Prognostic impact of MitraScore in elderly Asian patients with heart failure: sub-analysis of FRAGILE-HF
Abstract
Aims
MitraScore is a novel, simple, and manually calculatable risk score developed as a prognostic model for patients undergoing transcatheter edge-to-edge repair (TEER) for mitral regurgitation. As its components are considered prognostic in heart failure (HF), we aimed to investigate the usefulness of the MitraScore in HF patients.
Methods and results
We calculated MitraScore for 1100 elderly patients (>65 years old) hospitalized for HF in the prospective multicentre FRAGILE-HF study and compared its prognostic ability with other simple risk scores. The primary endpoint was all-cause deaths, and the secondary endpoints were the composite of all-cause deaths and HF rehospitalization and cardiovascular deaths. Overall, the mean age of 1100 patients was 80 ± 8 years, and 58% were men. The mean MitraScore was 3.2 ± 1.4, with a median of 3 (interquartile range: 2–4). A total of 326 (29.6%), 571 (51.9%), and 203 (18.5%) patients were classified into low-, moderate-, and high-risk groups based on the MitraScore, respectively. During a follow-up of 2 years, 226 all-cause deaths, 478 composite endpoints, and 183 cardiovascular deaths were observed. MitraScore successfully stratified patients for all endpoints in the Kaplan–Meier analysis (P < 0.001 for all). In multivariate analyses, MitraScore was significantly associated with all endpoints after covariate adjustments [adjusted hazard ratio (HR) (95% confidence interval): 1.22 (1.10–1.36), P < 0.001 for all-cause deaths; adjusted HR 1.17 (1.09–1.26), P < 0.001 for combined endpoints; and adjusted HR 1.24 (1.10–1.39), P < 0.001 for cardiovascular deaths]. The Hosmer–Lemeshow plot showed good calibration for all endpoints. The net reclassification improvement (NRI) analyses revealed that the MitraScore performed significantly better than other manually calculatable risk scores of HF: the GWTG-HF risk score, the BIOSTAT compact model, the AHEAD score, the AHEAD-U score, and the HANBAH score for all-cause and cardiovascular deaths, with respective continuous NRIs of 0.20, 0.22, 0.39, 0.39, and 0.29 for all-cause mortality (all P-values < 0.01) and 0.20, 0.22, 0.42, 0.40, and 0.29 for cardiovascular mortality (all P-values < 0.02).
Conclusions
MitraScore developed for patients undergoing TEER also showed strong discriminative power in HF patients. MitraScore was superior to other manually calculable simple risk scores and might be a good choice for risk assessment in clinical practice for patients receiving TEER and those with HF.
Introduction
The prevalence of heart failure (HF) in developed countries is ~2% of the adult population, with approximately 6 million people in the United States suffering from HF.1, 2 Although drug therapy has improved HF prognosis, approximately one in four patients will die from HF within 1 year, and one in two patients will die within 5 years.3, 4 Post-discharge readmission rate also remained significant, with approximately 24% of the patients readmitted within 30 days. Therefore, accurate risk stratification is imperative for the development of post-discharge treatment strategies.
MitraScore is a novel simple risk score developed for patients undergoing transcatheter edge-to-edge repair (TEER) for mitral regurgitation (MR).5 Although there are many scores reported for patients receiving TEER,6-8 MitraScore is easy to use in real-world clinical practice due to its simplicity. As a simple sum of eight criteria, it can be easily calculated at hand. Although the MitraScore was intended to predict mortality after TEER, it did not include any mitral valve-specific parameters, probably because the regurgitation itself was resolved by TEER. As a result, each component of this score is a parameter that has also been considered important in HF regardless of valvular heart disease. Therefore, we hypothesized that MitraScore may be broadly applicable to HF patients.
This study investigated the prognostic usefulness of the MitraScore in HF patients independent of valvular heat disease. Furthermore, we compared the performance of MitraScore to those of other simple risk scores, such as the GWTG-HF risk score,9 the BIOSTAT compact model,10 the AHEAD score,11 the AHEAD-U score,12 and the HANBAH score.13 Although the Seattle Heart Failure Model,14 the MAGGIC Risk Score,15 or EFFECT16 have excellent prognostic power, they are complicated and difficult to use in daily clinical practice.
Methods
Study subjects
We conducted a post hoc analysis of the FRAGILE-HF cohort study (prevalence and prognostic value of physical and social frailty in geriatric patients hospitalized for HF). This study enrolled 1332 patients hospitalized for decompensated HF, who were at least 65 years old and ambulatory at discharge, from 15 hospitals in Japan between September 2016 and March 2018. The design, population, and main results of this study were previously published elsewhere.17-19 The objective of the FRAGILE-HF study was to assess the prevalence and prognostic impact of multidomain frailty in older HF patients requiring hospitalization. HF decompensation was diagnosed based on the Framingham criteria.20 In this analysis, we included cases in which all predictive models could be used to compare multiple prognostic models. Exclusion criteria included previous cardiac transplantation or left ventricular assist device therapy, chronic peritoneal dialysis or haemodialysis, and acute myocarditis. Patients with no data on B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) and patients with BNP levels < 100 pg/mL or NT-proBNP levels < 300 pg/mL on admission were excluded because their diagnosis could be inappropriate on admission.
The study was conducted in compliance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. As this was an observational study without invasive interventions, written informed consent was not required. The research protocol was approved by the Ethics Committee of each participating hospital. Study information was made publicly available on the University Hospital Medical Information Network (UMIN-CTR, unique identifier: UMIN000023929) before the initial patient enrolment.
Data collection
Echocardiograms, blood samples, and medication information were obtained under stable conditions just before discharge. Although BNP and NT-proBNP values were not used for MitraScore calculation, BNP was converted to NT-proBNP using a previously reported conversion formula.21 MitraScore was calculated as previously reported.5 One point was given for each of the following criteria: age (>75 years), left ventricular ejection fraction (LVEF) < 40%, anaemia (<13.0 g/L for men and <12.0 g/L for women), renal dysfunction (estimated glomerular filtration rate < 60 mL/min/1.73 m2), peripheral artery disease, chronic obstructive pulmonary disease (COPD), high-dose diuretics (≥80 mg of furosemide/day or use of ≥2 diuretic agents excluding antialdosteronic drugs in accordance with previous reports5), and no therapy with renin angiotensin system (RAS) inhibitors. Patients were classified into three groups (0–2 points, low-risk group; 3–4 points, moderate-risk group; and 5–8 points, high-risk group) according to MitraScore. The GWTG-HF risk score was calculated according to a previous report.9 Calculation methods for other scores, including the BIOSTAT compact model, the AHEAD and AHEAD-U scores, and the HANBAH score, were summarized in Supporting Information, Table S1. Supporting Information, Table S2 showed the comparison of the variables in each score.
Study endpoints
Prospective data on the prognosis of the enrolled patients within 2 years of discharge were collected in March 2020. The primary endpoint was all-cause death, and the secondary endpoints were the composite of all-cause death and rehospitalization for HF readmission and cardiovascular death. Readmission events were defined as HF readmission only if they satisfied HF readmission criteria, as described in the American College of Cardiology/American Heart Association Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials.22 After discharge, patients were followed up in an outpatient clinic. For patients who were not followed up at the clinic, prognostic data were obtained through telephone interviews with medical record personnel and family members at other medical facilities.
Statistical analysis
Categorical and continuous variables were presented as numbers (percentages) and means (standard deviations) or medians [interquartile ranges (IQRs)], respectively. Group differences in baseline characteristics were examined using one-way analysis of variance, the Kruskal–Wallis test, or χ2 test.
To investigate the independent association of MitraScore with outcomes, multivariable Cox regression models were constructed, adjusting for variables selected based on previous studies according to their clinical importance, excluding those included in MitraScore: sex, current smoking status, history of HF (de novo case or not), hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation, systolic blood pressure, serum sodium level, serum albumin, log-transformed BNP, beta-blocker, mineralocorticoid receptor antagonist, and New York Heart Association class III/IV at discharge.
Logistic regression models were also constructed to assess the predictive ability of the model for primary and secondary outcomes at 2 years. Additionally, the Hosmer–Lemeshow test was used to validate the model fit to the 2 year event. Kaplan–Meier curves were drawn to examine the discriminative ability of MitraScore stratification, and log-rank tests and pairwise comparison using log-rank tests were used to assess differences in survival curves. Receiver operating characteristic (ROC) curves were drawn, the areas under the curve (AUCs) were measured for each endpoint, and the AUC of MitraScore was compared with those of other risk scores. We computed the net reclassification improvement (NRI) to compare MitraScore with other risk scores. All statistical analyses were performed using the R statistical software (Version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided P < 0.05.
Results
Patient characteristics
We enrolled 1332 HF patients admitted to 15 hospitals between September 2016 and March 2019. This population was previously described elsewhere.17 MitraScore was available for 1315 patients. Of these, 1100 had GWTG-HF risk score, BIOSTAT compact model, AHEAD score, AHEAD-U score, and HANBAH score, which were included in the analysis.
Baseline characteristics of the participants stratified by MitraScore are shown in Table 1. Overall, the mean patient age was 80 ± 8 years, and 58% (n = 637) were men. The mean LVEF was 46 ± 17%, and HF with reduced ejection fraction (HFrEF) (LVEF < 40%) and HF with preserved ejection fraction (HFpEF) (LVEF ≥ 50%) were 41% (n = 454) and 42% (n = 467), respectively. Atrial fibrillation and ischaemic heart disease represented 45% (n = 492) and 45% (n = 386) of the patient cohort, respectively. The average MitraScore was 3.2 ± 1.4, with a median of 3 (IQR: 2–4) (Supporting Information, Figure S3). A total of 326 (29.6%), 571 (51.9%), and 203 (18.5%) patients were classified into low-, moderate-, and high-risk groups, respectively. The high-risk group had higher rates of HFrEF, coronary artery disease, and COPD than other groups did. There were no significant differences among the three groups in terms of atrial fibrillation and diabetes mellitus. Laboratory data showed that the high-risk group had lower haemoglobin, serum albumin levels, and glomerular filtration rate and higher uric acid and NT-proBNP levels (Table 1).
| MitraScore | |||||
|---|---|---|---|---|---|
| Baseline characteristics | Overalla | 0–2a | 3–4a | 5–8a | P-valueb |
| N | 1100 | 326 | 571 | 203 | |
| Age, years | 80 ± 8 | 76 ± 8 | 82 ± 7 | 82 ± 6 | <0.001 |
| Male, n (%) | 637 (58%) | 185 (57%) | 312 (55%) | 140 (69%) | 0.002 |
| Female, n (%) | 463 (42%) | 141 (43%) | 259 (45%) | 63 (31%) | |
| NYHA class III/IV (%) | 161 (15%) | 34 (10%) | 94 (16%) | 33 (16%) | 0.037 |
| Height, cm | 156 ± 10 | 157 ± 10 | 155 ± 10 | 157 ± 10 | 0.006 |
| Weight, kg | 52 ± 11 | 55 ± 12 | 51 ± 10 | 51 ± 10 | <0.001 |
| Body mass index, kg/m2 | 21.3 ± 3.7 | 22.2 ± 4.0 | 21.1 ± 3.6 | 20.4 ± 3.1 | <0.001 |
| Systolic BP, mmHg | 113 ± 17 | 114 ± 18 | 114 ± 17 | 110 ± 16 | 0.006 |
| Diastolic BP, mmHg | 62 ± 11 | 64 ± 11 | 62 ± 11 | 61 ± 10 | 0.004 |
| Heart rate, b.p.m. | 71 ± 14 | 72 ± 14 | 70 ± 15 | 72 ± 12 | 0.039 |
| Past medical history | |||||
| Atrial fibrillation, (%) | 492 (45%) | 148 (45%) | 253 (44%) | 91 (45%) | 0.951 |
| Coronary artery disease, n (%) | 386 (35%) | 71 (22%) | 209 (37%) | 106 (52%) | <0.001 |
| Peripheral artery disease, n (%) | 88 (8.0%) | 5 (1.5%) | 40 (7.0%) | 43 (21%) | <0.001 |
| COPD, n (%) | 110 (10%) | 15 (4.6%) | 44 (7.7%) | 51 (25%) | <0.001 |
| Diabetes mellitus, n (%) | 405 (37%) | 119 (37%) | 206 (36%) | 80 (39%) | 0.693 |
| Hypertension, n (%) | 787 (72%) | 220 (67%) | 418 (73%) | 149 (73%) | 0.137 |
| Current smoking, (%) | 116 (11%) | 54 (17%) | 49 (8.6%) | 13 (6.4%) | <0.001 |
| Prior heart failure admission, n (%) | 451 (41%) | 85 (26%) | 237 (42%) | 129 (64%) | <0.001 |
| Prescription at discharge | |||||
| ACE-I/ARB, n (%) | 753 (68%) | 288 (88%) | 386 (68%) | 79 (39%) | <0.001 |
| Beta-blocker, n (%) | 819 (74%) | 261 (80%) | 403 (71%) | 155 (76%) | 0.006 |
| MRA, n (%) | 542 (49%) | 183 (56%) | 260 (46%) | 99 (49%) | 0.009 |
| Furosemide, n (%) | 610 (55%) | 169 (52%) | 313 (55%) | 128 (63%) | 0.038 |
| Azosemide, n (%) | 396 (36%) | 88 (27%) | 206 (36%) | 102 (50%) | <0.001 |
| Thiazides, n (%) | 59 (5.4%) | 6 (1.8%) | 30 (5.3%) | 23 (11%) | <0.001 |
| Torasemide, n (%) | 50 (4.5%) | 9 (2.8%) | 23 (4.0%) | 18 (8.9%) | 0.003 |
| Tolvaptan, n (%) | 265 (24%) | 22 (6.7%) | 126 (22%) | 117 (58%) | <0.001 |
| ≥80 mg of furosemide/day, n (%) | 52 (4.7%) | 2 (0.6%) | 23 (4.0%) | 27 (13%) | <0.001 |
| Use of ≥2 diuretic agents, n (%) | 336 (31%) | 23 (7.1%) | 161 (28%) | 152 (75%) | <0.001 |
| Laboratory data at discharge | |||||
| Haemoglobin, g/dL | 11.82 ± 2.03 | 13.02 ± 1.97 | 11.50 ± 1.90 | 10.79 ± 1.52 | <0.001 |
| Albumin, g/dL | 3.44 ± 0.48 | 3.58 ± 0.49 | 3.40 ± 0.47 | 3.36 ± 0.48 | <0.001 |
| Creatinine, mg/dL | 1.38 ± 0.82 | 1.02 ± 0.48 | 1.41 ± 0.81 | 1.87 ± 1.01 | <0.001 |
| eGFR, mL/min/1.73 m2 | 53 ± 22 | 69 ± 18 | 49 ± 20 | 37 ± 16 | <0.001 |
| BUN, mg/dL | 30 ± 14 | 23 ± 9 | 31 ± 14 | 37 ± 17 | <0.001 |
| Sodium, mEq/L | 139 ± 4 | 139 ± 4 | 139 ± 4 | 139 ± 4 | 0.252 |
| HbA1c | 6.25 ± 0.92 | 6.39 ± 1.08 | 6.19 ± 0.84 | 6.21 ± 0.85 | 0.007 |
| HDL, mg/dL | 48 ± 14 | 49 ± 15 | 49 ± 14 | 47 ± 13 | 0.127 |
| LDL, mg/dL | 92 ± 31 | 98 ± 29 | 91 ± 32 | 86 ± 29 | <0.001 |
| Triglyceride, mg/dL | 87 [65–115] | 91 [66–119] | 86 [64–113] | 86 [67–111] | 0.152 |
| Uric acid, mg/dL | 7.14 ± 2.08 | 6.64 ± 1.82 | 7.23 ± 2.01 | 7.66 ± 2.46 | <0.001 |
| BNP, pg/mL | 272 [139–499] | 206 [109–398] | 274 [138–471] | 405 [203–668] | <0.001 |
| NT-proBNP, pg/mL | 1098 [516–2299] | 708 [383–1291] | 1157 [579–2400] | 2223 [976–4801] | <0.001 |
| Echocardiographic parameter | |||||
| LVEF, % | 46 ± 17 | 48 ± 16 | 47 ± 16 | 38 ± 16 | <0.001 |
| Heart failure phenotypes | <0.001 | ||||
| HFrEF | 454 (41%) | 99 (30%) | 214 (37%) | 141 (69%) | |
| HFmrEF | 179 (16%) | 75 (23%) | 85 (15%) | 19 (9.4%) | |
| HFpEF | 467 (42%) | 152 (47%) | 272 (48%) | 43 (21%) | |
| Mitral regurgitation | <0.001 | ||||
| Moderate | 316 (29%) | 87 (27%) | 165 (29%) | 64 (32%) | |
| Severe | 82 (7.5%) | 19 (5.8%) | 38 (6.7%) | 25 (12%) | |
| Aortic stenosis | 0.002 | ||||
| Moderate | 69 (6.3%) | 12 (3.7%) | 41 (7.2%) | 16 (7.9%) | |
| Severe | 61 (5.5%) | 7 (2.1%) | 40 (7.0%) | 14 (6.9%) | |
| Risk scores | |||||
| MitraScore | 3.23 ± 1.41 | 1.55 ± 0.59 | 3.45 ± 0.50 | 5.34 ± 0.60 | <0.001 |
| GWTG-HF risk score | 44.8 ± 5.5 | 42.4 ± 5.4 | 45.1 ± 5.2 | 47.5 ± 5.2 | <0.001 |
| AHEAD score | 2.61 ± 1.08 | 1.90 ± 0.96 | 2.77 ± 0.98 | 3.32 ± 0.90 | <0.001 |
| AHEAD-U score | 2.83 ± 1.20 | 2.04 ± 1.00 | 3.01 ± 1.11 | 3.58 ± 1.01 | <0.001 |
| BIOSTAT risk score | 2.15 ± 1.14 | 1.36 ± 0.95 | 2.36 ± 1.03 | 2.84 ± 1.01 | <0.001 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
- a n; mean ± standard deviation; median [interquartile range]; n (%).
- b One-way analysis of variance; the Kruskal–Wallis rank sum test; Pearson's χ2 test.
Primary outcome
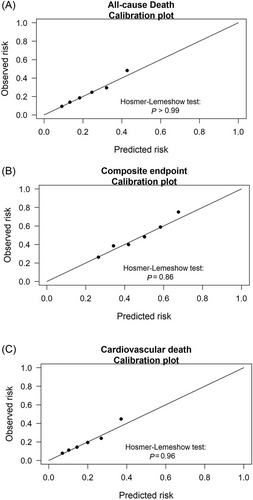
During a follow-up of 2 years, the primary endpoint of all-cause mortality was observed in 226 patients. The event rate increased in accordance with higher MitraScore scores (Supporting Information, Figure S4A). Figure 1 shows the Kaplan–Meier survival curves for the primary endpoint stratified by MitraScore. The log-rank test demonstrated a statistically significant difference in the survival curves of three MitraScore groups (P < 0.001). Univariate Cox proportional hazards analysis revealed that MitraScore was a significant predictor of the primary endpoint [hazard ratio (HR) per point increase: 1.39, 95% confidence interval (CI): 1.27–1.53, P < 0.001]. In multivariate analysis, MitraScore remained an independent predictor of the primary endpoint (adjusted HR: 1.22, 95% CI: 1.10–1.36, P < 0.001) after adjustment for the aforementioned variables (Table 2). The Hosmer–Lemeshow test results are shown in Figure 2, suggesting good calibration of the risk scores (P > 0.99).

| All-cause death | ||||||
|---|---|---|---|---|---|---|
| Groups | Unadjusted | Adjusteda | ||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Low-risk | 1 (reference) | 1 (reference) | ||||
| Moderate-risk | 1.96 | 1.36–2.82 | <0.001 | 1.52 | 1.02–2.25 | 0.039 |
| High-risk | 3.47 | 2.33–5.15 | <0.001 | 2.06 | 1.33–3.20 | 0.001 |
| MitraScore (continuous) | 1.39 | 1.27–1.53 | <0.001 | 1.22 | 1.10–1.36 | <0.001 |
| Composite endpoint | ||||||
|---|---|---|---|---|---|---|
| Groups | Unadjusted | Adjusteda | ||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Low-risk | 1 (reference) | 1 (reference) | ||||
| Moderate-risk | 1.46 | 1.16–1.83 | 0.001 | 1.22 | 0.95–1.56 | 0.13 |
| High-risk | 2.53 | 1.95–3.27 | <0.001 | 1.72 | 1.29–2.31 | <0.001 |
| MitraScore (continuous) | 1.30 | 1.21–1.38 | <0.001 | 1.17 | 1.09–1.26 | <0.001 |
| Cardiovascular death | ||||||
|---|---|---|---|---|---|---|
| Groups | Unadjusted | Adjusteda | ||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Low-risk | 1 (reference) | |||||
| Moderate-risk | 1.87 | 1.25–2.81 | 0.003 | 1.35 | 0.87–2.09 | 0.18 |
| High-risk | 3.63 | 2.35–5.61 | <0.001 | 1.96 | 1.21–3.18 | 0.006 |
| MitraScore (continuous) | 1.43 | 1.29–1.58 | <0.001 | 1.24 | 1.10–1.39 | <0.001 |
- Bold values indicate P < 0.05.
- CI, confidence interval; HR, hazard ratio.
- a Adjusted for sex, current smoking status, history of heart failure (de novo case or not), hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation, systolic blood pressure, serum sodium level, serum albumin, log-transformed B-type natriuretic peptide, beta-blocker, mineralocorticoid receptor antagonist, and New York Heart Association class III/IV at discharge.
Secondary outcomes
The composite endpoint of HF rehospitalization and all-cause mortality was observed in 478 patients. The event rate increased in accordance with higher MitraScore scores (Supporting Information, Figure S4B). Figure 1 shows the Kaplan–Meier survival curves for the composite endpoint stratified by MitraScore, with higher scores indicating greater event rates. In both univariate and multivariate Cox analyses, the association between MitraScore and the composite endpoint was significant (HR for the univariate analysis: 1.30, 95% CI: 1.21–1.38, P < 0.001; adjusted HR: 1.17, 95% CI: 1.09–1.26, P < 0.001) (Table 2). The Hosmer–Lemeshow test results showed good calibration of the score with P = 0.86 for the 2 year composite endpoint (Figure 2).
Cardiovascular death occurred in 183 patients. The event rate increased in accordance with higher MitraScore scores (Supporting Information, Figure S4C). Figure 1 shows the Kaplan–Meier survival curve for cardiovascular death stratified by MitraScore, with lower survival in the high-risk group. In both univariate and multivariate Cox analyses, the association between MitraScore and cardiovascular death was significant (HR for the univariate analysis: 1.43, 95% CI: 1.29–1.58, P < 0.001; adjusted HR: 1.24, 95% CI: 1.10–1.39, P < 0.001) (Table 2). The Hosmer–Lemeshow test results showed good calibration of the score with P = 0.96 for cardiovascular death (Figure 2).
Subgroup analyses: left ventricular ejection fraction subgroup and valvular heart diseases
We further investigated the prognostic ability of the MitraScore in patients with HFrEF (LVEF < 40%), HF with mildly reduced ejection fraction (HFmrEF) (LVEF ≥ 40% and LVEF < 50%), and HFpEF (LVEF ≥ 50%). Supporting Information, Tables S5–S7 show the results of univariate and multivariate Cox analyses of MitraScore for the HFrEF, HFmrEF, and HFpEF subgroups, respectively. For HFrEF, MitraScore was significantly correlated with all endpoints (adjusted HR: 1.22, 95% CI: 1.04–1.44, P = 0.014 for all-cause mortality; adjusted HR: 1.20, 95% CI: 1.07–1.35, P = 0.002 for composite endpoint; and adjusted HR: 1.27, 95% CI: 1.06–1.52, P = 0.011 for cardiovascular death). Similarly, for HFpEF, significant correlations were observed for all endpoints (adjusted HR: 1.27, 95% CI: 1.05–1.54, P = 0.013 for all-cause mortality; adjusted HR: 1.22, 95% CI: 1.08–1.39, P = 0.002 for composite endpoint; and adjusted HR: 1.32, 95% CI: 1.07–1.63, P = 0.009 for cardiovascular death). On the other hand, for HFmrEF, MitraScore was not correlated with all endpoints.
Additionally, we tested whether the results were similar, regardless of valvular heart disease. There were 162 patients with severe valvular heart disease (61, 82, and 19 for aortic stenosis, aortic regurgitation, and MR, respectively) whose prognosis might be dramatically changed by valve-related surgical interventions. However, the results were similar when these patients were excluded (Supporting Information, Table S8).
Comparison with other scores
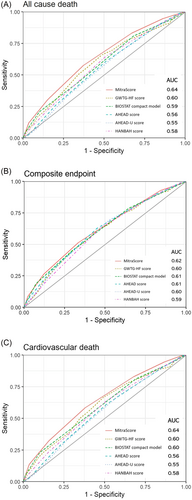
The prognostic performance of MitraScore and other risk scores was compared using ROC curves and AUC (Figure 3), Akaike information criterion (AIC), and NRI for all-cause mortality, composite endpoint, and cardiovascular death at 2 years. For the primary endpoint, MitraScore consistently had a larger AUC and smaller AIC than the BIOSTAT compact model and the AHEAD, AHEAD-U, and HANBAH scores. NRI results showed that MitraScore improved the reclassification of these scores (Table 3).
| (A) All-cause death | ||||||
|---|---|---|---|---|---|---|
| MitraScore | GWTG | BIOSTAT | AHEAD | AHEAD-U | HANBAH | |
| AUC | 0.64 | 0.60 | 0.59 | 0.56 | 0.55 | 0.58 |
| P-value | 0.1113 | 0.0209 | <0.001 | <0.001 | 0.0071 | |
| AIC | 1065.4 | 1092.4 | 1092.5 | 1105.1 | 1108.4 | 1096.9 |
| cNRI | 0.1993 | 0.2218 | 0.3927 | 0.3921 | 0.2913 | |
| P-value | 0.0075 | 0.0027 | <0.001 | <0.001 | <0.001 | |
| (B) Composite endpoint | ||||||
|---|---|---|---|---|---|---|
| MitraScore | GWTG | BIOSTAT | AHEAD | AHEAD-U | HANBAH | |
| AUC | 0.62 | 0.60 | 0.61 | 0.61 | 0.60 | 0.59 |
| P-value | 0.2683 | 0.5407 | 0.5341 | 0.2883 | 0.0999 | |
| AIC | 1435.4 | 1458.0 | 1444.0 | 1444.8 | 1454.4 | 1458.6 |
| cNRI | 0.2039 | −0.0105 | 0.0919 | 0.1298 | 0.1409 | |
| P-value | <0.001 | 0.8627 | 0.1328 | 0.0331 | 0.0204 | |
| (C) Cardiovascular death | ||||||
|---|---|---|---|---|---|---|
| MitraScore | GWTG | BIOSTAT | AHEAD | AHEAD-U | HANBAH | |
| AUC | 0.64 | 0.60 | 0.60 | 0.56 | 0.55 | 0.58 |
| P-value | 0.1589 | 0.0761 | <0.001 | <0.001 | 0.0098 | |
| AIC | 944.1 | 968.1 | 965.3 | 981.5 | 983.5 | 974.4 |
| cNRI | 0.2032 | 0.2215 | 0.4162 | 0.4043 | 0.2939 | |
| P-value | 0.0119 | 0.0058 | <0.001 | <0.001 | <0.001 | |
- Bold values indicate P < 0.05.
- AIC, Akaike information criterion; AUC, area under the curve; cNRI, continuous net reclassification improvement.
For the composite endpoint, MitraScore consistently showed numerically larger AUCs and smaller AICs; however, the differences were not significant in terms of discriminatory power. MitraScore showed reclassification improvement from the GWTG-HF risk score, the AHEAD-U score, and the HANBAH score (Table 3).
For cardiovascular death, MitraScore also showed better discrimination for cardiovascular death than the AHEAD, AHEAD-U, and HANBAH scores. MitraScore tended to be larger than that of the BIOSTAT compact model, although the AUC was not significant. MitraScore showed a significant improvement in reclassification as compared with all other scores (Table 3).
Discussion
This study is the first external validation of MitraScore in an HF cohort, showing that a high MitraScore was strongly associated with adverse outcomes, including all-cause mortality. Furthermore, MitraScore was as good as or better than several other manually calculable prognostic models for HF.
Applicability of MitraScore for transcatheter edge-to-edge repair to heart failure patients
MitraScore is a simple score that can be calculated from several clinical, historical, and echocardiographic parameters. It was developed using backward stepwise regression analysis based on data from 1109 patients enrolled in the international MIVNUT registry, a European and Canadian registry investigating the correlation between nutritional status and prognosis in TEER patients.23 Interestingly, despite the reported correlation of post-operative residual MR with worse prognosis in TEER patients,24, 25 this score, developed for TEER patients, does not include a parameter regarding MR. This might be explained by the high procedural success rate of 94.2% for TEER in the development cohort, which diminished the prognostic effect of MR in most patients.23 Once MR is resolved, it is not surprising that TEER patients share common comorbidities and risk factors because those receiving TEER are elderly, sick, and have more comorbidities, all of which also characterize HF patients. Indeed, HF risk factors were reportedly useful in patients undergoing TEER.26, 27 The excellent performance of MitraScore in HF patients further supported this hypothesis.
Superiority of MitraScore
In this study, MitraScore was even better than other risk scores, including the well-established, yet complicated, GWTG-HF risk score. Considering the components of the score (Supporting Information, Tables S1 and S2), MitraScore has several unique parameters that are not included in other scores. Particularly, the prescription of high-dose diuretics and RAS inhibitors may play an important role in HF patients as these drugs have been repeatedly reported as important prognostic factors in HF patients.28-30 The group with a higher MitraScore had a lower proportion of medications for HF, represented by RAS inhibitors, despite the fact that more patients had impaired cardiac function. The high-risk group may have been unable to optimize drug therapy for HF because of their older age, smaller body size, and impaired renal function. The use of estimated glomerular filtration rate instead of creatinine level for kidney function might be also important in elderly patients. As creatinine level depends on the patient's age, sex, and muscle mass,31, 32 it may not accurately represent kidney function and risk in a wide range of patients. Patients with high MitraScore had smaller body mass index. This might be due to the fact that components of MitraScore, such as old age, anaemia, and COPD, are frequently associated with sarcopenia and a lower body mass index.
Clinical usefulness
One of the strengths of MitraScore is its simplicity. Each parameter is easily ascertained in HF patients and has the same weight, making it manually calculable and easy to apply in clinical practice. The GRASP score7 and the COAPT score,6 prognostic models for post-operative TEER, have been externally validated. However, the GRASP score is difficult to calculate without a dedicated application, and the COAPT score contains TEER-specific parameters that cannot be adopted for patients with HF. The MITRALITY score8 is a machine-learning model with excellent prognostic power, but it requires a dedicated application. Despite its simplicity, MitraScore outperformed other scores. As mentioned above in the introduction, although risk stratification for HF patients is of great importance, these risk scores are underused in clinical practice and complexity is a major barrier. Learning about multiple risk factors and calculating them for each disease are not practical for busy clinicians. Furthermore, there are many risk factors for each disease and condition, such as the GWTG-HF risk score for in-hospital mortality in acute HF and the MAGGIC risk score15 for HF hospitalization in chronic HF. In our results, the simple MitraScore was applicable not only in patients receiving TEER but also in HF patients in multiple endpoints, including all-cause mortality, composite outcomes, and cardiovascular deaths. Therefore, MitraScore might reduce the cost of learning multiple and complicated risk factors while maintaining a high risk-prediction performance. Machine-learning approaches might provide promising predictive ability, and online applications will provide access to these predictive models. However, in reality, people do not frequently use such online calculators for acute HF, although they exist. Even though these tools can simplify the scoring process, there are still some barriers to clinical implementation: not all healthcare providers have access to the internet in their office, these applications require installation and configuration on computers or smartphones, using these applications generally takes longer than manual calculation, and the black-box nature of machine-learning models prevents interpretation of the model outputs. As a result, especially in the risk management of patients with acute illnesses including acute HF, manually calculable risk scores still play an important role. Future studies comparing these machine-based complex models and simple manual risk scores in real-world settings are warranted.
Limitation
This study had several limitations. First, it was conducted in a cohort of elderly (>65 years old) Asian patients. MitraScore does not include race as a component, whereas other risk scores include it. The performance of such scores with the inclusion of race might be underestimated in our analysis, although it does not influence the validity of MitraScore. Further investigations should be performed to confirm these findings in different patient populations. NT-proBNP was frequently missing from our dataset because BNP was used. BNP values were converted to NT-proBNP using a previously reported formula that estimates NT-proBNP values from BNP, age, body mass index, haemoglobin, and creatinine clearance.21 As high correlations have been reported in the validation cohort and BNP/NT-proBNP is not used to calculate MitraScore, we do not think this approach will significantly affect the results of this study. In this study, characteristics of patients with high and low MitraScore were significantly different across a number of parameters, and there might be observed or non-observed confounders that were not considered in the multivariable models.
Conclusions
In conclusion, MitraScore was validated to predict all-cause mortality, a composite of death and HF hospitalization, and cardiovascular death in HF patients, regardless of presence of MR. MitraScore was superior to other manually calculable simple risk scores and might be a good choice for risk assessment in clinical practice for patients receiving TEER and those with HF.
Conflict of interest
Dr N.K. received an honorarium from Otsuka Pharmaceutical Co. and Novartis Japan and received research grants from EchoNous Inc. and AMI Inc. for work outside this study. Dr N.K. and Dr Ta.K. are affiliated with a department funded by Philips Healthcare, Asahi KASEI Corporation, Inter Reha Co., Ltd., and Toho Holdings Co., Ltd. based on collaborative research agreements. Dr Y.M. received an honorarium from Otsuka Pharmaceutical Co., Novartis Japan, AstraZeneca Japan, and Bayer Japan and a collaborative research grant from Pfizer Inc. and Nippon Boehringer Ingelheim Co., Ltd.
Funding
This study was partly supported by Japan Society for the Promotion of Science KAKENHI, grant number 21K18086. FRAGILE-HF was supported by Novartis Pharma Research Grants and a Japan Heart Foundation Research Grant.




