Protective effect of secretory APE1/Ref-1 on doxorubicin-induced cardiotoxicity via suppression of ROS and p53 pathway
This work was performed at the Research Institute for Medical Sciences, Chungnam National University Hospital, College of Medicine, Chungnam National University, Daejeon, Republic of Korea.
Soo Yeon An and Seon-Ah Jin contributed equally to this manuscript.
Abstract
Aims
The clinical application of doxorubicin (DOX), a potent anthracycline anticancer drug that effectively treats various malignancies, is limited by its side effects, such as cardiomyopathy. Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) is a multifunctional protein that can be secreted and is a promising target for the reduction of DOX-induced inflammation and oxidative stress. We aimed to investigate the protective role of secretory APE1/Ref-1 against DOX-induced cardiac injury.
Methods and results
Designated adenoviral preprotrypsin-leading sequence APE1/Ref-1 (Ad-PPTLS-APE1/Ref-1) was used to overexpress secretory APE1/Ref-1 and assess its role in preventing DOX-induced cardiomyopathy in vitro. Our findings revealed that exposure to secretory APE1/Ref-1 significantly decreased N-terminal pro-B-type natriuretic peptide levels in DOX-treated H9C2 cells. In addition, secretory APE1/Ref-1 reduced the severity of cardiomyocyte injury and apoptosis in both in vitro and in vivo DOX-induced cardiotoxicity models. The observed cardioprotective effects of secretory APE1/Ref-1 were mediated via inhibition of the p53 signalling pathway and enhancement of cell viability through attenuation of oxidative stress in DOX-treated cardiomyocytes.
Conclusions
Our study provides evidence that secretory APE1/Ref-1 has the potential to inhibit DOX-induced cardiac toxicity by inhibiting oxidative stress and p53 related apoptosis both in vitro and in vivo. These findings suggest that secretory APE1/Ref-1 supplementation is a promising strategy to attenuate DOX-induced cardiomyocyte damage in a preclinical model. Further clinical investigations are essential to validate the therapeutic efficacy and safety of the intervention in human subjects.
Introduction
Doxorubicin (DOX) is a widely used anthracycline chemotherapeutic agent with potent therapeutic efficacy against various malignancies.1 DOX-induced cardiotoxicity is a severe adverse effect observed in chemotherapy-treated patients, resulting in increased mortality risk and poor prognoses.2 DOX also induces cardiac dysfunction in asymptomatic patients; however, except for preventive measures, no efficient clinical treatments are currently available.3 The efficacy of DOX is mediated via multiple mechanisms, such as DNA intercalation, inhibition of transcription by topoisomerase II, and induction of cytotoxicity through the formation of reactive oxygen species (ROS).4 ROS generation, regulated by p53, is the most commonly accepted mechanism of DOX-induced cardiomyopathy resulting from increased oxidative stress.5
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1), a multifunctional protein that encodes two functional domains, plays a significant role in redox activity and DNA base excision repair. The redox activity of APE1/Ref-1 regulates various transcription factors, and its DNA repair activity increases cell survival.6 APE1/Ref-1 is transported between the cytoplasm and nucleus in response to oxidative stress; it regulates numerous transcription factors, including p53, by reducing disulfide bonds in the cysteine residue, thereby facilitating p53 DNA binding.7 By regulating p53, APE1/Ref-1 prevents cell senescence and improves cell survival.8 APE1/Ref-1 overexpression in progenitor cells renders apoptosis resistance and improves viability.9 Furthermore, APE1/Ref-1 exerts protective effects against vascular ischaemia–reperfusion injury and myocardial hypoxia-reoxygenation injury by alleviating oxidative stress.10, 11
Studies have demonstrated that, in response to increased oxidative and cytotoxic stress, APE1/Ref-1 is secreted extracellularly, reducing inflammation and ROS generation.13, 12, 14, 15 Using a secretory adenovirus encoding preprotrypsin leading sequence-APE1/Ref-1 (Ad-PPTLS-APE1/Ref-1), Joo et al. showed that APE1/Ref-1 regulates inflammation levels in lipopolysaccharide-stimulated macrophages by reducing disulfide bonds on interleukin (IL)-1 and Toll-like receptors.16 Ad-PPTLS-APE1/Ref-1 injection in mice also resulted in the inactivation of p53, p21, and nuclear factor-κB pathways.17 These findings suggest that secretory APE1/Ref-1, similar to its nuclear counterpart, regulates cellular activities and promotes cellular homeostasis by attenuating inflammation and preventing apoptosis.
APE1/Ref-1 is a promising target for reducing DOX-induced cardiac inflammation and oxidative stress, and p53 activation plays a crucial role in DOX-induced cardiomyocyte apoptosis. Therefore, we aimed to confirm the protective role of secretory APE1/Ref-1 against DOX-induced cardiac injury and apoptosis related to p53.
Methods
Cell culture
H9C2 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM, Welgene, Kyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% penicillin (Lonza BioScience, Basel, Switzerland). Cells were cultured at a ratio of 1:3 every 3 days and plated at a density of 5 × 105 cells per well in a medium until they reached approximately 80% confluence at the time of transfection.
Adenoviral vector transfection to purify secretory APE1/Ref-1
Adenoviruses encoding-galactosidase (Adβ-gal), full-length human APE1/Ref-1 (Ad-APE1/Ref-1), or PPTLS-APE1/Ref-1 (Ad-PPTLS-APE1/Ref-1) with secretory signal sequences were prepared according to previously described methods,16-18 and their treatment effects were compared. Human embryonic kidney-293 T cells (HEK293T) with the large T-antigen of Simian virus 40 (ATCC, Manassas, VA, USA) were used to amplify and purify the adenoviruses. HEK293T cells were seeded in plates at 2.5 × 105 cells/mL and maintained at 37°C in a humidified incubator with 5% CO2. HEK293T cells were infected with 500 multiplicity of infection (particle-forming units per cell) of the adenovirus containing Adβ-gal, Ad-APE1/Ref-1, or Ad-PPTLS-APE1/Ref-1 and incubated for 48 h. The adenovirus was purified using the Adeno-X maxi Purification Kit (Takara Bio, San Jose, CA, USA). The cell culture-amplified adenovirus was quantified using an Adeno-X rapid titre kit (Takara Bio). APE1/Ref-1 sandwich enzyme-linked immunosorbent assay (ELISA; MediRedox, Daejeon, Korea) was used to quantify secretory APE1/Ref-1 in the culture supernatant or whole-cell lysates, as previously described.19
Determination of thiol activity with 5,5′-dithio-bis(2-nitrobenzoic acid)
The thiol activity of secretory APE1/Ref-1 in the culture medium was measured using the fluorometric Measure-iT™ Thiol assay kit (Cat. No. M30550, Invitrogen, Paisley, UK) following the manufacturer's protocol.
In vitro experiment in a cell model of DOX-induced cardiotoxicity
The dose-dependent effects of DOX on cell viability were evaluated to select the adequate drug dose to induce in vitro cardiotoxicity. H9C2 cells were treated with varying concentrations of DOX (0.2, 0.5, 1.0, 2.0, and 5.0 μM). Then, suspended H9C2 cells were examined under a microscope for cell death and morphological changes. Anti-p53 (Cat. No. 126; Santa Cruz Biotechnology, Dallas, TX, USA; 1:1000 dilution) and APE1/Ref-1 ELISA kit (MediRedox) were used to quantify cellular p53 and APE1/Ref-1 expression depending on different concentrations of DOX. Next, the time-dependent effects of DOX on cell viability were evaluated; cell viability was determined after 12, 24, and 48 h of DOX treatment at concentrations of 1.0, 2.0, and 5.0 μM.
HEK293T cells were transfected with adenovirus (2 × 109 particles) encoding Adβ-gal, Ad-APE1/Ref-1 or Ad-PPTLS-APE1/Ref-1 and DMEM at a ratio of 3:2 for 48 h. The supernatant of HEK293T cell culture media was prepared by centrifuging for 5 min at 1500 rpm at 4°C and discarding the precipitates. The purified supernatant was used as the conditioned media to pre-treat H9C2 cells for 24 h to assess the effect of secretory APE1/Ref-1. Subsequently, the pre-treated H9C2 cells were challenged with 2 μM DOX (HPLC purity > 98%; Sigma-Aldrich, St. Louis, MO, USA) for 24 h to establish the in vitro model of DOX-induced cardiotoxicity (Figure 1).
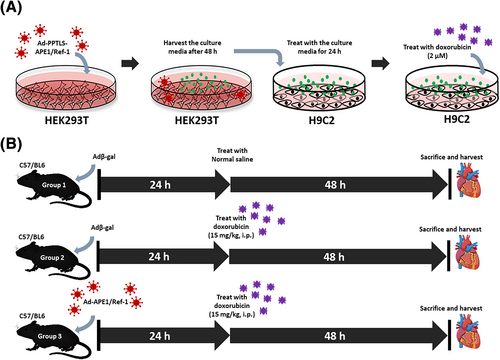
In vivo experiment in a mouse model of DOX-induced cardiotoxicity
We did pilot study to make a mouse model of DOX-induced cardiotoxicity as described before.20, 21 Finally eight-week-old male C57BL/6 mice (Koatech, Pyeongtaek, Korea, n = 16) were randomly divided into (1) a DOX group that received a single dose of DOX [15 mg/kg; DOX (Sigma-Aldrich) was dissolved in normal saline and injected intraperitoneally (i.p.)]; (2) a transfection group that received 5 × 109 virus particles of Ad-APE1/Ref-1 24 h before DOX (15 mg/kg; i.p.) treatment; and (3) a control group that received a normal saline injection and Adβ-gal (5 × 109 particles; i.p. for 24 h). Mice were sacrificed under anaesthesia on day 3. The experimental design to establish the in vivo mouse model of DOX-induced cardiotoxicity is shown in Figure 1.
All animal experiments were conducted in accordance with the principles of animal experimentation at Chungnam National University, College of Medicine (Daejeon, Korea). The Animal Ethics Committee approved the animal protocols according to the institutional guidelines for animal care and use of the Chungnam National University-IACUC (CNUH-019-A0057).
Analysis of plasma levels of N-terminal pro-B-type natriuretic peptide and APE1/Ref-1
The plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) level was determined as a cardiotoxicity marker in the serum using an ELISA kit (NBP2-76775, Novus Biologicals, Centennial, CO, USA) and a fluorescence microplate reader (FilterMax™ F5 Multi-Mode Microplate Reader). APE1/Ref-1 levels in the plasma were measured using an APE1/Ref-1 sandwich ELISA kit (MediRedox).
Cell viability
Cell viability was assessed using the Cell Counting Kit (CCK)-8 assay (DoGen Bio, Seoul, Korea) and manual counting. Serial dilutions of 1:20 were made by adding 10 μL of cell suspension into 190 μL of diluting fluid to a final dilution of 8000. The suspension was prepared at a concentration of 5 × 103 cells/mL in 96-well plates and incubated at standard conditions (37°C, 5% CO2, and 95% humidity). Subsequently, 10 μL CCK-8 solution was added to each well and incubated for 3 h at 37°C. The absorbance at 450 nm was measured using a microplate reader (FilterMax™ F5 Multi-Mode Microplate Reader). A 100 μL cell suspension volume was combined with 40 μL of a 0.08% trypan blue solution. The trypan blue-infused cell suspension was pipetted into the well of the counting chamber and examined under a microscope (Leica-Microsystems, Wetzlar, Germany) set at a magnification of 10 times. Manual counting was performed to determine the number of unstained cells, indicative of cell viability, and the number of stained cells, which signified cell death. Morphological changes were noted, including cell necrosis, mononuclear infiltration, vacuolation, and degenerative changes.
Western blot analysis
Total cellular protein was extracted using total protein extraction lysis buffer (10 mM Tris HCl (pH 7.4), 150 mM NaCl, and 1 mM EDTA). The homogenates were cleared by centrifugation at 14,000× g for 15 min, and the supernatants were precipitated. Protein concentrations were measured using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal amounts of protein samples were separated using 10% sodium dodecyl-sulphate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% fat-free milk in Tris-buffered saline for 1 h, the membranes were incubated with appropriate primary antibodies overnight at 4°C. The membranes were then incubated with the appropriate secondary antibodies at 24°C for 2 h. The band signals were normalized to β-gal as an internal control and visualized using an enhanced chemiluminescence detection kit (Pierce Biotechnology, Rockford, IL, USA). Band intensity was quantified by densitometry using Fuji ImageJ software. The following antibodies were used: Anti-p53 (Cat. No. 126; Santa Cruz Biotechnology, Dallas, TX, USA; 1:1000 dilution), anti-Bax (Cat. No. 2772; Cell Signalling Technology, Danvers, MA, USA; 1:1000 dilution), anti-Bcl-2 (Cat. No. 3498; Cell Signalling Technology; 1:1000 dilution), and anti-caspase-3 (Cat. No. 9662; Cell Signalling Technology; 1:1000 dilution).
Flow cytometry analysis of ROS
Intracellular ROS generation was measured by staining the cells with 20 μM 2′,7′-dichlorofluorescein-diacetate using a ROS detection assay kit (Cat. No. 113851; Abcam, Cambridge, UK). After incubation for 30 min at 37°C, cells were washed in FBS buffer. The suspension was analysed using flow cytometry using a BD Accuri-C6 flow cytometer (BD, Bedford, MA, USA) and fluorescence microscope (BX51, Olympus, Miami, FL, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 26.0 (IBM Co., Chicago, IL, USA). Continuous variables were compared using Student's t-test or one-way analysis of variance for multiple comparisons. All P-values were two-sided, and a P-value < 0.05 was considered statistically significant. All data are presented as means ± standard error of the mean (SEM).
Results
Secretory APE1/Ref-1 demonstrated antioxidant activity in vitro
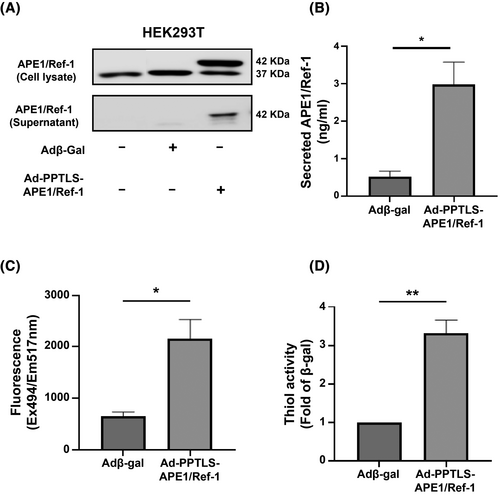
Transfection of HEK293T cells with Ad-PPTLS-APE1/Ref-1 resulted in persistent and elevated expression of secretory APE1/Ref-1 in the culture media (Figure 2). Cellular APE1/Ref-1 was ubiquitously expressed with or without adenoviral vector transfection. With Ad-PPTLS-APE1/Ref-1 transfection, HEK293T cells expressed both cellular APE1/Ref-1 (37 kDa) and secretory APE1/Ref-1 (42 kDa), and the secretory APE1/Ref-1 was detected in the cell supernatant. The secretory APE1/Ref-1 concentration (2.98 ± 0.62 ng/mL) in the culture media was significantly higher than that of β-gal (0.53 ± 0.14 ng/mL; P < 0.05). The supernatant containing secretory APE1/Ref-1 showed significantly higher fluorescence (APE1/Ref-12165 ± 374.1 RFU vs. β-gal 653.8 RFU; P < 0.01; Figure 2) and thiol activity (3.31 ± 0.34-fold of β-gal, P < 0.01; Figure 2), suggesting that the transfection of Ad-PPTLS-APE1/Ref-1 effectively increased redox activity in culture media.
DOX increased p53 expression with reduced cell viability
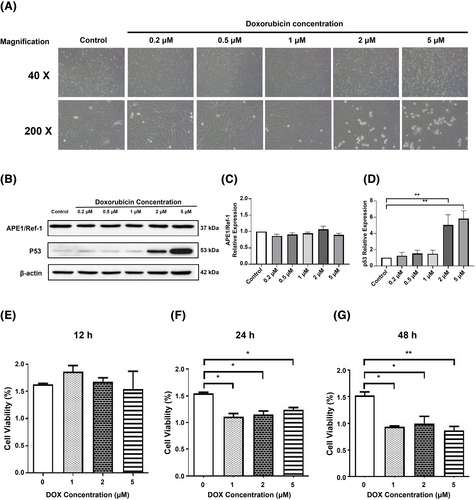
Exposure of the isolated H9C2 cells to various concentrations of DOX revealed consistent cell death and morphological changes under the microscope at concentrations beyond 2 μM (Figure 3). As shown in Figure 3, relative expression of cellular APE1/Ref-1 did not differ significantly at different concentration of DOX. Nevertheless, p53 expression was markedly elevated in the cells treated with DOX at 2 μM (5.04 ± 1.25-fold of control; P < 0.01) and 5 μM (5.81 ± 1.70 fold of control; P < 0.01) (Figure 3). Furthermore, the viability of H9C2 cells started to decrease after 24 h of treatment with DOX at a concentration greater than 1 μM (P < 0.05) and consistently decreased after 48 h (P < 0.05; Figure 3).
Secretory APE1/Ref-1 protected cardiomyocytes against DOX-induced cell death
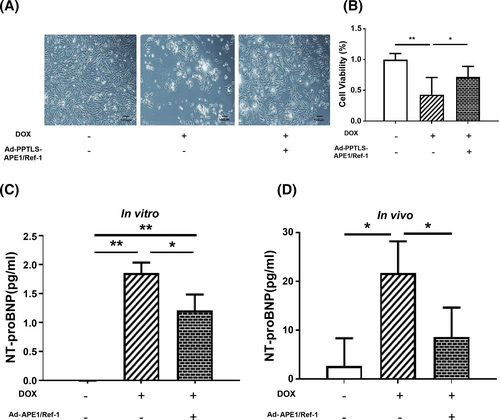
DOX-treated H9C2 cells disintegrated and were vacuolated with apoptotic cell debris (second image in Figure 4). The total cell mass and numbers of H9C2 decreased, and cell viability was 43% ± 28% (P < 0.01). Remarkably, pre-treatment of secretory APE1/Ref-1 reinstated viable cells (72% ± 17%, P < 0.05), partially inhibiting cell death caused by DOX (Figure 4). Cells pre-treated with secretory APE1/Ref-1 partially recovered cell arrays (third image in Figure 4). Therefore, secretory APE1/Ref-1 inhibits DOX-induced cell death in H9C2 cells in vitro.
Secretory APE1/Ref-1 decreased DOX-induced cardiotoxicity in vitro and in vivo
In our in vitro model, DOX (2 μM) significantly increased the NT-proBNP level in H9C2 cells (1.86 ± 0.18 pg/mL) compared with that in the control (0.00 ± 0.003 pg/mL; P < 0.01). In contrast, pre-treatment of H9C2 cells with secretory Ad-PPTLS-APE1/Ref-1 before DOX treatment significantly inhibited the DOX-induced increase in NT-proBNP level (1.22 ± 0.27 pg/mL; P < 0.05; Figure 4).
In our mouse model of DOX-induced cardiotoxicity, NT-proBNP level was increased (21.60 ± 6.55 pg/mL) after 48 h compared with that in the control (2.65 ± 5.69 pg/mL; P < 0.05). In contrast, Ad-APE1/Ref-1 overexpression alleviated the DOX-induced increase in NT-proBNP levels (8.52 ± 6.09 pg/mL; P < 0.05 vs. DOX; Figure 4). Taken together, these results indicate that secretory APE1/Ref-1 alleviates the damaging effects of DOX in vitro and in vivo.
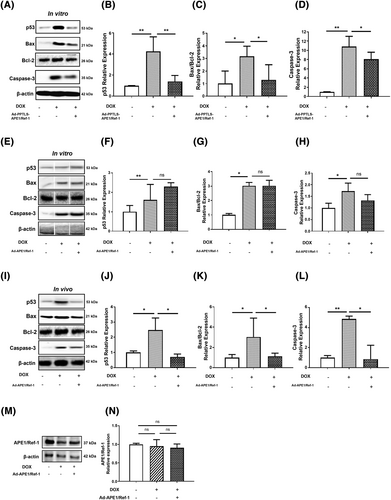
Secretory APE1/Ref-1 alleviated p53-mediated apoptosis in vitro and in vivo
Previous studies have shown that p53, an apoptosis regulator, is an intermediary in the pathophysiology of many cardiovascular diseases, including heart failure and DOX cardiotoxicity.22 When triggered by various signals and cellular stress, p53 and its downstream effectors, such as Bax, Bcl-2, and caspases, induce apoptosis and prevent further inflammatory damage.23 Therefore, we assessed the effects of APE1/Ref-1 on the expression of these apoptosis-related proteins to assess the effects of APE1/Ref-1 on p53-mediated apoptosis in vitro and in vivo.
In vitro, DOX increased the expression of apoptosis-related proteins p53, Bax/Bcl-2, and caspase-3 in H9C2 cells, which were significantly improved by Ad-PPTLS-APE1/Ref-1 transfection (Figure 5). However, transfection with Ad-APE1/Ref-1 did not reduce p53 or apoptosis-related protein levels (Figure 5). In vivo, overexpression of APE1/Ref-1 decreased the expression of p53 (P < 0.05), Bax/Bcl-2 (P < 0.05), and caspase-3 (P < 0.05) in the cardiac tissues of DOX-treated mice (Figure 5). In addition, endogenous APE1/Ref-1 expression did not change after transfection of DOX-treated mice with Ad-APE1/Ref-1 (Figure 5). Our results show that in vivo transfection of Ad-APE1/Ref-1 expressing APE1/Ref-1 is effective in regulating p53-related apoptosis in the DOX cardiotoxicity model, indicating the inhibitory effect of secretory APE1/Ref-1.
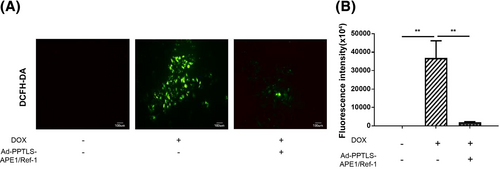
Secretory APE1/Ref-1 alleviated oxidative stress in DOX-treated H9C2 cells
The critical trigger for DOX-induced apoptosis is oxidative DNA damage caused by the formation of free radicals. DNA damage leads to H2O2 generation indirectly through the activation of downstream signalling, resulting in caspase-3 activation.4 DOX-induced cardiotoxicity can be attributed to p53-mediated ROS formation and subsequent cardiomyocyte loss.5
Therefore, we examined the changes in oxidative stress induced by DOX. The generation of ROS was significantly increased in H9C2 cells treated with 2 μM DOX (36877 ± 9436 RFU, P < 0.01). However, pre-treatment of the cell media with secretory APE1/Ref-1 induced by Ad-PPTLS-APE1/Ref-1 transfection decreased the fluorescence intensity of intracellular ROS (1836 ± 397 RFU; P < 0.01), as shown in flow cytometry and fluorescence microscopy images (Figure 6). Our study demonstrates that secretory APE1/Ref-1 attenuates oxidative stress by decreasing ROS generation.
Discussion
Our study demonstrates that secretory APE1/Ref-1 plays a regulatory role in p53-mediated apoptosis and ROS generation in vitro and in vivo model of DOX-induced cardiotoxicity. Secretory APE1/Ref-1 can lessen DOX-induced ROS generation and oxidative stress when administered before the toxic insult. This result suggests that preconditioning with APE1/Ref-1 overexpression before DOX exposure will result in a cardioprotective window. The overexpression of secretory APE1/Ref-1 to inhibit p53 signalling could be a viable strategy to alleviate cardiac injury, especially in those who are at high risk of DOX-induced cardiotoxicity.
Current therapeutic interventions for DOX-induced cardiotoxicity are limited to angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, statins, and iron chelators.24 Dexrazoxane, an iron chelator of anthracyclines, is the only cardioprotective agent currently approved for patients with a high and very high risk of chemotherapy-related cardiotoxicity.25, 26 Also, dexrazoxane is administered to patients with advanced breast cancer who have already received a minimum cumulative dose of 300 mg/m2 DOX. Dexrazoxane has demonstrated effectiveness, it targets a specific mechanism of cardiotoxicity by chelating iron from the DOX-iron complex.27 In contrast, DOX-induced cardiotoxicity involves multiple pathways, such as oxidative stress, calcium homeostasis disruption, and mitochondrial dysfunction. The need for additional cardio-protective agents for broader use arises due to the complex and multifactorial nature of DOX cardiotoxicity. DOX alters the function of TNF-α receptors I and II in modulating p53-mediated apoptosis in H9C2 cells.28 Therefore, comprehensive antioxidant and anti-apoptotic strategies are necessary to counter these intricate cardiotoxic mechanisms and provide optimal cardio-protection. Our data support that secretory APE1/Ref-1 could exert a protective effect, potentially mitigating cell death and preserving cardiac function by inhibiting p53-mediated apoptosis. These observations highlight the potential importance of secretory APE1/Ref-1 as a protective agent under pathological heart conditions by attenuating cardiac inflammation and remodelling.
Secretory APE1/Ref-1 exhibits diverse anti-inflammatory and antioxidant functions in response to endothelial dysfunction, inflammatory disorders, and sepsis.29 APE1/Ref-1 decreases the binding capacity of receptors for tumour necrosis factor-alpha (TNF-α), a cytokine known to mediate cardiac inflammation.29, 12, 30 In the context of DOX-induced cardiotoxicity, it is plausible that secretory APE1/Ref-1 may exert a similar anti-inflammatory effect within the heart. By reducing inflammatory signals, secretory APE1/Ref-1 could potentially contribute to preserving cardiac function and preventing further damage. In our study utilizing the adenoviral overexpression of secretory APE1/Ref-1 via Ad-PPTLS-APE1/Ref-1, we observed significantly lower levels of NT-proBNP, a marker of cardiac dysfunction. This finding further supports the notion that secretory APE1/Ref-1 protects against inflammation-associated cardiac injury in DOX cardiotoxicity.
Although these studies demonstrate the APE1/Ref-1-mediated inhibition of p53-mediated apoptosis, the precise mechanism by which secretory APE1/Ref-1 affects and controls the transportation and expression of p53 in cells remains unclear. The present study showed that the increased expression of apoptotic proteins and cell death were concurrent with p53-mediated apoptosis and elevated ROS generation, consistent with previous studies on DOX cardiotoxicity.31 Overexpression of secretory APE1/Ref-1 significantly reduced p53 expression and apoptosis-related proteins, whereas cellular APE1/Ref-1 expression did not change. These findings suggest that the decreased expression of downstream molecules involved in p53-mediated apoptosis plays a central role in the protective effect of secretory APE1/Ref-1 in DOX-induced cardiomyopathy. The effects of DOX on the heart are blocked by secretory APE1/Ref-1 via its antioxidant activity, suggesting that secretory APE1/Ref-1 inhibits apoptosis and ROS generation during cell injury.
The present study had several limitations characterize that should be highlighted. First, the scope of the study was primarily on the therapeutic effects of secretory APE1/Ref-1 in doxorubicin-induced cardiotoxicity, without exploring the mechanistic aspects of the transmembrane transport and molecular signalling pathways related to the nuclear and mitochondrial p53 pathways of cellular apoptosis. Second, the use of adenovirus for overexpressing secretory APE1/Ref-1 is associated with inconsistent transduction and expression rates, which can affect consistent administration. In addition, an immunologic response to adenovirus could have affected the responses to secretory APE1/Ref-1 administration. Third, although this study demonstrated a potential application of secretory APE1/Ref-1 in DOX-induced cardiotoxicity, its broader efficacy in providing cardiac protection undergoing other anthracycline chemotherapy and cardiotoxic treatments, such as trastuzumab and radiation therapy, remains uncertain. Fourth, we did not compare protective role of secretory APE1/Ref-1 and reference treatment with dexrazoxane. It is pertinent to note the contrasting mechanisms of action between secreted APE1/Ref-1 and dexrazoxane. Dexrazoxane functions as an iron chelator, binding or removing free iron from the doxorubicin-iron complex, thus preventing the formation of oxygen free radicals and potentially mitigating some cardiotoxic effects. In contrast, secreted APE1/Ref-1 predominantly operates extracellularly within myocardial tissues, where it potentially interacts with membrane proteins, utilizing its reducing capabilities to alleviate oxidative stress induced by doxorubicin. Further investigations are warranted to validate and expand therapeutic pathways of both secreted APE1/Ref-1 and dexrazoxane in cardio-protection. Lastly, the study is still in an early preliminary stage, with potential adverse reactions associated with the administration of secretory APE1/Ref-1, which should be explored in future studies.
In conclusion, this study demonstrated that secretory APE1/Ref-1 protects against DOX-induced cardiomyocyte injury and oxidative stress. APE1/Ref-1, secreted from the adenovirus-mediated overexpression of PPTLS-APE1/Ref-1, exhibits antioxidant abilities in vitro and functions as a reducing protein. Our research showed that secretory APE1/Ref-1 reduced cardiomyocyte injury in both in vitro and in vivo models of DOX-induced cardiotoxicity by inhibiting inflammation, oxidative stress, p53 related apoptosis and increasing the viability. These findings suggest that APE1/Ref-1 supplementation shows promise in reducing DOX-induced cardiomyocyte damage in a preclinical model. Further clinical investigations are essential to validate the therapeutic efficacy and safety of the intervention in human subjects.
Conflict of interest
None declared.
Funding
This work was supported by the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (grant number #HI22C0610) and the Basic Science Research Program of the National Research Foundation (NRF), funded by the Ministry of Education of Korea (grant numbers NRF-2014R1A6A1029617 and NRF-2018R1C1B5041472).




