Pleural effusion in severe aortic stenosis: marker of an adverse haemodynamic constellation and poor prognosis
Abstract
Aim
Pleural effusion (PE) is a common chest radiography (CXR) finding in patients with advanced cardiac disease. The pathophysiology and clinical value of PE in this setting are incompletely defined. We aimed to assess the haemodynamic correlates and prognostic impact of PE in patients with severe aortic stenosis (AS).
Methods and results
We studied 471 patients (mean age 74 ± 10 years) with severe AS (indexed aortic valve area 0.42 ± 0.12 cm2/m2, left ventricular ejection fraction 58 ± 12%) undergoing right heart catheterization and upright CXR prior to aortic valve replacement (AVR). Two radiologist independently evaluated all CXR for the presence of bilateral PE, unilateral, or no PE, blinded to any other data. There were 49 (10%) patients with bilateral PE, 32 (7%) patients with unilateral PE, and 390 (83%) patients with no PE. Patients with bilateral PE had the highest mean right atrial pressure, mean pulmonary artery wedge pressure (mPAWP), and pulmonary vascular resistance, and had the lowest stroke volume index while those with unilateral PE had intermediate values. In the multivariate analysis, mPAWP was an independent predictor of any PE and bilateral PE. After a median (interquartile range) post-AVR follow-up of 1361 (957–1878) days mortality was highest in patients with bilateral PE (2.7 times higher than in patients without PE), whereas patients with unilateral PE had similar mortality as those without PE.
Conclusions
In severe AS patients, the presence of PE, particularly bilateral PE, is a marker of a poor haemodynamic constellation. Bilateral PE is associated with a substantially increased post-AVR mortality.
Introduction
The conventional chest radiography (CXR) as a broadly available tool has been used over many decades for the diagnosis and management of cardiac diseases.1 Pleural effusion (PE) is a common CXR finding that is usually interpreted as a sign of heart failure. However, the pathophysiology underlying PE formation, in particular the detailed contribution of haemodynamics, is incompletely understood. Given that PE has been found to be associated with an increased risk of readmission in patients with heart failure,2, 3 a better understanding of the mechanisms contributing to PE formation is required. In clinical practice, it would be very helpful to know the typical haemodynamic constellation of a patient presenting with PE as this may guide the further diagnostic and therapeutic approach. Data from small studies using right heart catheterization revealed higher pulmonary artery wedge pressure (PAWP) in patients with PE compared with those without.4, 5 However, there are no larger studies evaluating detailed invasive haemodynamics in patients with versus without PE.
In the present study, we systematically assessed the presence of PE by CXR in 471 patients with severe aortic stenosis (AS) undergoing cardiac catheterization prior to aortic valve replacement (AVR). The aim of the study was to determine the relationship between PE and detailed invasive haemodynamics and the long-term prognostic impact of PE in these patients.
Methods
Study population
This is a retrospective analysis of systematically collected data on cardiac catheterization in consecutive patients with severe AS undergoing a highly standardized evaluation process prior to AVR in a single center between January 2011 and January 2016 with a post-AVR follow-up of several years. At our institution, right heart catheterization is performed on a routine basis is patients with severe AS prior to AVR. For this analysis, we included 471 patients in whom an upright CXR of sufficient imaging quality had been obtained prior to cardiac catheterization (in the vast majority on the day before). All patients subsequently underwent surgical or transcatheter AVR. The study was approved by the local ethics committee. A waiver of consent was granted.
Conventional chest radiography reading
All CXR were digitally stored. Images were systematically re-evaluated and scored by two radiologists who were blinded to all clinical data including haemodynamics. The two radiologists independently rated all CXR for the presence of no PE, unilateral PE, or bilateral PE. In case of disagreement, a conservative rating was applied (e.g. no PE if one rating was unilateral PE, and the other one was no PE). Apart from PE scoring, the two radiologists independently applied a modified version of a previously described CXR congestion score.6 The original score is composed of six items including redistribution of lung vessels, enlarged cardiac silhouette and peribronchial cuffing (each scored with 0 to 1 points), pleural effusion and Kerley's lines (each scored with 0 to 2 points) and lung opacity (scored with 0 to 3 points). Thus, the maximal score would be 10 points.6 Given that PE is part of the original score, a modified score without the PE item was calculated with a maximum of eight points. This modified CXR congestion score is shown in Table S1. The CXR congestion score used for the analysis was the average of the ratings of the two radiologists. Thus, also half points were possible, for example, the score was 1.5 if radiologist A scored one point, and radiologist B scored two points. The rationale for the exclusion of the PE item from the score is to provide a rough measure of pulmonary congestion without a priori attributing PE a specific pathophysiological role.
Cardiac catheterization
Procedures were generally (>95%) performed in the morning in the fasting state and after withholding loop diuretics and renin-angiotensin system inhibitors. Patients underwent coronary angiography using five or six French catheters via the femoral or radial artery and right heart catheterization using six French Swan Ganz catheters via femoral or brachial access. The midthoracic level was used as zero reference point. Right atrial pressure, right ventricular pressure, pulmonary artery pressure, and pulmonary artery wedge pressure were measured. The wedge position was confirmed by fluoroscopy and waveform analysis. Measurements were obtained at end-expiration, the mean PAWP (mPAWP) was calculated over the entire cardiac cycle, and v waves were included to determine mPAWP. This practice leads to higher values compared with the measurement of the end-diastolic PAWP.7 However, for the estimation of the impact of the left heart contribution to pulmonary pressures and calculation of pulmonary vascular resistance (PVR) respectively, the mPAWP is preferred.8, 9 In patients with atrial fibrillation, at least five cardiac cycles were used to assess pulmonary artery pressure and pulmonary artery wedge pressure (sinus rhythm: usually three cycles). Cardiac output was assessed by the indirect Fick method based on blood gases, which were collected simultaneously and in duplicate from the arterial catheter and the pulmonary artery. After completion of right heart catheterization a coronary or a pigtail catheter was advanced into the ascending aorta for measurement of systolic, diastolic, and mean aortic pressure. In approximately 2/3 of the population, the aortic valve was crossed with a stiff wire, and the left ventricular end-diastolic pressure was measured using a pigtail catheter within a few minutes after the right heart catheter measurements and before coronary angiography. All pressure readings were double-checked by the operator by manual review of the pressure tracings before they were entered into the report and used for haemodynamic calculations respectively. The transpulmonary gradient was calculated as mean pulmonary artery pressure (mPAP) minus mPAWP, PVR was calculated as transpulmonary gradient divided by cardiac output, and pulmonary artery compliance was calculated as stroke volume divided by the differences of systolic and diastolic pulmonary artery pressure, where stroke volume is cardiac output divided by heart rate.
Echocardiography
All patients had an echocardiogram prior to cardiac catheterization as a basis for the referral. Echocardiograms were performed by experienced cardiologists according to contemporary guidelines but not according to a specific study protocol. All echocardiograms were retrospectively evaluated, and all data were manually extracted from the reports by a research fellow.
Laboratory analyses
A venous blood sample was obtained in all patients on the day prior to cardiac catheterization. Analysis was performed immediately after blood collection in the local biochemistry laboratory. Plasma albumin concentration was used as a marker of plasma oncotic pressure.
Follow-up
Patients underwent surgical (73%) or transcatheter (27%) AVR after a median (interquartile range) interval of 21 (12–35) days post-catheterization. Information on long-term follow-up was obtained by a research fellow from patients, general practitioners, and hospital or practice cardiologists. The clinical endpoint was all-cause mortality.
Statistical analysis
Categorical data are presented as numbers and percentages, and continuous data are reported as mean ± standard deviation or median (interquartile range; IQR) as appropriate. Clinical characteristics and echocardiographic and haemodynamic data in patients with bilateral PE, unilateral PE und no PE were compared using analysis of variance, Kruskal–Wallis tests, or χ2 tests as appropriate. Bonferroni post hoc tests for analysis of variance and Bonferroni corrections for the other analyses were applied. Survival rates of patients with bilateral PE, unilateral PE und no PE were compared using Kaplan–Meier plots and log-rank tests. Univariate and multivariate logistic regression was performed to identify predictors of any PE (vs. no PE) and bilateral PE (vs. no PE or unilateral PE). Univariate and multivariate Cox regression was applied to describe the time-dependent association between any PE and bilateral PE and mortality. A P-value <0.05 was considered statistically significant. Analyses were performed using SPSS statistical package version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
Study population
The mean age of the entire study population was 74 ± 10 years, and 273 (58%) were males. The mean indexed aortic valve area was 0.42 ± 0.12 cm2/m2, and the mean left ventricular ejection fraction (LVEF) was 58 ± 12%. Detailed clinical, echocardiographic, and haemodynamic characteristics of the entire study population are shown in Tables 1 and 2.
| All (n = 471) | Bilateral PE (n = 49) | Unilateral PE (n = 32) | No PE (n = 390) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 74 ± 10 | 79 ± 8* | 75 ± 12 | 73 ± 10 | <0.001 |
| Gender (male) | 273 (58%) | 26 (53%) | 22 (69%) | 225 (58%) | 0.36 |
| Body mass index (kg/m2) | 27.9 ± 5.2 | 26.9 ± 5.1 | 27.3 ± 4.3 | 28.1 ± 5.2 | 0.22 |
| eGFR (mL/min/1.73 m2) | 74 ± 30 | 56 ± 25* | 65 ± 27 | 77 ± 29 | <0.001 |
| Haemoglobin (g/L) | 135 ± 16 | 125 ± 21* | 133 ± 18 | 137 ± 15 | <0.001 |
| Albumin (g/L) | 39.2 ± 14.5 | 38.0 ± 3.2 | 37.9 ± 4.5 | 38.7 ± 5.1 | 0.44 |
| Diabetes | 98 (21%) | 11 (22%) | 6 (19%) | 81 (21%) | 0.92 |
| Stroke | 27 (6%) | 6 (12%) | 1 (3%) | 20 (5%) | 0.11 |
| Chronic obstructive lung disease | 52 (11%) | 9 (18%) | 7 (22%) | 36 (9%) | 0.02 |
| FEV1 (% predicted) | 87 ± 20 | 78 ± 19* | 76 ± 22# | 89 ± 19 | <0.001 |
| Heart rhythm | 0.02 | ||||
| Sinus rhythm | 408 (87%) | 38 (78%) | 23 (72%) | 347 (89%) | |
| Atrial fibrillation | 46 (10%) | 9 (18%) | 8 (25%) | 29 (7%) | |
| Pacemaker | 17 (3%) | 2 (4%) | 1 (3%) | 14 (4%) | |
| Heart rate (b.p.m.) | 69 ± 13 | 74 ± 14* | 70 ± 13 | 69 ± 12 | 0.01 |
| Medication | |||||
| Oral anticoagulation | 89 (19%) | 14 (29%) | 9 (28%) | 66 (17%) | 0.06 |
| Aspirin | 290 (62%) | 29 (59%) | 17 (53%) | 244 (63%) | 0.54 |
| Loop diuretics | 226 (48%) | 42 (86%)* | 21 (66%)# | 163 (42%) | <0.001 |
| Beta-blocker | 226 (48%) | 23 (47%) | 15 (47%) | 188 (48%) | 0.98 |
| ACEI/ARB | 260 (55%) | 21 (43%) | 19 (59%) | 220 (56%) | 0.18 |
| Digoxin | 28 (6%) | 11 (22%)* | 2 (6%) | 15 (4%) | <0.001 |
| Spironolactone | 23 (5%) | 5 (10%) | 1 (3%) | 17 (4%) | 0.18 |
| Modified CRX congestion score | 1 (0–2) | 3 (2–4.5)*§ | 2 (1–3) # | 1 (0–2) | <0.001 |
| Symptoms | |||||
| Dyspnoea NYHA class | * | # | <0.001 | ||
| I | 95 (20%) | 3 (5%) | 3 (9%) | 89 (23%) | |
| II | 230 (49%) | 12 (25%) | 16 (50%) | 202 (52%) | |
| III | 125 (27%) | 22 (45%) | 10 (32%) | 93 (24%) | |
| IV | 21 (4%) | 12 (25%) | 3 (9%) | 6 (1%) | |
| Mode of AVR | * | # | <0.001 | ||
| Surgical AVR | 343 (73%) | 21 (43%) | 16 (50%) | 306 (78%) | |
| Transcatheter AVR | 128 (27%) | 28 (57%) | 16 (50%) | 84 (22%) | |
- Data are given as numbers and percentages, mean ± standard deviation, or median (interquartile range).
- ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AVR, aortic valve replacement; CRX, chest radiography; eGFR, estimated glomerular filtration rate: FEV1, forced expiratory volume within the first second; NYHA, New York Heart Association.
- * P < 0.05 versus no PE; post hoc test.
- # P < 0.05 versus no PE; post hoc test.
- § P < 0.05 versus unilateral PE; post hoc test.
| All (n = 471) | Bilateral PE (n = 49) | Unilateral PE (n = 32) | No PE (n = 390) | P-value | |
|---|---|---|---|---|---|
| Echocardiography | |||||
| Left ventricular end-diastolic diameter (mm) | 48 ± 8 | 48 ± 10 | 47 ± 6 | 48 ± 8 | 0.90 |
| Indexed left ventricular end-diastolic diameter | 26 ± 4 | 265 | 25 ± 2 | 25 ± 4 | 0.30 |
| Left ventricular ejection fraction (%) | 58 ± 12 | 48 ± 16* | 55 ± 13 | 59 ± 11 | <0.001 |
| E/e′ | 16.8 ± 8.6 | 23.0 ± 10.5* | 17.5 ± 8.8 | 16.0 ± 8.0 | <0.001 |
| Left atrial area (cm2) | 25 ± 7 | 27 ± 5 | 28 ± 8 | 25 ± 7 | 0.15 |
| Indexed left atrial area (cm2/m2) | 13.4 ± 3.8 | 14.9 ± 3.4 | 14.5 ± 3.9 | 13.0 ± 3.8 | 0.05 |
| Tricuspid annular plane systolic excursion (mm) | 21 ± 5 | 20 ± 5 | 19 ± 6 | 22 ± 5 | 0.07 |
| Estimated sPAP (mmHg) | 39 ± 13 | 45 ± 12* | 47 ± 16# | 37 ± 12 | 0.001 |
| Mean aortic valve gradient (mmHg) | 47 ± 17 | 43 ± 18 | 44 ± 17 | 48 ± 17 | 0.09 |
| Aortic valve area (cm2) | 0.79 ± 0.24 | 0.74 ± 0.25 | 0.83 ± 0.29 | 0.80 ± 0.23 | 0.18 |
| Indexed aortic valve area (cm2/m2) | 0.42 ± 0.12 | 0.40 ± 0.13 | 0.44 ± 0.13 | 0.42 ± 0.12 | 0.38 |
| Mitral regurgitation | * | <0.001 | |||
| No | 224 (47%) | 8 (16%) | 12 (38%) | 204 (52%) | |
| Mild | 201 (43%) | 28 (57%) | 14 (44%) | 159 (41%) | |
| Moderate | 36 (8%) | 11 (23%) | 5 (15%) | 20 (5%) | |
| Severe | 10 (2%) | 2 (4%) | 1 (3%) | 7 (2%) | |
| Coronary angiography | 0.57 | ||||
| No coronary artery disease | 249 (53%) | 23 (47%) | 20 (63%) | 206 (53%) | |
| 1-vessel disease | 80 (17%) | 8 (17%) | 3 (9%) | 69 (18%) | |
| 2-vessel disease | 66 (14%) | 9 (18%) | 2 (6%) | 55 (14%) | |
| 3-vessel disease | 76 (16%) | 9 (18%) | 7 (22%) | 60 (15%) | |
| Invasive haemodynamics | |||||
| mRAP (mmHg) | 7 ± 4 | 8 ± 4* | 8 ± 5# | 6 ± 3 | <0.001 |
| Right ventricular end-diastolic pressure (mmHg) | 9 ± 4 | 10 ± 5* | 9 ± 4 | 8 ± 3 | <0.001 |
| sPAP (mmHg) | 39 ± 15 | 51 ± 15* | 46 ± 17# | 37 ± 13 | <0.001 |
| dPAP (mmHg) | 15 ± 7 | 21 ± 8* | 18 ± 8# | 14 ± 7 | <0.001 |
| mPAP (mmHg) | 25 ± 10 | 34 ± 10* | 30 ± 12# | 24 ± 9 | <0.001 |
| mPAWP (mmHg) | 16 ± 8 | 23 ± 8*§ | 18 ± 8# | 15 ± 7 | <0.001 |
| Transpulmonary gradient (mmHg) | 9 ± 5 | 11 ± 5* | 11 ± 6# | 9 ± 5 | 0.001 |
| Pulmonary vascular resistance (Wood units) | 2.1 ± 1.2 | 2.8 ± 1.3* | 2.7 ± 1.9# | 1.9 ± 1.3 | <0.001 |
| Pulmonary artery compliance (mL/mmHg) | 3.4 ± 1.8 | 2.0 ± 1.0* | 2.9 ± 1.6 | 3.6 ± 1.8 | <0.001 |
| Left ventricular end-diastolic pressure (mmHg), n = 327 | 21 ± 8 | 25 ± 8* | 21 ± 7 | 21 ± 8 | 0.02 |
| Systolic aortic pressure (mmHg) | 145 ± 25 | 135 ± 26* | 147 ± 31 | 146 ± 24 | 0.02 |
| Diastolic aortic pressure (mmHg) | 68 ± 12 | 64 ± 13* | 68 ± 10 | 69 ± 11 | 0.03 |
| Mean aortic pressure (mmHg) | 99 ± 14 | 92 ± 14* | 99 ± 14 | 99 ± 14 | 0.006 |
| Arterial oxygen saturation (%) | 95 (94–97) | 95 (92–97) | 94 (93–97) | 95 (94–97) | 0.24 |
| Mixed venous oxygen saturation (%) | 69 (64–72) | 62 (57–66)*§ | 65 (62–70) # | 69 (66–73) | <0.001 |
| Cardiac output (L/min) | 4.7 ± 1.0 | 3.9 ± 0.8*§ | 4.5 ± 1.0 | 4.8 ± 1.0 | <0.001 |
| Cardiac index (L/min/m2) | 2.5 ± 0.5 | 2.2 ± 0.4* | 2.4 ± 0.5 | 2.6 ± 0.5 | <0.001 |
| Stroke volume (mL) | 70 ± 19 | 54 ± 15*§ | 67 ± 20 | 73 ± 19 | <0.001 |
| Stroke volume index (mL/m2) | 37 ± 10 | 30 ± 8*§ | 36 ± 11 | 39 ± 9 | <0.001 |
- Data are given as numbers and percentages, mean ± standard deviation, and/or median (interquartile range).
- dPAP, diastolic pulmonary artery pressure; E/e′, ratio of peak early mitral inflow velocity to peak early mitral annular velocity; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure; mRAP, mean right atrial pressure; sPAP, systolic pulmonary artery pressure.
- * P < 0.05 versus no PE; post hoc test.
- # P < 0.05 versus no PE; post hoc test.
- § P < 0.05 versus unilateral PE; post hoc test.
Conventional chest radiography findings
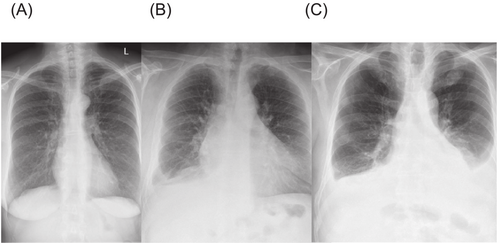
The median (IQR) interval between CXR and cardiac catheterization was 1 (1-1) days, that is, in the vast majority of patients the CXR was obtained on the day prior to cardiac catheterization. In only 87 patients (18%), the interval was more than 1 day. Among the 471 patients, 49 (10%) had bilateral PE, 32 (7%) had unilateral PE, and 390 (83%) had no PE. The median CXR congestion in the entire population was 1 (0–2) points. The score was highest in patients with bilateral PE, lowest in those without PE, and intermediate in those with unilateral PE, with the difference being statistically significant for all comparisons between the three groups (Table 1). In Figure 1, a CXR example from each patient category is shown.
Comparison of clinical characteristics of patients with bilateral pleural effusion versus unilateral pleural effusion versus no pleural effusion
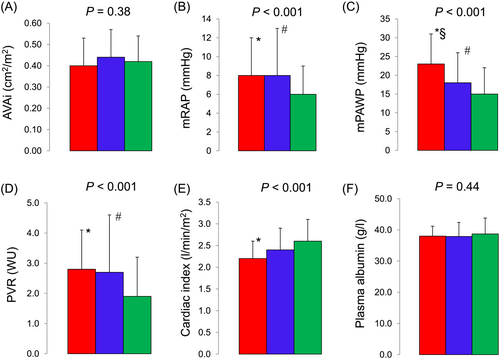
Patients with bilateral PE had the highest age, had the lowest estimated glomerular filtration rate, haemoglobin, and forced expiratory volume with the first second, and had the highest heart rate and the worst symptoms (Table 1). However, there was no difference in the proportion of patients with diabetes and plasma albumin concentrations (Table 1, Figure 2) across the three groups. Patients with bilateral PE were most likely to take digoxin and loop diuretics (Table 1).
Comparison of echocardiographic findings and invasive haemodynamics of patients with bilateral pleural effusion versus unilateral pleural effusion versus no pleural effusion
Patients with bilateral PE had the lowest LVEF and the most severe mitral regurgitation. In contrast, the severity of AS expressed as the indexed aortic valve area did not differ across the three groups. Patients with bilateral PE had the highest mean right atrial pressure, right ventricular end-diastolic pressure, mPAP, mPAWP, left ventricular end-diastolic pressure, and PVR, and they had the lowest pulmonary artery compliance, cardiac index, and stroke volume index. Notably, there was a statistically significant difference in mPAWP across all three groups, that is, mPAWP has higher in patients with bilateral PE compared with unilateral PE, and it was higher in bilateral and unilateral PE compared with no PE (Table 2, Figure 2).
Predictors of pleural effusion
Lower estimated glomerular filtration rate, lower haemoglobin, lower forced expiratory volume within the first second (expressed as percentage of the predicted value), lower cardiac index (68% lower likelihood of PE with every 1 L/min/m2 increase) and higher mPAWP (4% higher likelihood of PE with every 1 mmHg increase) were identified as independent predictors of any PE (Supplemental Table S2).
Lower haemoglobin, higher NYHA class, lower LVEF (4% lower likelihood of bilateral PE with every 1% increase), and higher mPAWP (7% higher likelihood of bilateral PE with every 1 mmHg increase) were independent predictors of bilateral PE (Supplemental Table S3).
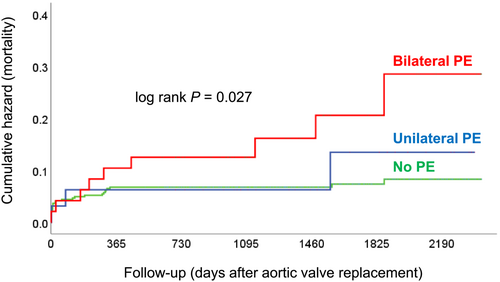
Prognostic impact of bilateral and unilateral pleural effusion
After a median post-AVR follow-up of 1361 (957–1878) days there were 40 (8%) deaths. Mortality was highest in patients with bilateral PE and lowest in those without PE (Figure 3). Mortality in patients with bilateral PE was nearly 2.7-fold higher compared with those without PE [hazard ratio 2.69 (95% confidence interval 1.27–5.70); P = 0.01]. In contrast, mortality did not significantly differ between patients with unilateral PE and no PE [hazard ratio 1.28 (95% confidence interval 0.39–4.20); P = 0.69]. Patients with any PE had an approximately two-fold mortality compared with patients without PE [hazard ratio 2.11 (95% confidence interval 1.07–4.14); P = 0.03]. Patients with bilateral PE had an approximately 2.6-fold higher mortality compared with patients with unilateral PE or no PE [hazard ratio 2.63 (95% confidence interval 1.25–5.53); P = 0.01]. In a multivariate analysis including only non-invasive parameters, chronic obstructive pulmonary disease, oral anticoagulation, and mitral regurgitation severity but not PE were independently associated with mortality (Supplemental Table S4).
Discussion
The present study systematically evaluating the association between PE and invasive haemodynamics in 471 patients with severe AS revealed the following key findings: First, the presence of PE, in particular bilateral PE, was a marker of an unfavourable haemodynamic constellation with high right-sided and left-sided filling pressures and low cardiac output. Second, bilateral PE was associated with more severe symptoms before AVR and increased mortality after AVR.
In accordance with two previous small studies4, 5 we found a significantly higher mPAWP in patients with PE (highest in those with bilateral PE), and mPAWP was identified as an independent predictor of any PE and bilateral PE in the multivariate analysis. In patients with AS, high mPAWP is the result of the interplay of left ventricular and left atrial dysfunction, functional mitral regurgitation, and in advanced cases atrial fibrillation, and represents the driver for post-capillary pulmonary hypertension.10 However, patients with PE also had higher PVR, right ventricular end-diastolic pressure and mean right atrial pressure, that is, already more advanced AS-related ‘cardiac damage’11, 12 with combined pre- and post-capillary pulmonary hypertension in many cases. The fluid balance in the pleural space depends not only on left atrial pressure but also on right atrial pressure because a higher right atrial pressure limits lymphatic drainage.13 Notably, there was no evidence of a difference in oncotic pressure between patients with and without PE, and therefore central haemodynamics were most likely the key drivers of pleural fluid formation in the present cohort (Figure 2). For a long time right ventricular dysfunction/failure and high PVR were thought to protect the lung from pulmonary congestion/edema.14 However, as recently shown in two seminal studies on the haemodynamic basis of pulmonary congestion in patients with heart failure with preserved ejection fraction and the pathophysiology of combined pre- and post-capillary pulmonary hypertension respectively right atrial pressure elevation contributes to pulmonary congestion.15, 16 The present study was performed in a cohort of severe AS patients. However, the proposed principles of the pathophysiology underlying PE may also be applicable to other heart failure syndromes, in particular heart failure with preserved ejection fraction, which apart from the disease of the aortic valve itself shares many features with the heart in AS. The findings from this large and relatively unique cohort may therefore contribute to a better understanding of the role of PE in patients with cardiac disease and may be clinically relevant also outside of the AS setting.
Although AS patients with PE received more intense medical therapy (i.e. diuretics and digoxin) and all patients underwent AVR, the finding of bilateral PE in the pre-AVR CXR was a marker of an increased long-term post-AVR mortality, which represents an intriguing finding. We cannot adjudicate the exact mechanism of PE in all patients. The vast majority of PE were small, and information on drainage and pleural fluid analysis is not available. There were no patients with advanced cancer in our cohort. Still we cannot exclude unrecognized cancer in some patients. The association between low haemoglobin and PE would be plausible in this context. On the other hand, the association between impaired renal function and mild anaemia may also be explained by advanced heart failure, and unilateral PE is well known to occur in certain heart failure patients.13 Furthermore, unilateral PE, which is the typical scenario in the context of malignancy, was not significantly associated with all-cause mortality, which we would expect if cancer would have been the driver of mortality. In contrast, bilateral PE, typically associated with heart failure, and associated with a particularly poor haemodynamic status in our patients, was associated with substantially increased mortality. This together with a lack of difference in plasma albumin between patients with and without PE and a gradual increase in the CXR congestion score from no PE to unilateral and bilateral PE suggests that in the present cohort, PE was mainly a marker of heart failure. A previous study had found an association between diabetes and PE,17 which we could not reproduce here. However, in our study, all patients had severe AS, and in this selected population the role of diabetes may be less important.
From a clinical point of view it is important to realize that PE is not a benign accidental finding but represents a marker of poor prognosis although PE was not an independent predictor of mortality in the multivariate analysis. In this analysis, PE was likely outperformed by (functional) mitral regurgitation, which typically is a marker of an advanced cardiac damage stage characterized by left ventricular and left atrial dysfunction, atrial fibrillation, left atrial hypertension, and pulmonary vascular disease.11 This most likely explains why mitral regurgitation rather than PE emerged as an independent predictor of mortality.
Guidelines on the diagnosis and management of heart failure recommend (class I) CXR to confirm heart failure and to assess differential diagnoses in patients with shortness of breath,18 while current valve disease guidelines do not provide a specific recommendation.19 The present data suggest that a close look at the standard CXR provides a window to the haemodynamic constellation in a patient with AS and even information on prognosis. We acknowledge that our observational study is hypothesis-generating only and does not allow clear recommendations for clinical practice. Still, it suggests that an increased awareness for PE in patients with AS is required. In clinical practice, not only CXR but also ultrasound or computed tomography (routinely performed in transcatheter AVR candidates) may be used for this purpose. Further research will be required to define how to implement knowledge on PE and cardiac disease patients in clinical practice.
Study limitations
The study has limitations. First, the number patients was relatively small for a prognostic study. However, patients were very well characterized including very detailed data on invasive haemodynamics. To the best of our knowledge, this study represents the by far largest study investigating the relationship between PE and haemodynamics. Second, there was an interval of approximately 1 day between CXR and cardiac catheterization. We acknowledge that this may not be optimal with regard to the assessment of the CXR congestion score. In contrast, PE formation occurs relatively slowly, and therefore, we consider this not a critical limitation. Third, to assess cardiac output, we have employed the indirect Fick method, which may be subject to error, as oxygen consumption is often inaccurately estimated.20 This likely affects all cardiac output-based measurements, including cardiac index and PVR. It must, however, be noted that this technique is routinely used in clinical practice.
Conclusions
In patients with severe AS, the presence of PE, particularly bilateral PE, is a marker of a poor haemodynamic constellation. Bilateral PE is associated with a substantially increased post-AVR mortality.
Acknowledgements
None.
Conflict of interest
None.
Funding
None.




