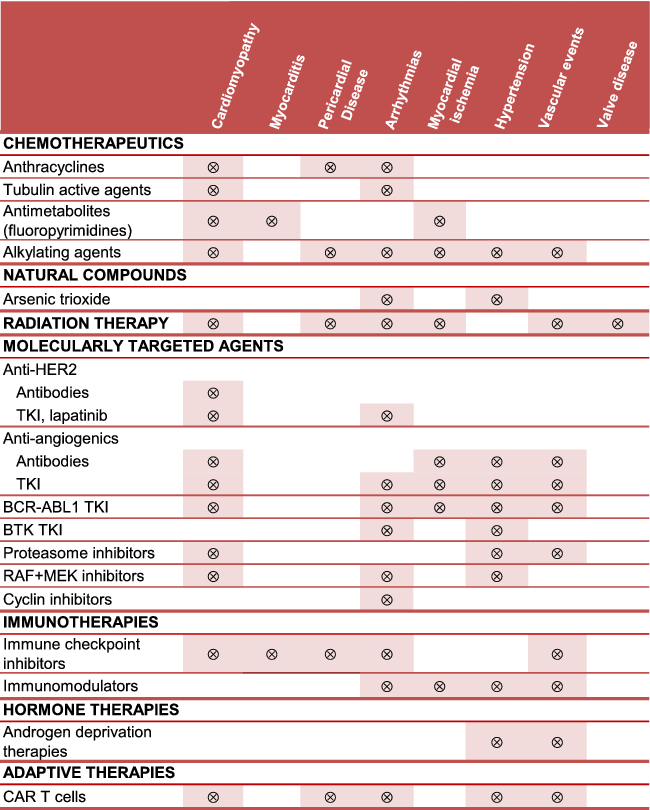 |
- Arrhythmias (atrial fibrillation, ventricular tachyarrhythmias, atrio-ventricular block, long QTc); cardiomyopathy (mainly dilative, restrictive in the case of radiation therapy); hypertension [mainly systemic, pulmonary in the case of dasatinib (BCR-ABL1 TKI), MEK inhibitors, CAR-T cells]; myocardial ischaemia (acute coronary syndrome, angina, coronary artery disease); vascular event (arterial thrombosis, venous thromboembolism, vasculitis). Cyclin inhibitors includes cyclin-dependent kinase (CDK) 4 and 6 inhibitors.
- BCR-ABL1, breakpoint cluster region-Abelson kinase 1; BTK, Bruton tyrosine kinase; CAR, chimeric antigen receptor; HER2, human epidermal growth factor receptor-2; TKI, tyrosine kinase inhibitor.
Risk stratification and mitigation
In oncology patients the risk factors for cardiotoxicity include many of the traditional risk factors for CVD that are seen in the general population. The concept of hidden cardiotoxicity was therefore introduced to emphasize that CV toxicity from a given drug may manifest only or predominantly if that particular drug is administered to patients with CV risk factors.20 Data suggest that the development of CVD and cancer, respectively, may actually share common predisposing risk factors (smoking, age, obesity, hyperlipidaemia),3, 5, 21 as well as newly identified genetic risk factors such as clonal haematopoiesis.22 Table 2 shows some of the traditional and unique risk factors that may increase the risk for CV toxicity in patients receiving cancer treatments.3, 10, 23-28 Risk stratification proformas have been proposed to help clinicians identify patients at low, medium, high or very high risk for treatment-related CV events.28
| Demographic factors | |
|
|
| Lifestyle factors | |
|
|
| Patient history factors | |
|
|
| • Comorbidities: | |
|
|
| Treatment-related factors | |
|
|
| CV-related factors | |
|
|
| Cancer-related factors | |
|
|
- AF, atrial fibrillation; AV, atrioventricular; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; CV, cardiovascular; CVAD, central venous access device; CVD, cardiovascular disease; HF, heart failure; LQTS, long QT syndrome; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; PCI, percutaneous coronary intervention; RT, radiotherapy; VHD, valvular heart disease; VTE, venous thromboembolism.
Anthracyclines
Anthracyclines have improved the prognosis of patients with many cancer types, including breast cancer, lymphomas, leukaemias, sarcomas and some gastrointestinal (GI) cancers, and withholding these agents for fear of cardiotoxic effects may impact survival. The cardio-oncology expert plays a key role in identifying patients at risk, offering mitigation strategies and cardioprotective therapies, and providing long-term follow-up.
Anthracyclines cause distinctive morphologic and functional damage to cardiac myocytes and anthracycline regimens are associated with a three- to five-fold greater risk for cardiotoxicity compared with non-anthracycline regimens.29, 30 In addition to common baseline risk factors (Table 2), additional factors for anthracycline cardiotoxicity include cumulative dose, combination therapy and genetic factors.3, 7, 27, 29, 31-33 Cumulative dose is especially important: the risk for heart failure (HF) increases from 3–5% at 400 mg/m2 to 18–48% at 700 mg/m2.34 In trials conducted in patients with solid tumours, HF risk was higher with bolus vs. continuous infusions,29 showing that cardiotoxicity is influenced by peak plasma concentration; however, the cardioprotective effects of replacing bolus with continuous infusions were not observed in children with acute lymphoblastic leukaemia.35 Strategies for reducing the risk for cardiotoxicity include the use of liposomal vs. non-liposomal anthracycline formulations and the use of cardioprotective agents (see the section on strategies for the prevention and treatment of cardiotoxicities).36-38 Another option may be to consider less toxic but equally active alternatives. A 7-year follow-up of a randomized controlled trial found that docetaxel/cyclophosphamide therapy had superior disease-free survival with a lower risk for cardiotoxicity compared with doxorubicin/cyclophosphamide in both older and younger women with early breast cancer.39
Fluoropyrimidines
Fluoropyrimidines such as 5-fluorouracil (5-FU) and capecitabine are frequently prescribed for GI, breast, colorectal, and head and neck cancers. Fluoropyrimidines have been associated with a significant risk for chest pain, presenting as atypical chest pain, angina on exertion or rest, and acute coronary syndromes including myocardial infarction.40 The overall incidence of fluoropyrimidine-associated cardiac events is high enough to classify 5-FU as the second most common chemotherapeutic associated with cardiotoxicity after anthracyclines.40 The risk becomes higher with continuous infusions compared with bolus regimens, which contrasts with that associated with anthracyclines and indicates that cardiotoxicity is influenced primarily by plasma exposure over time. Uncertainty exists around precisely how CVD risk factors influence fluoropyrimidine cardiotoxicity, and the role of dihydropyrimidine dehydrogenase (DPD) activity (i.e. the main enzyme for inactivation of fluoropyrimidines).40 About 3–8% of the population is partially deficient in DPD activity and potentially exposed to increased haematologic and non-haematologic toxicity of 5-FU and capecitabine.41 Genetic polymorphisms in DPYD (i.e. the gene encoding for DPD) can result in low DPD activity and pre-emptive screening for DPYD variants and DPYD genotype-guided dose adjustments have been recommended to minimize toxicity.41 Information about the efficacy of this strategy against fluoropyrimidine cardiotoxicity is nonetheless limited at this point in time.
Trastuzumab
The anti-HER2 monoclonal antibody, trastuzumab, has revolutionized the management of HER2-overexpressing breast tumours and heralded the era of drugs targeted at receptors overexpressed in tumours. HER2, a member of the epidermal growth factor receptors family, is expressed in approximately 20–25% of breast tumours, but is constitutively expressed also in the heart, serving multiple functions in energy metabolism, sarcomere proteins turnover, and adaptation to chemical and haemodynamic challenges.42 Trastuzumab has been associated with left ventricular (LV) dysfunction in up to 20% of patients, with significant declines in global longitudinal strain (GLS) seen at 3-month follow-up.3, 43 The rate of trastuzumab cardiotoxicity is increased when an anthracycline is given before trastuzumab, and even more so when the drugs are given concurrently (however, this is no longer clinical practice).7 When administered after an anthracycline, trastuzumab causes cardiotoxicity that differs dramatically from that seen with anthracyclines. Trastuzumab cardiotoxicity is in fact characterized by only minor structural myocardial changes, is more likely to be reversible, is not cumulative dose-dependent, and generally does not recur with subsequent stressors.7 Trastuzumab cardiotoxicity also shows good responses to HF therapy.7 Other anti-HER2-targeted therapies, such as the antibodies pertuzumab and trastuzumab emtansine (T-DM1), and the tyrosine kinase inhibitor (TKI) lapatinib, appear to share this pattern of cardiotoxicity.3 In contrast, the use of anthracycline-free regimens can reduce the risk for trastuzumab cardiotoxicity.3
Early discontinuation of trastuzumab should always be weighed against oncologic risk as it represents an independent predictor of overall survival even after adjusting for cardiotoxicity. Early discontinuation has been associated with a two- to three-times greater risk for clinically significant relapse of breast cancer, and greater risk for both CV and non-CV death.44, 45
Product labelling recommends that treatment be withheld until the cardiac status has stabilized if LV ejection fraction (LVEF) drops by ≥10% and to <50%.46 Resumption of trastuzumab, supported by HF therapy and close cardio-oncology monitoring, can be considered in patients in whom trastuzumab therapy has been interrupted, whose LVEF is ≥40% and/or whose signs and symptoms of HF have resolved. In a retrospective database review of patients who experienced an asymptomatic LVEF decline to <50%, there was no significant difference in LVEF at follow-up between patients who continued vs. those who interrupted trastuzumab therapy.47 Furthermore, the prospective SAFE-HEaRt study demonstrated that interruption of HER2 therapies may not be necessary in patients with cardiac dysfunction (i.e. LVEF of 40–49% and no HF symptoms).48 Patients were closely monitored and received HF therapies, and 90% were able to complete the planned course of therapy without a cardiac event or worsening of LVEF. When considering the interruption of trastuzumab, the risk–benefit assessment of prognosis from cancer vs. HF should be discussed with the multidisciplinary team.24
Tyrosine kinase inhibitors
Tyrosine kinase inhibitors are indicated for the treatment of a wide variety of haematologic malignancies and solid tumours, and are often prescribed for prolonged periods. They are classified by target groups including human epidermal growth factor receptor inhibitors, vascular endothelial-derived growth factor receptor (VEGFR) inhibitors, and breakpoint cluster region-Abelson kinase 1 (BCR-ABL1) inhibitors.5 Many TKIs can be designed to target the kinase domain of a given growth factor receptor. For example, TKIs directed at VEGFR include drugs such as sunitinib, sorafenib, pazopanib, axitinib, vandetanib, cabozantinib, lenvatinib and regorafenib. Some TKIs, like ponatinib, can target both VEGFR2 and BCR-ABL1, showing that different receptors and kinases share residues liable to pharmacologic targeting.49 Hence, TKIs may also cause off-target effects in the CV system and other organs.
By targeting kinases involved in controlling survival signalling, energy homeostasis and excitation–contraction coupling, TKIs have been linked to cardiomyopathy, HF and QT prolongation, as well as vascular toxicities.5, 50 In pooled analyses of clinical trial data, the increased relative risk for cardiotoxic events was 1.7 for high-grade HF (HF requiring intervention or life-threatening dysfunction), 2.7 for any HF (high-grade and HF not requiring intervention),51 2.7 for high-grade QTc prolongation (>500 ms or serious/life-threatening arrhythmias) and 8.7 for any QTc prolongation (high-grade and prolongation of 450–500 ms).52 Subgroup analysis showed that only sunitinib and vandetanib (especially at higher doses) were associated with significant risk for QTc prolongation. It is worth noting that most cases were of low clinical significance, but caution is recommended when patients are treated off clinical trials and receive other drugs that also prolong QTc. Nilotinib, which was not included in the analysis, has also been associated with QTc prolongation, and electrocardiogram (ECG) monitoring is recommended.53
Ibrutinib, a Bruton's tyrosine kinase, which is used mainly in certain leukaemias and lymphomas, has been associated with a risk for atrial fibrillation (AF).54 In a pooled analysis of 16 studies, the incidence of ibrutinib-associated AF was 5.77 per 100 person-years, which was much higher than in the general adult population.54 In clinical trials, additional risk factors included history of AF and age over 65 years.11 AF was managed with anticoagulant medications, with a low risk for serious bleeds, and a low incidence of discontinuation of therapy. Ibrutinib has also been associated with ventricular arrhythmias,55 as well as the development of hypertension.56 In a cohort study, 78% of ibrutinib users developed new-onset or worsened hypertension (systolic blood pressure ≥130 mmHg). This was associated with a two-fold higher risk for major adverse coronary events (MACEs), which was lowered by 60% with antihypertensive therapy.56 The CV issues with ibrutinib are underscored by a pharmacovigilance study that documented 303 CV deaths associated with ibrutinib therapy.57 Pharmacological strategies to prevent and manage AF and hypertension are discussed in more detail in the following sections.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs), such as atezolizumab, durvalumab, ipilimumab, nivolumab and pembrolizumab, are used to treat a range of cancers, and indications for their use continue to expand. Cardiotoxicities have been reported early after initiation of therapy, and can include pericardial disease, vasculitis, temporal arteritis and HF. The reported incidence of ICI-related myocarditis is low (0.04–1.14%), but it is associated with a high mortality rate (25–50%).12, 13, 58-60 Data suggest that most cases of myocarditis will present early after the initiation of ICI treatment, although it has also been reported in patients on long-term ICI therapy.61 A review of 101 cases found that 64% occurred after the first or second ICI dose.59 Another study also found some cases to have occurred after the first ICI dose.58
Use of combination ICI therapy seems to significantly increase the risk for myocarditis (from 0.06% to 0.27% in one case series).12, 58 Other risk factors are poorly defined, but may include underlying autoimmune disease, diabetes mellitus and pre-existing CVD.12, 60, 62, 63
The diagnosis of myocarditis can be challenging; in case series, elevated troponin and abnormal ECG findings were common.12, 64 In the presence of myocardial oedema, cardiac magnetic resonance (CMR), with late gadolinium enhancement (LGE), is useful in the early course of the disease for diagnosis and risk assessment.64, 65 Endomyocardial biopsy is the reference standard for diagnosis but is often underused as a result of its invasive nature, risk for complications, and a lack of expertise in many clinical centres.64-66 Registry data showed that although LGE was present in >80% of patients with non-ICI myocarditis, it was present in <50% of those with ICI-associated myocarditis.65 Therefore, CMR with LGE should not be relied on as the sole diagnostic approach; rather, biopsy should be used in patients with suspected myocarditis and negative CMR findings.65
The mechanism of cardiotoxicity has been related to activation of immune cells (T cells).58 Myocarditis has been shown to respond to high doses of corticosteroids, or use of immunosuppressants.12, 14 A case report suggested that the immunosuppressive and antirheumatic drug abatacept may be useful in treating ICI-associated myocarditis.67
Radiotherapy
Radiotherapy may cause damage to the pericardium, vascular tree, valve structure, endocardium and myocardium, which can manifest in days, months or years.16, 68 Women with breast cancer who received radiotherapy had a 30% greater risk for coronary heart disease, and a 38% greater risk for cardiac death compared with those who did not.69 The risk for cardiotoxicity is increased when radiotherapy is used with concomitant anthracyclines.70, 71 Radiotherapy-specific risk factors include the volume and dose to which the heart and its substructures are irradiated.72, 73 The risk for LV injury was 1.4 times higher in patients with left-sided vs. right-sided breast cancer.74 In one study, the rate of MACEs (i.e. myocardial infarction, coronary revascularization or CV death) increased linearly by 7.4% per Gray (Gy) increase in mean heart dose.75
An analysis showed that the LV volume receiving 5 Gy was a better predictor of MACEs than mean heart dose.76 Several prediction models for MACEs in patients with breast cancer treated with radiotherapy have been proposed. A model based on three-dimensional (3D) dose distributions to cardiac substructures uses mean heart dose, age and the presence of traditional CVD risk factors to calculate the risk for cardiotoxicity.76 In addition, a high pre-treatment coronary artery calcium score (CACS) has been associated with MACEs in patients treated with radiotherapy.77 After correction for age, history of ischaemic heart disease, diabetes, obesity, mean heart dose, hypercholesterolaemia and hypertension, the hazard ratio for MACE for the low CACS was 1.4, and for the combined intermediate and high CACS was 5.0 compared with a CACS of zero.
Risk mitigation strategies are focused on reducing the heart's exposure to radiation.72, 78, 79 Techniques include cardiac displacement manoeuvres (e.g. prone positioning and deep inspiratory breath holding), personalized heart block, intensity-modulated techniques, intraoperative irradiation and/or brachytherapy, and proton irradiation.
Early diagnosis of cardiotoxicities
Guidance from the European Society of Cardiology (ESC), the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) recommends a thorough assessment of baseline CV risk factors (Table 2) and a measurement of baseline cardiac function in patients who will receive potentially cardiotoxic treatments.3, 24, 80 For patients with risk factors, including signs or symptoms of current cardiac dysfunction, the guidelines recommend the further assessment of risk using biomarkers (troponins, natriuretic peptides) and evaluation of LVEF. Whenever possible, the same biomarker assays and imaging modalities should be used at baseline and throughout ongoing follow-up to ensure comparable information.3
The timing and frequency of surveillance will depend upon the specific cancer treatment, including the cumulative anthracycline dose, delivery protocol and duration, as well as the patient's baseline CV risk.3 Patients identified as being at high risk should be referred to a cardio-oncology specialist.3
Biomarkers
Although the routine use of cardiac biomarkers is not well established in patients with cancer, guidelines recommend the measurement of high-sensitivity (hs)-cardiac troponins (TnI or TnT), and natriuretic peptides [B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP)] for those at high risk or undergoing potentially cardiotoxic therapies.3, 24, 80
A meta-analysis of 61 trials including over 5500 patients assessed the utility of cardiac troponins and BNP/NT-proBNP to predict cancer therapy-related LV dysfunction.81 The odds of LVEF impairment were higher in patients with cancer therapy-related elevation of troponin compared with those without [odds ratio (OR) 11.9, 95% confidence interval (CI) 4.4–32.1], and troponin had a negative predictive value of 93%. The odds were highest with high-dose regimens (OR 97.9, 95% CI 52.2–183.8), anthracyclines (OR 7.0, 95% CI 1.4–34.1), and HER2 inhibitor therapy (primarily trastuzumab) (OR 10.1, 95% CI 2.1–48.9). Conversely, although mean BNP/NT-proBNP levels were increased in patients post-cancer therapy, these biomarkers did not consistently predict LV dysfunction (OR 1.7, 95% CI 0.7–4.2). It was recently suggested that post-cancer therapy elevations of NT-proBNP might herald early diastolic dysfunction rather than LV systolic dysfunction.82
Troponin elevation was also useful to monitor the efficacy of preventive therapy, with lower levels seen with angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) therapy (OR 9.8, 95% CI 2.6–37.1) and, to a lesser extent, beta-blocker therapy (OR 2.1, 95% CI 1.3–3.6).81 However, a multicentre prospective study, the International CardioOncology Society (ICOS)-one trial, did not demonstrate prevention of troponin elevations by ACE inhibitor therapy,83 whereas both the PRevention of cArdiac Dysfunction during Adjuvant breast cancer therapy (PRADA)84 and Carvedilol Effect in Preventing Chemotherapy-Induced Cardiotoxicity (CECCY)85 trials demonstrated an attenuation of the troponin response to anthracycline therapy by beta-blocker therapy. The utility of circulating biomarkers, and imaging with two-dimensional (2D) echocardiography, computed tomography (CT) coronary angiography, and CMR for the early detection of radiotherapy-associated cardiotoxicity is being prospectively assessed in the European multicentre MEDIRAD EARLY HEART Study.86
Patients receiving cardiotoxic cancer therapies who have elevated troponin levels should be referred to cardio-oncology specialty services and may benefit from preventive therapies (see the section on strategies for the prevention and treatment of cardiotoxicities).3, 24, 80
Imaging
Guidelines recommend the thorough assessment of baseline cardiac function in patients who will receive potentially cardiotoxic treatments.3, 24, 80 An echocardiogram before the initiation of therapy is generally recommended to provide a quantitative measure of LVEF and diastolic function to help identify individuals at high risk and to establish a baseline, should symptoms develop during therapy.3, 24, 80
Several studies have shown that 3Dechocardiography and CMR provide more accurate measurements of LVEF and LV volumes than 2D-echocardiography87, 88 or multi-gated acquisition (MUGA).88, 89 However, quantitative 2D-echocardiography may be used when issues of availability, cost or expertise prohibit the use of 3Dechocardiography or CMR imaging. The same imaging modality and the same equipment should be used for baseline and longitudinal follow-up to minimize variability and to ensure the consistent interpretation of results.3
The frequency of surveillance imaging should be determined based on clinical judgement and patient circumstances.3, 24, 80 For example, patients who are identified as being at low risk (normal baseline echocardiography, no clinical risk factors) may require less frequent surveillance compared with those with reduced LVEF, or structural heart disease on baseline echocardiography, or with clinical risk factors.3 Survivors who received highly cardiotoxic cancer therapies or who developed cardiotoxicity during therapy are candidates for long-term surveillance echocardiography.
Echocardiography-based strain imaging may be particularly useful.80, 90 In a meta-analysis of 21 studies including over 1700 patients with different malignancies and treated with anthracyclines with and without trastuzumab, reduced GLS was associated with a 12-fold greater risk for cardiotoxic events (clinically significant change in LVEF with or without new-onset HF symptoms).91 A reduction of GLS of >15% from baseline is generally considered abnormal and a marker of early LV subclinical dysfunction.3 In a 2020 case–control study conducted in patients on ICIs, GLS demonstrated significant decreases in those who developed myocarditis compared with those who did not, and the risk for MACEs was higher with lower GLS.92 In addition, a prospective follow-up study showed that GLS was responsive to cardioprotection, improving in patients receiving beta-blocker therapy among patients treated with anthracyclines with or without trastuzumab.93 The on-going Strain sUrveillance of Chemotherapy for improving Cardiovascular Outcomes (SUCCOUR) is a randomized controlled trial that will assess whether GLS-guided cardioprotective therapy will improve cardiac function in high-risk patients undergoing cardiotoxic chemotherapies.94
Cardiovascular magnetic resonance imaging may be particularly useful to evaluate vascular cardiotoxicities. Stress echocardiography, stress CMR, CT and positron emission tomography are options for the evaluation of ischaemia in patients receiving therapies that have the potential to cause vasospasms or accelerate atherosclerosis.90
Strategies for the prevention and treatment of cardiotoxicities
Pharmacological interventions
In patients with high baseline risk for cardiotoxicity, pre-existing risk factors (Table 2) should be identified and strictly controlled, and prophylactic cardioprotective medication against drug-induced cardiotoxicity should be considered as warranted.3, 80 Recommendations for the use of cardioprotective pharmacotherapies in patients with cancer vary across different guidelines.3, 24, 80 The ESMO guidelines recommend initiation of cardioprotective treatments (ACE inhibitors, ARBs and/or beta-blockers) in patients receiving cardiotoxic therapy who exhibit a decrease in LVEF, a decrease in GLS, or an elevation in cardiac troponin, with a statin being considered in those with existing coronary artery disease (CAD).24 ASCO guidelines recommend dexrazoxane for prevention of cardiotoxicity in patients planning to receive high-dose anthracyclines (e.g. doxorubicin ≥250 mg/m2).80 The data were deemed insufficient to make formal recommendations regarding other cardioprotective strategies (e.g. ACE inhibitors, beta-blockers, ARBs and statins). Guidance from the ESC suggests the use of cardioprotective drugs (ACE inhibitors, beta-blockers, ARBs) in patients with pre-existing clinical HF or significant LV dysfunction at baseline, and the initiation of cardioprotection in patients with a troponin increase during treatment with high-dose anthracycline regimens.3 Patients with anthracycline-related or idiopathic cardiomyopathy treated with optimized HF therapy have been shown to have similar mortality rates, which suggests that these patients should be treated with current guideline-directed therapies.95 Dexrazoxane is the only drug formally approved for the prevention of anthracycline cardiotoxicity; however, both the US Food and Drug Administration and the European Medicines Agency recommend using dexrazoxane only in breast cancer patients with prior high-dose anthracycline and candidates for continued anthracycline-based therapy.3 Children who are candidates for high-dose anthracycline can receive dexrazoxane starting from the first anthracycline dose. Meta-analyses of clinical trial data assessing the efficacy of cardioprotective interventions in patients with cancer are shown in Table 3.37, 96-99
| Study | Cancer treatment | Cardioprotective intervention | Results (95% CI) |
|---|---|---|---|
|
Li et al. 202096 Network meta-analysis (7 studies, n = 628) |
Anthracyclines, trastuzumab, taxanes, platinum agents, others |
ACEi vs. PBO ACEi vs. control |
LVEF MD 6.79 (2.11–11.48) MD 7.76 (2.64–12.88) |
|
Fang et al. 202097 Meta-analysis (9 studies, n = 1095) (5 studies; n = 799) (4 studies; n = 647) |
Anthracyclines ± trastuzumab | ACEi/ARB vs. PBO |
LVEF MD 4.24 (1.53–6.95; P = 0.002) Chemotherapy-related cardiotoxicitya RR 0.63 (0.30–1.31; P = 0.22) Hypotension RR 3.94 (95% CI 1.42–10.90; P = 0.008) |
|
Li et al. 202096 Network meta-analysis (9 studies, n = 722) |
Anthracyclines, trastuzumab, taxanes, platinum agents, others | Beta-blockers vs. PBO |
LVEF MD 4.00 (0.87–7.14) |
|
Huang et al. 201998 Meta-analysis (5 studies, n = 495) |
Anthracyclines | Beta-blockers vs. PBO |
LVEF MD 1.74 (−0.18 to 3.66; P = 0.08) Clinically overt cardiotoxicitya OR 0.42 (0.20–0.89; P = 0.02) |
|
Wang et al. 202099 Meta-analysis (3 studies, n = 1465) |
Chemotherapy | DOACs vs. PBO |
VTE RR 0.53 (0.36–0.78; P = 0.001) Pulmonary embolism RR 0.50 (0.28–0.89; P = 0.02) |
|
Macedo et al. 201937 Meta-analysis (9 studies, n = 2177) |
Anthracyclines | Dexrazoxane vs. PBO or no cardioprotective intervention |
HF RR 0.19 (0.09–0.40; P < 0.001) Cardiac events RR 0.36 (0.27–0.49; P < 0.001) |
|
Li et al. 202096 Network meta-analysis (2 studies, n = 91) |
Anthracyclines | Statins vs. control |
LVEF MD 8.35 (1.11–15.59) |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; DOAC, direct oral anticoagulant (e.g. rivaroxaban, apixaban, edoxaban. dabigatran); HF, heart failure; LVEF, left ventricular ejection fraction; MD, mean difference; OR, odds ratio; PBO, placebo; RR, risk ratio; VTE, venous thromboembolism.
- a Definitions used by included studies: LVEF reduction ≥10–20%, LVEF decreased to <40–55%, chemotherapy interrupted as a result of cardiotoxicity, and cardiomyopathy.
The prevention and management of CV conditions in patients with cancer should generally follow published guidelines for specific conditions, such as HF,100, 101 hypertension,102, 103 AF,104-106 and venous thromboembolism (VTE).107-109 However, there are some specific considerations, and contraindications such as drug interactions, to be aware of in patients with cancer.
Patients receiving anthracyclines, HER2 inhibitors and TKIs, or undergoing radiotherapy, have a high risk for the development or worsening of LV dysfunction and HF. ESC guidelines for the diagnosis and treatment of acute and chronic HF in the general population recommend pre- and post-therapy assessment of LVEF.100 In general, the pharmacological treatments recommended for the general population of patients with symptomatic HF with reduced LVEF can be used in patients with cancer. These include ACE inhibitors, beta-blockers and certain diuretics if required. Recommendations to prevent the development or worsening of HF in asymptomatic patients include the use of ACE inhibitors and beta-blockers, and management of risk factors (Table 2), such as hypertension, dyslipidaemia, smoking, alcohol use and obesity. In patients with cardiac dysfunction caused by high-dose anthracycline, early treatment with ACE inhibitors and beta-blockers, possibly in combination, has been shown to be effective in the full, or at least partial, recovery of cardiac function.110 More recent studies using lower contemporary anthracycline doses have shown more modest effects.111 A retrospective study suggested that sacubitril–valsartan could improve echocardiographic functional and structural parameters, biomarker levels and symptomatic status compared with baseline.112 Sacubitril–valsartan was also effective in recovering LVEF in a long-term childhood cancer survivor who developed severe HF some 30 years after anthracycline exposure.113 In patients with cancer receiving anthracyclines, dexrazoxane may be useful but its use is limited by the aforesaid prescribing restrictions (Table 3).
Hypertension has been reported in up to 35% of patients receiving TKI therapy and may occur within the first months after the initiation of therapy.9, 103 For this reason, the 2018 ESC/European Society of Hypertension (ESH) guidelines for the management of arterial hypertension recommend weekly monitoring of blood pressure during the first cycle of cancer therapy and at least every 2–3 weeks thereafter.103 Patients who develop hypertension (≥140/90 mmHg), or an increase in diastolic blood pressure of ≥20 mmHg should initiate or optimize antihypertensive therapy with ACE inhibitors, ARBs, calcium channel blockers or combination therapy. The calcium channel blockers diltiazem and verapamil should be avoided because they block the CYP3A4 isoenzyme that is involved in the metabolism of TKIs, such as sorafenib and sunitinib, which can lead to increased drug levels.114
Cancer therapies have been associated with AF rates of up to 20% of patients.8 Guidelines for the management of AF in the general population provide no specific recommendations for patients with cancer.104-106 However, cancer is recognized as a risk factor for bleeding in patients receiving anticoagulation therapy.104 In the general population, oral anticoagulation is recommended in patients with AF at risk for stroke. Contraindications for the use of therapeutic anticoagulants in patients with cancer include active major bleeding, liver failure and severe bleeding disorders.109 Drug interactions with concomitant potent P-glycoprotein or CYP3A4 inhibitors or inducers are common with non-vitamin K oral anticoagulants (NOACs). In addition, ibrutinib causes platelet dysfunction, which can result in a higher risk for bleeding.54 Drug–drug interactions that can increase plasma exposure to ibrutinib may therefore increase bleeding risk.54, 115
For rhythm or rate control, first- and second-line drug treatments include a variety of drugs such as dronedarone, flecainide, propafenone, amiodarone, ibutilide, beta-blockers, diltiazem, verapamil and digoxin.104 Therapy is recommended after careful evaluation of patients in the general population and those with underlying CVD.104 These drugs can interact with TKIs and other cancer therapies, resulting in increased concentrations of either the CV medication or the TKI.114 Increased QT interval has been reported and electrophysiologic actions by antiarrhythmic drugs may be pro-arrhythmic and cause bradycardia and ventricular tachyarrhythmias (e.g. torsades de pointes). Cancer patients in whom therapy with antiarrhythmic drugs or anticancer drugs associated with QT prolongation is initiated should be monitored with ECGs and serum electrolytes to avoid hypokalaemia according to guidelines (Table 4).3, 24, 80
| Who to screen | When and how |
|---|---|
| ASCO guidelines80 | |
|
|
|
|
| ESMO guidelines24 | |
|
|
|
|
|
|
| ESC guidance3 | |
|
|
|
|
|
|
|
|
|
|
- 3D, three-dimensional; ASCO, American Society of Clinical Oncology; BNP, B-type natriuretic peptide; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CVD, cardiovascular disease; ECG, electrocardiography; ESC, European Society of Cardiology; ESMO, European Society for Medical Oncology; HF, heart failure; hs-TnI or TnT, high-sensitivitycardiac troponins (I or T); LV, left ventricular; LVEF, left ventricular ejection fraction; MUGA, multi-gated acquisition; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Cancer is a well-recognized predisposing factor for VTE, but it is also a risk factor for major bleeding with oral anticoagulation.107 The ASCO guidelines for the prevention and treatment of VTE in patients with cancer recommend thromboprophylaxis in all hospitalized patients, and selected high-risk outpatients, including low-molecular-weight heparin (LMWH) and some NOACs.108, 109 In particular, in the context of thalidomide- or lenalidomide-based regimens for multiple myeloma, thromboprophylaxis with LMWH should be offered to higher-risk patients, and either aspirin or LMWH to lower-risk patients.109
Meta-analyses of the use of anticoagulation for the treatment of VTE in patients with cancer concluded that NOACs are more effective, but are associated with a higher risk for bleeding compared with vitamin K antagonists and LMWHs.116, 117 However, data evolve and the recently completed CARAVAGGIO trial showed that oral apixaban was non-inferior to dalteparin for the treatment of cancer-associated VTE and did not increase the risk for major bleeding.118 Possible interactions of apixaban with CYP3A4 and P-glycoprotein substrates/inhibitors should nonetheless be considered.119
There are additional considerations in the management of CV toxicities in older patients, including comorbidity (multiple diseases), frailty (vulnerability to adverse outcomes), and disability (difficulty or dependency in carrying out activities essential to independent living).120 Therapy should be tailored to physiological status, with consideration of a patient's life expectancy independently of any cancer, organ function, performance score, comorbidity and quality of life. Patients assessed as fit may be more suitable and willing to undergo curative cancer therapies, despite cardiotoxicities, whereas the priority for those who are unfit or frail may be to achieve a balance between efficacy and tolerability, or palliative therapy with minimal toxicity. Alternative or reduced-intensity regimens may be needed, and the mechanisms of drug elimination and metabolism become more important.121 For this patient group, there is a need for collaboration between not only the cardiology and oncology specialties, but also geriatrics.122
Exercise and lifestyle interventions
Patients receiving cancer therapies demonstrate significant and persistent declines in cardiorespiratory fitness (CRF) levels [e.g. peak oxygen consumption (VO2peak)].123-125 Poor CRF levels have been associated with a greater symptom burden (fatigue, impaired health-related quality of life),124 increased incidence of treatment-related toxicities (both acute and chronic),126 and increased risk for both cancer-related and all-cause mortality.127 Chemotherapy, particularly anthracycline-based therapy, may cause up to 10–30 years of physiological ageing, in terms of a decline in VO2peak, compared with healthy control subjects.123, 128
The toxicities of anticancer therapies extend beyond the heart to affect the entire CV–skeletal muscle axis and involve effects such as cognitive impairment, immune dysfunction, anaemia, GI events, skeletal muscle atrophy and bone demineralization.129, 130 Evidence suggests that exercise training to improve CRF levels in patients with cancer can lead to multi-system improvements (Figure 2).125, 131-133 Numerous observational studies in cancer patients have demonstrated that self-reported exercise is associated with significant reductions in morbidity and all-cause mortality, even after controlling for traditional risk factors.131, 132 There is also growing evidence from randomized exercise intervention studies. A meta-analysis of 48 randomized controlled trials including over 3600 patients with adult-onset cancers found that exercise is an effective adjunctive therapy to improve CRF.134 However, few studies have assessed the effects of exercise on CV endpoints beyond CRF.129
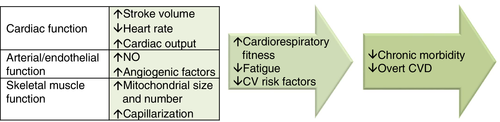
Exercise training is an important element of cardiac rehabilitation programmes. Programmes should be tailored to the individual patient, and should include aerobic and resistance exercise, warm-up and cool-down activities, and flexibility/stretching exercises.125 These programmes should also include lifestyle interventions, such as nutrition counselling and weight management, smoking cessation and psychosocial interventions.125
Long-term follow-up of cancer survivors
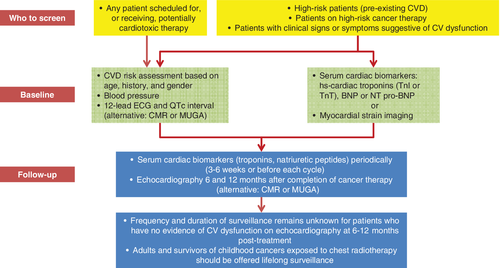
Table 4 summarizes current recommendations regarding testing strategies for the early identification and long-term monitoring of patients with cancer receiving potentially cardiotoxic therapies.3, 24, 80 Figure 3 provides a visual summary of the strategies for baseline and long-term CV monitoring in patients with cancer.3, 24, 80
Data from multiple registries of survivors of childhood cancer have shown an elevated risk for the development of CV events during long-term follow-up compared with that in the general population (Table 5).4, 135-138 Chemotherapy and radiotherapy at a young age appeared to accelerate the ageing process in survivors of childhood cancer, accelerating the onset of age-related cognitive impairments, physical impairments and fatigue.139 In one registry, 24-year-old survivors had the same cumulative incidence of grade 3–5 health conditions (including CV conditions) as their 50-year-old siblings.139
| Registry (n-value) | Patient population at assessment, median (range) | Frequency of cardiac events |
|---|---|---|
| CCSS4 (n = 10 724) |
5-year survivors Age 33.7 years (11–59) Follow-up 25.6 years (7.4–39.3) |
Cumulative incidence at age 45 years CAD (grade ≥ 3): 5.3% (95% CI 4.4–6.1) HF (grade ≥ 3): 4.8% (95% CI 4.1–5.6) |
| SJLIFE135 (n = 1807) |
10-year survivors Age 31 years (18–65) Follow-up 23 years (10–48) |
Prevalence LVEF <50%: 5.8% Systolic dysfunction detected by GLS: 31.8% |
| BCCSS136 (n = 34 489) |
5-year survivors Age, mean 29.6 (5.5–85.6) Follow-up, mean 18.0 (0.0–68.7) |
Standardized mortality ratios Overall cardiac mortality: 3.4 (95% CI 2.9–3.9) Ischaemic heart disease: 2.5 (95% CI 2.0–3.1) Cardiomyopathy/HF: 5.9 (95% CI 4.4–7.7) |
| PanCareSurFup137 (n = 39 152) |
5-year survivors Follow-up 20 years (12–28) |
Prevalence ≥1 cardiac eventa: 2.6% |
| DCOG-LATER138 (n = 5845) |
5-year survivors Age 27.3 years (5.1–65.2) Follow-up 19.9 years (5.0–50.4) |
Cumulative 40 years risk HF (grade ≥ 3): 4.4% (95% CI 3.4–5.5) |
- BCCSS, British Childhood Cancer Survivor Study; CAD, coronary heart disease; CCSS, Childhood Cancer Survivor Study; CI, confidence interval; DCOG-LATER, Dutch Childhood Oncology Group–Long-Term Effects After Childhood Cancer; GLS, global longitudinal strain; HF, heart failure; LVEF, left ventricular ejection fraction; PanCareSurFup, pan-European PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies; SJLIFE, St Jude Lifetime Cohort Study.
- a Predominantly symptomatic HF, cardiac ischaemia, pericarditis, valvular disease, or arrhythmia.
An international harmonized guideline has been developed to provide recommendations for cardiomyopathy surveillance in survivors of childhood cancer.140 Echocardiography is the recommended surveillance modality, and CMR and MUGA are considered reasonable alternatives. Biomarkers are recommended in conjunction with imaging studies, but not as a sole strategy in patients in whom cardiomyopathy is suspected. Surveillance beginning within 2 years after therapy, and repeated every 5 years thereafter, is recommended in patients treated with >250 mg doxorubicin equivalents/m2). Surveillance is also recommended or deemed reasonable in patients treated with lower anthracycline doses with and without chest radiation, respectively.
Finally, important questions remain around the issue of whether patients who have received cardiotoxic medications should be monitored by oncologists, cardiologists or primary care physicians. Here, dedicated cardio-oncology services become critical, especially for patients who may have been exposed at younger ages, and have an extended remaining life expectancy. For older patients, geriatricians with expertise in patient-centred rather than organ- or disease-specific approaches may need to play a larger role.122
Future directions
Unmet needs: research opportunities and strategies for cardio-oncology
Some of the ongoing unmet needs that the field of cardio-oncology will need to address are summarized in Table 6. The optimal monitoring strategy, including frequency and modality, remains unclear. As Table 4 shows, guidelines provide inconsistent or only tentative recommendations for long-term surveillance. For example, the ASCO guidelines recommend that the frequency of surveillance is determined by clinicians based on clinical judgement and patient circumstances.80 The ESC guidance states that the exact interval is not established, and the frequency of surveillance depends on patient and treatment characteristics.3 In addition, current recommendations are mainly specific to patients treated with anthracyclines, with or without trastuzumab or chest radiation, and further stratified by anthracycline dose (e.g. <250 mg/m2 or >250 mg2).80, 140 For patients receiving any of the many newer oncology agents, limited firm recommendations exist. Clinical trial data are needed to define the impacts of different surveillance strategies on both CV and oncological clinical outcomes, as well as their relative cost-effectiveness.80 A retrospective review of 600 early-stage breast cancer patients found that baseline echocardiography for CV screening before anthracycline- or trastuzumab-based therapy rarely identified abnormalities that impacted treatment plans, and few CV events occurred during follow-up (average of 4 years).141 The data suggested that baseline CV testing may have limited utility in young patients with no history of cardiac disease and may not be cost-effective at a population level. This highlights the importance of evaluating monitoring strategies in a controlled trial setting.
|
- CV, cardiovascular.
Randomized clinical trials specifically designed to assess the prevention and management of adverse CV effects of cancer therapy are needed. Generally, CV trials have excluded patients with comorbidities such as cancer, particularly those receiving cancer therapy. Similarly, oncology trial designs focus on cancer treatment efficacy and tend to exclude patients with CV risk factors, such as those with an LVEF of <50% and older populations. As a result, safety issues develop post-approval when cancer drugs are used in unselected patients from the general population. Integrated trials that concurrently evaluate CV and cancer outcomes, and assess the balance between the risk for asymptomatic cardiotoxicity or the worsening of pre-existing CVD, and the benefits of cancer therapy are required. The current trend towards the de-escalation of therapy through lower doses and shorter treatment durations, as well as the use of more highly targeted drugs, may be further developed to improve the balance between benefit and CV risk.
Cardio-oncology trials must include sufficient numbers of patients at risk for potential adverse CV outcomes. Key elements of these trials should include rigorous baseline and ongoing assessment of CV risk using clinical and surrogate endpoints such as biomarkers and imaging.142 Cardioprotective agents should be introduced at different time-points during therapy, and adverse event data systematically collected. There is a need to harmonize and standardize the definitions of cardiac endpoints in oncology trials, as there continue to be issues in the comparison of data across different studies.143 Cardiac outcomes, and the frequency and modality of cardiac monitoring to detect these outcomes, need to be precisely defined, using standard criteria across all trials. Criteria for adjudicating events also require to be standardized across trials. Whereas CV trials may be adequately powered for the CV outcomes of interest, oncological trials may be statistically under-powered to assess the true nature of CV adverse events.
Finally, longer follow-up beyond cancer therapy is critical, as demonstrated by the high lifetime risk for CV events among survivors of childhood and adult cancers treated with cardiotoxic therapies.3, 4, 24, 80, 135-138
There is a particular need for randomized trials evaluating the use of CV drugs in patients receiving newer cancer treatments because much of the current body of evidence is based on data sourced from patients treated with anthracyclines. New MTAs cause CV toxicities with very different mechanisms of action and therefore the effects of CV medications may also differ. Moreover, accelerated clinical development and fast-track evaluation processes often lead to the approval of drugs of uncertain CV liability and at unnecessarily high dosages, which paves the road to post-approval surges of CV toxicities.144 Certain drugs are designed for long-term treatment of chronic diseases (such as chronic leukaemias), but the on-trial time from treatment to end of follow-up may be in the order of just a few months. Again, trials should incorporate longer follow-up and biomarker-driven CV prophylaxis should be evaluated.
Finally, there is a critical need for the greater education of both patients and physicians, and a greater number of cardio-oncology clinics. Long-term survivors of childhood cancers have high rates of risky health behaviours, including smoking (14%), drinking (15%) and physical inactivity (24%).145 Long-term follow-up shows that those who are seen in specialized centres have a higher likelihood of receiving indicated echocardiograms for CV screening compared with those seen by primary care physicians (53% vs. 22%).145, 146 Thus, there is a need for clinical trials of shared care models, and specifically designed programmes, to successfully transition the care of cancer survivors from oncology or cardio-oncology centres to primary care physicians. Most primary care physicians will see very few cancer survivors in their practice; therefore, there is an obvious need to develop methods of providing physicians with patient-specific education, perhaps through registries.
Academic, clinical and industry partnerships to facilitate clinical research
There is a need to include pharmaceutical industry partners early in the research design and drug development stages. Industry may be best suited to identify early cardiotoxicity signals in preclinical models and Phase 1 and 2 studies, which will help to incorporate adequate identification and monitoring protocols into clinical trials. As described earlier, ‘hidden cardiotoxicity’ should be considered in the preclinical phase of cancer drug development20 and the cardiac safety of new drugs should be evaluated in cellular147 and animal148 models of CV risk factors.
Industry partners may also facilitate the design and conduct of trials that require multidisciplinary approaches for the evaluation of new MTAs.
Funding opportunities
Funding opportunities for cardio-oncology trials exist in the European Commission's Horizon Europe programme. Cancer is one of the five main mission areas in the €100 bn research and innovation programme proposed by the European Commission as the successor to the prior Horizon 2020 programme. Several studies relating to CVD and cancer were included in Horizon 2020, including 14 projects linking the two diseases and seven projects specifically examining cardiotoxicity. In the USA, the National Institutes of Health (NIH) has funded several initiatives to support the capture of CV endpoints in cancer trials.142 Researchers should engage in these opportunities.
Awareness
With the need for long-term follow-up, the field of cardio-oncology should expand substantially. The referral of patients to a cardio-oncology clinic would ensure prompt evaluation of CV risk and optimization of CV therapy, and result in improvements in cardiac function, follow-up and high rates of continuation of cancer therapy.149, 150 A survey conducted in 2020 of 104 centres in 35 countries around the world found that oncology patients with CV adverse effects were being cared for within a dedicated cardio-oncology unit in just 14% of centres.151 Care was provided jointly by both cardiologists and oncologists in 56% of responding centres, by cardiologists alone in 41%, and by oncologists alone in 3% of centres. In 60% of centres, cancer is not included in CV protocols as a risk factor, routine monitoring of long-term cancer survivors is not performed, and CV assessment is based on development of symptoms. This reinforces the call for more cardio-oncology units and increased education and awareness among both clinicians and patients. In the absence of an available cardio-oncology clinic, knowledgeable primary care physicians will have to be prepared to manage cancer patients during long-term follow-up, with referral to specialty care as needed.
In a health care environment of limited resources, there is a critical need to identify patients at increased risk, who warrant closer long-term follow-up. In addition, there is a need for increased awareness of the long-term CV risk among patients who receive cancer therapy, at all levels, including among specialists (e.g. oncologists, cardiologists and geriatricians), primary care physicians, industry, government and the general population. The need for increased awareness and education extends to patients themselves as many cancer survivors do not know they are at increased risk for CV morbidity and mortality as a result of their treatment. Advocacy groups such as the European Cancer Patient Coalition (ECPC) and ESC advocacy initiatives are helping to increase awareness throughout the care continuum. The ECPC is leading a Cancer Related Complications and Comorbidities Initiative with a primary focus on CV morbidity. The initiative is intended to raise awareness among European Union-based policymakers (European Parliament and Commission) on existing gaps in research and integrated care.
Registries, such as the EURObservational Registry Programme (EORP), also play an important role in identifying long-term CV outcomes, especially those that are uncommon. The EORP is a registry for CV and rare diseases. It uses an international protocol and shares data with the medical community, regulatory bodies and health care stakeholders. This allows for the comparison of data on the management of major CVDs globally, which can help improve patient care. The Cardiac Oncology Toxicity (COT) Registry was launched by the European Association of Cardiovascular Imaging/Heart Failure Association (EACVI/HFA) as an EORP multicentre registry of patients affected by breast cancer for assessment of cancer therapy-related cardiotoxicity.152 The goal of the registry is to provide information on outcomes of cardiotoxicities, to describe their time course in relation to chemotherapy, and to help design standards for the diagnosis, management and follow-up of these patients. However, there remains a need for additional dedicated cardio-oncology registries to identify and monitor cardiotoxicities, including the global linking of these registries. In 2018, the ESC created the Cardio-Oncology Council, which is tasked with bringing experts from different specialties to work together to better define the needs and problems associated with the care of cardio-oncology patients.153 Education, research and the development of best practice are among the tasks that will help to define a better future and quality of life for these patients.
Acknowledgements
This article was generated from discussions during a Cardiovascular Round Table (CRT) workshop organized in January 2020 by the European Society of Cardiology (ESC). The ESC CRT is a strategic forum for high-level dialogue between 20 industry companies (pharmaceutical, devices and diagnostics) and the ESC leadership to identify and discuss key strategic issues for the future of cardiovascular health in Europe.
The authors would like to thank Pauline Lavigne and Steven Portelance (unaffiliated, supported by the ESC) for their contributions to the writing and editing of this manuscript.
Conflict of interest: C.G. reports salary received as an employee of AstraZeneca, outside the submitted work. J.J.B. reports the receipt of personal fees as a speaker for Abbott and Edwards Lifescience, outside the submitted work. P.F. reports the receipt of grants from Semmelweis University and the Pharmahungary Group during the conduct of the study, is the founder and chief executive officer of the Pharmahungary Group, a group of research and development companies developing cardioprotective drug therapeutics and digital therapeutics related to drug safety, outside the submitted work, and reports an issued software copyright for www.mirnatarget.com, and a pending software copyright for www.vigilace.com. T.L.F. reports the receipt of personal fees from Janssen, Servier, Amgen, Philips, MSD and Daiichi Sankyo, outside the submitted work. C.P.G. reports his position as chair of the European Society of Cardiology EurObservational Research Programme Oversight Committee. T.O. reports the receipt of non-financial support from Novartis, grants and personal fees from Roche and Abbott, grants and stock from CardiNor, personal fees from Siemens and Bayer, and non-financial support from Singulex and SomaLogic, outside the submitted work. All other authors have nothing to disclose.
Appendix
Additional participants
Stephan Achenbach (ESC Board; University of Erlangen), Neeraja Balachander (Bristol-Myers Squibb), Anthony Chan (Pfizer), Jason Deeken (Philips), Christina Dimopoulou (ESC), Friedrich Fuchs (Siemens Healthineers), José Luis García López (AstraZeneca), Alexandra Goncalves (Philips), Christina Grundt (Boehringer-Ingelheim), Geeta Gulati (University of Oslo), Claudia Kaiser-Albers (MSD), Maciej Kostrubiec (European Medicines Agency; Medical University of Warsaw), Cecilia Linde (ESC Board; Karolinska University Hospital), Rosalinda Madonna (G. D'annunzio University), Alessandro Ortisi (Siemens Healthineers), Grzegorz Owsianik, European Commission, DG Research & Innovation), Giuseppe Rosano (St George's Hospital), Stefan Schröder (Bayer AG), Kerry Short (Philips), David Soergel (Novartis Pharma AG), Alphons Vincent (Medtronic), André Ziegler (Roche Diagnostics International Ltd).