Distinct myocardial triglyceride lipolysis pathways in primary and idiopathic triglyceride deposit cardiomyovasculopathy
Yoshihiko Ikeda, Mikio Shiba, and Hiroyuki Yamamoto equally contributed to this work.
Triglyceride (TG) is a major energy source for a normal heart. Myocardial lipolysis is a crucial step to hydrolyse TG and release long-chain fatty acid (LCFA) for mitochondrial β-oxidation to produce ATP. Therefore, it is of significance to elucidate its regulatory pathway(s) in order to understand pathophysiology of heart failure (HF). Triglyceride deposit cardiomyovasculopathy (TGCV) is a rare, emerging cardiovascular disorder first reported in patients with HF with massive cardiomyocyte steatosis who required cardiac transplantation.1 In TGCV, defective intracellular TG lipolysis results in TG deposition and energy failure, mainly in cardiomyocytes and coronary smooth muscle cells.2, 3 TGCV is classified into primary and idiopathic types with and without genetic mutation of PNPLA2 encoding adipose triglyceride lipase (ATGL), respectively.3 In both types, defective TG lipolysis can be evaluated using scintigraphy by calculating the washout rates (WR) of 123I-β-methyl-p-iodophenyl-pentadecanoic acid (BMIPP),2, 3 an established radiopharmaceutical of LCFA analogue.4, 5 After intravenous administration, BMIPP is taken up by cell surface transporters including CD36. Approximately 90% of cellular BMIPP form TG-BMIPP and are incorporated into TG pool. Then, TG-BMIPP is hydrolysed by intracellular lipases including ATGL, and eventually, its catabolites are washed out from cardiomyocytes.2-4 Thus, BMIPP-WR, defined as a % count reduction between early and delayed images in myocardial scintigraphy,5 reflects lipolysis of TG in the heart.2, 3
We developed the diagnostic criteria for TGCV in which low BMIPP-WR was included as an essential item and have identified ~200 patients by December 2021. Patients with TGCV exhibit adult-onset HF, diffuse coronary artery disease (CAD), and ventricular arrhythmia with high mortality in our recent registry study.6 Although they received standard medical and interventional therapies for HF and CAD, the 5-year-overall and cardiovascular event-free survival rates of patients with TGCV were approximately 70% and 53%, respectively.6 The major cause of death was cardiovascular events. Non-fatal cardiovascular events included revascularization, stroke, and hospitalization of HF or device implantation.6 For a possible specific treatment, a phase IIb/III clinical trial with a first-in-class orphan drug, of which active ingredient is tricaprin/trisdecanoin, is underway (jRCT2051210177), after proving that tricaprin/trisdecanoin facilitated myocardial lipolysis in patients with TGCV.7, 8
In primary TGCV (P-TGCV), inherited in the autosomal recessive fashion, a homozygous genetic defect of ATGL, an essential molecule to hydrolyse intracellular TG and release LCFA for mitochondrial β-oxidation, causes cellular steatosis.1-3 However, the mechanism(s) underlying defective intracellular lipolysis and subsequent TG deposition remains unknown in idiopathic TGCV (I-TGCV). Here, we report pathological characteristics of endomyocardial biopsy (EMB) specimens from TGCV patients and discuss the regulation of myocardial TG deposition in I-TGCVs compared with that in P-TGCVs.
A series of 18 consecutive EMB specimens from patients with TGCV (1 primary and 17 idiopathic types) referred from 7 hospitals to the Department of Pathology, National Cerebral and Cardiovascular Center (NCVC), the core laboratory of myocardial pathology for the Japan TGCV study group, between August 2020 and December 2021 were analysed. BMIPP scintigraphy and measurement of BMIPP-WR were performed according to the recommendation by the Japan Society of Nuclear Cardiology.5 The P-TGCV patient was a 34-year-old man with HF, homozygous for a large deletion of PNPLA2 (Gene ID: LC508023). The patient's BMIPP-WR was −4.5% (<10% BMIPP-WR, the cutoff for TGCV diagnosis5). The 17 I-TGCV patients included 15 men and 2 women (mean age: 66.1 ± 12.7 years). They all presented with HF or diffuse CAD, and the mean BMIPP-WR was 2.0 ± 6.7%. Following routine analyses with haematoxylin and eosin (H&E) staining, paraffin-embedded sections of EMB specimens were immunostained, and the area fractions of immunoreactivities (AFIs) were evaluated using two antibodies: ATGL responsible for P-TGCV and perilipin-2 (PLIN2), a protein coating lipid droplets in non-adipocytes to detect intracellular lipid deposition in cardiomyocytes, alternative to lipid staining owing to limited availability of frozen EMB samples. Correlations between PLIN2- or ATGL-AFI and BMIPP-WR were evaluated using Pearson's correlation analysis in I-TGCV. P-values <0.05 were considered statistically significant, and statistical analysis was performed using JMP version 14.3.0 (SAS Institute Inc., Tokyo, Japan). This study was approved by the Ethics Committee of NCVC.
EMB specimens from the P-TGCV patient exhibited severely hypertrophied cardiomyocytes full of multiple large lipid droplets negative for ATGL (Figure 1A). The PLIN2-AFI was 54.2 ± 2.9% (mean ± SD from three different high power fields). Cardiomyocytes in the I-TGCV group were smaller than those from P-TGCV, but possessed numerous numbers of lipid droplets positive for both PLIN2 and ATGL (Figure 1B). Lipid droplets in I-TGCV were small and sometimes rarely observed using H&E staining. Correlation analyses in I-TGCV patients demonstrated negative correlations between BMIPP-WR and PLIN2-AFI (r = −0.6292, P = 0.0068). ATGL-AFI positively correlated with PLIN2-AFI (r = 0.5337, P = 0.0274). No correlation existed between BMIPP-WR and ATGL-AFI, indicating that defective myocardial lipolysis is likely independent of ATGL expression in I-TGCV.
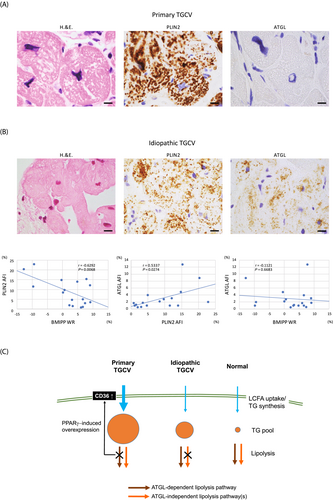
Myocardial lipid deposition is determined by substrate uptake, TG synthesis, and lipolysis (Figure 1C). In P-TGCV, as reported previously,9 massive TG accumulation was caused by the defect in ATGL-dependent lipolysis and increased LCFA uptake through peroxisome proliferated activated receptor-γ-induced CD36 overexpression. In I-TGCV specimens investigated, the ATGL expression was conserved and positively correlated with the degree of lipid deposition (PLIN2-AFI). Even though ATGL was overexpressed possibly by metabolic adaptation to prevent excess TG deposition, intracellular TG deposition and very low BMIPP-WR were observed. These results indicated that a putative ATGL-independent lipolysis pathway may be involved in regulating TG deposition in I-TGCV. The existence of such an ATGL-independent lipolysis pathway is also supported by the evidence that tricaprin/trisdecanoin improved myocardial BMIPP-WR and reduced TG deposition in ATGL-knockout mice.10 Further studies are required to identify the molecule(s) involved in ATGL-independent lipolysis, particularly causing I-TGCV.
The present study indicates distinct myocardial TG lipolysis pathways in primary and idiopathic TGCV, which is a rare but important cause of HF. Our findings provide information to understand heterogeneity of myocardial TG lipolysis pathways and complexed regulatory mechanisms for lipid droplet formation in HF.
Acknowledgements
The authors thank Drs Atsuo Nakajima (Shizuoka City Shizuoka Hospital), Shohei Yoshida (Kanazawa University), and Shoko Ohura (Iwate Prefectural Isawa Hospital) for providing EMB samples.
Funding
This study was partially supported by rare disease research grants from the Japan Agency of Medical Research and Development (A-MED) (22ek0109479h0003) and the Ministry of Health, Labour, and Welfare of Japan (grant No. 20FC1008) to KH.
Conflict of interests
KH has held the position of Joint Research Chair in collaboration with Toa Eiyo Ltd. (Tokyo, Japan) since February 2021 and has served as a medical advisor for Toa Eiyo Ltd. since December 2021. KH has a pending patent. The other authors have no competing interests.




