Role of vericiguat in management of patients with heart failure with reduced ejection fraction after worsening episode
Abstract
Worsening heart failure (HF) is a vulnerable period in which the patient has a markedly high risk of death or HF hospitalization (up to 10% and 30%, respectively, within the first weeks after episode). The prognosis of HF patients can be improved through a comprehensive approach that considers the different neurohormonal systems, with the early introduction and optimization of the quadruple therapy with sacubitril–valsartan, beta-blockers, mineralocorticoid receptor antagonists, and inhibitors. Despite that, there is a residual risk that is not targeted with these therapies. Currently, it is recognized that the cyclic guanosine monophosphate deficiency has a negative direct impact on the pathogenesis of HF, and vericiguat, an oral stimulator of soluble guanylate cyclase, can restore this pathway. The effect of vericiguat has been explored in the VICTORIA study, the largest chronic HF clinical trial that has mainly focused on patients with recent worsening HF, evidencing a significant 10% risk reduction of the primary composite endpoint of cardiovascular death or HF hospitalization (number needed to treat 24), after adding vericiguat to standard therapy. This benefit was independent of background HF therapy. Therefore, optimization of treatment should be performed as earlier as possible, particularly within vulnerable periods, considering also the use of vericiguat.
Introduction
Heart failure (HF) affects more than 60 million people worldwide, with an estimated current prevalence of 2% in the adult population. Even it is expected that these numbers will increase in the next decades.1, 2 Despite traditional treatments, mortality rates remain high, reaching 20% after 1 year of HF diagnosis.3 In addition, the risk of hospitalization is important in HF population. It has recently been shown that in Spain, the incidence of hospitalization for HF is around 232 per 1000 person-years in patients with HF and even higher in patients with HF with reduced ejection fraction (HFrEF) (267 per 1000 person-years).4 In fact, HF represents the most important cause of admission in older patients in developed countries.1, 2 Moreover, health care costs associated with HF are enormous, being HF hospitalizations the most important determinant.5, 6
Patients with chronic HF may deteriorate, either suddenly or slowly, and require hospital admission or treatment with intravenous diuretic therapy in the outpatient setting. These periods of decompensation represent an inflexion point in the evolution of patients as they indicate that HF is progressing, with a greater risk of adverse events, particularly death and HF hospitalization.7, 8
The quadruple therapy with sacubitril–valsartan, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 (SGLT2) inhibitors has been shown to be effective in reducing the rates of mortality and HF hospitalization.9 Therefore, an early implementation of these drugs, together with optimizing doses to maximum tolerability to reach target doses stated in the guidelines, is mandatory in patients with HFrEF.7, 8, 10 However, residual risk persists despite the use of these drugs. Recent data have shown that despite quadruple therapy, one in seven patients with HFrEF will develop cardiovascular death or HF hospitalization over a median follow-up as short as 18.2 months.11 In this context, a new comprehensive approach is warranted.8, 12
Need for a comprehensive treatment of patients with heart failure with reduced ejection fraction
HF is a complex syndrome in which the ventricular filling and/or contractile function are impaired. As a result of left ventricular dysfunction, there is a general hypoperfusion that affects the different organs and systems of the organism, which leads to the activation of different compensatory mechanisms, particularly neurohormonal systems. Classically, the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS) have been involved in the pathogenesis of HF. Although both systems initially try to maintain homeostasis, finally, they result deleterious in a self-perpetuating vicious circle unless the appropriate treatment is prescribed.13, 14
Thus, the overactivation and perpetuation of the sympathetic nervous system are associated with myocyte hypertrophy, increased ventricular mass, fibrosis, ventricular remodelling, oxidative stress, and inflammation, leading to a decrease in the contractile capacity of the heart.15, 16 In contrast, beta-blockers reduce heart rate and blood pressure, increase the duration of diastole, improve myocardial filling, and reduce oxygen demand.15, 16 Different clinical trials have demonstrated the benefits of treating patients with HFrEF with beta-blockers in reducing mortality and HF hospitalization and improving symptoms.17-22
On the other hand, the sustained activation of RAAS produces deleterious effects, including ventricular and vascular hypertrophy, fibrosis, arterial stiffness, vasoconstriction, endothelial dysfunction, increased oxidative stress, inflammation, and activation of sympathetic nervous system. At the end, all these mechanisms promote myocardial failure.13, 14, 23-25 Angiotensin-converting enzyme inhibitors (ACE-Is) were the first RAAS inhibitors to demonstrate a reduction in the risk of mortality and HF hospitalization and an improvement in functional class.26-28 The level of recommendation with angiotensin II receptor blockers (ARBs) has changed over time as no clinical trial has demonstrated a reduction of all-cause mortality with these drugs in patients with HFrEF and now, they are recommended when ACE-I or sacubitril–valsartan cannot be prescribed due to side effects.29, 30 Finally, mineralocorticoid receptor antagonists (spironolactone or eplerenone) added to standard therapy have also demonstrated reduced mortality and HF hospitalization in clinical trials.31, 32
In recent years, several clinical trials have shown that treatment of HFrEF should not be limited to targeting only sympathetic nervous system and RAAS but also other neurohormonal systems. Thus, natriuretic peptides promote salt (natriuresis) and water (diuresis) loss, produce vasodilation, and are a counterregulatory mechanism of the sympathetic nervous system and RAAS. Natriuretic peptides are degraded by neprilysin. Sacubitril inhibits neprilysin and allows natriuretic peptides to interact with their receptors, increasing the intracytoplasmic amount of cyclic guanosine monophosphate (cGMP), which leads to vasodilation, decreased fibrosis/hypertrophy, and increased natriuresis and diuresis.33 In this context, the combination of sacubitril with valsartan (an ARB) was compared with enalapril (an ACE-I) in the PARADIGM-HF trial. This study included symptomatic patients with left ventricular ejection fraction (LVEF) ≤ 40% (changed from ≤35% after amendment) and elevated natriuretic peptides. After a follow-up of 2.3 years, compared with enalapril, treatment with sacubitril–valsartan was associated with a reduction of cardiovascular death or first HF hospitalization by 20% (P < 0.001), the risk of death by 16% (P < 0.001), the risk of cardiovascular death by 20% (P < 0.001), and the risk of HF hospitalizations by 21% (P < 0.001).34
The benefits of SGLT2 inhibitors go beyond their ability to reduce blood glucose and restore metabolic disorders associated with diabetes. Multiple mechanisms that would explain their protective effects in patients with HFrEF have been described, including a reduction of glucotoxicity, more efficient use of cardiac energy sources, increase of diuresis and, secondarily, decreasing plasma volume, reduction of blood pressure, inflammation, oxidative stress, fibrosis, and arterial stiffness, and also providing a positive regulation of sympathetic nervous system, renal protection, increased nitric oxide availability, and an improvement of oxygen transport.35, 36 The DAPA-HF and EMPEROR-Reduced trials included symptomatic patients with LVEF ≤ 40%. After 1.5 years of follow-up, dapagliflozin reduced the risk of cardiovascular death or HF worsening by 26% (P < 0.001) and the risk of cardiovascular death by 18%, compared with placebo. In contrast, empagliflozin reduced the risk of cardiovascular death or HF hospitalization by 25% (P < 0.001) and the total number of HF hospitalizations by 30% (P < 0.001) compared with placebo after 1.3 years of follow-up.11, 37
Role of vericiguat in post-event management in heart failure with reduced ejection fraction
Patients with HFrEF develop endothelial cell dysfunction that leads to a decreased nitric oxide production and insufficient stimulation of soluble guanylyl cyclase. This fact translates into a reduction of cGMP formation that has deleterious consequences at different levels, such as the heart (ventricular hypertrophy, fibrosis, and myocardial stiffness), blood vessels (vasoconstriction and arterial stiffness), and the kidneys (decreased renal blood flow and increased sodium and water retention), promoting HF progression.12, 38-40 Although nitrates or phosphodiesterase inhibitors up-regulate cGMP levels, they have failed to provide robust clinical benefits in the HFrEF population.12 In contrast, vericiguat is an oral stimulator of soluble guanylate cyclase that restores the relative cGMP deficiency in the nitric oxide-soluble guanylate cyclase signalling pathway, independently and synergistically with nitric oxide, reducing the adverse effects of cGMP deficiency, through the promotion of ventricular relaxation, a decrease of ventricular filling pressures, improving the vascular function, and also providing renal protection (Figure 1).12, 38-40 The SOCRATES-REDUCED trial showed a dose–response relationship between vericiguat doses and more significant reductions in N-terminal pro-brain natriuretic peptide (NT-proBNP) levels among patients with reduced LVEF and worsening chronic HF.41 The VICTORIA trial included 5050 patients with symptomatic chronic HF, LVEF < 45%, and recent worsening HF, defined such <3 months after HF hospitalization (67%), 3–6 months after HF hospitalization (17%), and those requiring outpatient intravenous diuretic therapy (16%). Patients were randomized to receive vericiguat (target dose, 10 mg once daily) or placebo in addition to standard medical therapy. The primary outcome was a composite of death from cardiovascular causes or first HF hospitalization. After 10.8 months of follow-up, the addition of vericiguat to standard therapy translated into a significant 10% risk reduction of the primary composite endpoint of cardiovascular death or HF hospitalization [hazard ratio (HR) 0.90; 95% confidence interval (CI) 0.82–0.98; P = 0.02; number needed to treat (NNT) 24], a 9% risk reduction of total HF hospitalization (HR 0.91; 95% CI 0.84–0.99; P = 0.02), and a 10% risk reduction of first HF hospitalization and all-cause death (HR 0.90; 95% CI 0.83–0.98; P = 0.02). No differences were observed in total nor cardiovascular mortality between groups. Symptomatic hypotension and syncope similarly occurred in both groups.42
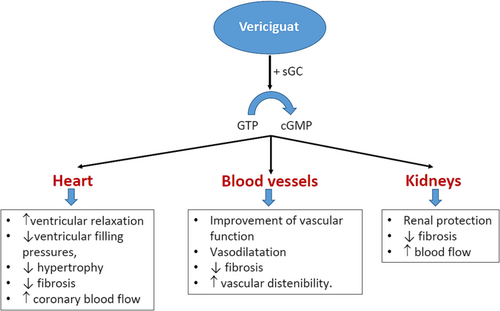
Therefore, all the treatments that have demonstrated to positively modify the clinical course of HF have complementary mechanisms of action and provide additional benefits when they are used concomitantly, including the nitric oxide-soluble guanylate cyclase signalling pathway.9, 11, 12 However, not all stimulators of soluble guanylate cyclase have been shown to be effective. Thus, intravenous administration of cinaciguat, a first-generation soluble guanylate cyclase activator, did not show an improvement of dyspnoea or cardiac index.43 In contrast, recent studies have reported that adding vericiguat to quadruple therapy would reduce the risk of adverse events in patients with HF, particularly those with recent worsening HF.44, 45
Of note, although the relative benefit of vericiguat seems lower than that of recent HF clinical trials, such as PARADIGM-HF, DAPA-HF, or EMPEROR-Reduced, it should be considered that the patients included in the VICTORIA trial were at higher risk (recent worsening HF episode) and had a greater absolute risk of events. In this context, it is important to consider the absolute benefit rather than relative effects across clinical trials. Thus, the NNT to prevent one additional primary endpoint was very similar between the PARADIGM-HF, DAPA-HF, EMPEROR-Reduced, and VICTORIA trials (Figure 2).11, 34, 37, 42 All these data show that to reduce HF burden, it is necessary to implement all disease-modifying HF therapies, adjusted according to the clinical profile of patients.46
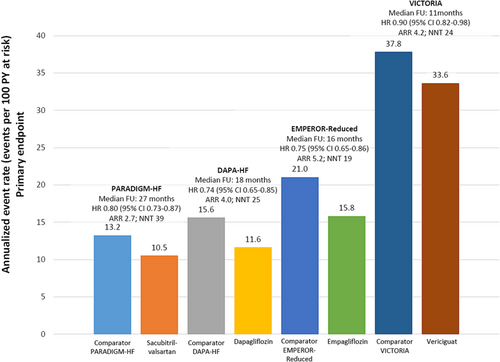
Source: Figure performed with data from references 11, 34, 37, 42.
Practical aspects of vericiguat
Vericiguat takes once daily, at a starting dose of 2.5 mg and a target dose of 10 mg (up-titration may be made approximately every 2 weeks). No dose adjustment is required according to age, renal, or hepatic function. The risk of drug–drug interactions is low, as vericiguat is not a substrate of CYP3A or P-gp. Vericiguat may reduce systolic blood pressure by approximately 1–2 mmHg. In case of symptomatic hypotension or systolic blood pressure < 90 mmHg, down-titration or discontinuation should be considered; furthermore, vericiguat should not be started in patients with systolic blood pressure < 100 mmHg.47
Vericiguat and current guidelines
Previous guidelines had proposed a step-by-step approach in managing patients with HFrEF, intensifying the treatment if the patient remained symptomatic, including up-titration to target doses.48 However, this approach delays the implementation of disease-modifying therapies, reducing the benefits obtained by these therapies.10, 49
In contrast, the current 2021 European Society of Cardiology guidelines recommend among patients with HFrEF, the early initiation and up-titration of first-line disease-modifying therapies, including sacubitril–valsartan (preferred)/ACE-I, beta-blockers, SGLT2 inhibitors, and mineralocorticoid receptor antagonists. If the patient remains symptomatic, second-line therapies should be considered (Class IIa recommendation). Second-line therapies include the use of ARB in patients unable to tolerate an ACE-I or sacubitril–valsartan, ivabradine in patients in sinus rhythm, and a resting heart rate ≥ 70 b.p.m. despite treatment with a beta-blocker (or unable to take it), and also vericiguat, in patients who have had worsening HF, to reduce the risk of cardiovascular mortality or HF hospitalization (recommendation IIbB) (Figure 3 and Table 1). In addition, implantable cardioverter-defibrillator and resynchronization therapy should be considered in selected patients.7
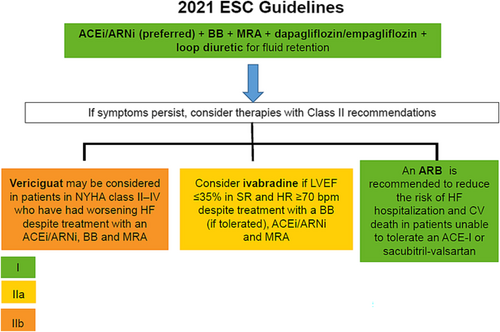
Source: Figure performed with data from reference 7.
| Drugs | Class of recommendation/level of evidence |
|---|---|
| An ACE-I is recommended to reduce the risk of HF hospitalization and death | IA |
| A beta-blocker is recommended to reduce the risk of HF hospitalization and death | IA |
| An MRA is recommended to reduce the risk of HF hospitalization and death | IA |
| Dapagliflozin or empagliflozin is recommended to reduce the risk of HF hospitalization and death | IA |
| Sacubitril/valsartan is recommended as a replacement for an ACE-I to reduce the risk of HF hospitalization and death | IB |
| An ARB is recommended to reduce the risk of HF hospitalization and CV death in patients unable to tolerate an ACE-I or sacubitril–valsartan | IB |
| Ivabradine should be considered in patients in sinus rhythm and a resting heart rate ≥ 70 b.p.m. despite treatment with a beta-blocker* to reduce the risk of HF hospitalization and CV death | IIaB |
| *Unable to tolerate or have contraindications for a beta-blocker | *IIaC |
| Vericiguat may be considered in patients who have had worsening HF to reduce the risk of CV mortality or HF hospitalization | IIbB |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CV, cardiovascular; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist.
The 2022 American (American College of Cardiology/American Heart Association/Heart Failure Society of America) guidelines recommend in all patients with HFrEF, the treatment with beta-blockers, mineralocorticoid receptor antagonists, SGLT2 inhibitors, and sacubitril–valsartan [in New York Heart Association (NYHA) II–III patients] or ACE-I/ARB (in NYHA II–IV patients). In addition, these guidelines state that in selected high-risk patients with HFrEF and recent worsening HF, vericiguat may be considered to reduce HF hospitalization and cardiovascular death [recommendation 2b; level of evidence B-R (moderate quality from one or more randomized clinical trials)].50
In summary, in current guidelines, the level of recommendation for using vericiguat is IIb. However, considering that this recommendation is provided from a robust clinical trial that has demonstrated a moderate benefit in reducing HF hospitalization, some authors consider that the recommendation should be upgraded to IIaB.51 However, it should be considered that whereas the PARADIGM-HF, DAPA-HF, and EMPEROR-Reduced trials were performed in stable patients with HFrEF, the VICTORIA trial was explicitly developed in patients with recent worsening HF episodes, showing benefits in a significant clinical scenario that other clinical trials have not been well assessed.11, 34, 37, 42
Expert opinion
Despite the importance of assessing the best approach in patients with worsening HF, very few small studies with less important endpoints have analysed the role of some drugs in this clinical setting. The EMPULSE trial, which included 530 patients with HF, showed that the addition of empagliflozin to standard treatment between Days 1 and 5 of acute HF hospitalization reduced the risk of all-cause death, number of worsening HF events, and change in Kansas City Cardiomyopathy Questionnaire symptom score at 90 days, regardless of the presence of diabetes or LVEF.52 The SOLOIST-WHF trial included 1222 patients with type 2 diabetes, recently hospitalized for worsening HF. Starting sotagliflozin, during hospitalization or at early discharge, reduced the risk of cardiovascular death and hospitalizations and urgent visits for HF (first and recurrent events) by 33%.53 In addition, the PIONEER-HF trial showed in 881 patients with HFrEF who had been hospitalized for acute decompensated HF that in-hospital initiation of sacubitril–valsartan reduced the levels of NT-proBNP and the number of rehospitalizations for HF compared with enalapril.54
However, the VICTORIA trial is the first positive larger trial that has exclusively included patients with HFrEF within the vulnerable period after a worsening HF episode, demonstrating a 10% risk reduction of cardiovascular death or HF hospitalization after only 0.9 years of follow-up.42 Importantly, this positive effect was independent of baseline HF therapy.55 In addition, the benefits of vericiguat were independent of the index event, suggesting that vericiguat can be used in patients with recent worsening HF, regardless of underlying HF treatment, from an inpatient or outpatient point of view, as CIs of subgroups were crossing and consequently, the value of P interaction was not significant (Figure 4).56 Remarkably, vericiguat does not have a negative impact on renal function, and the benefits of vericiguat were consistent across the full range of renal function.57 However, vericiguat seems less effective in those patients with higher levels of NT-proBNP (>5314 pg/mL), suggesting a lower efficacy in those patients with more advanced disease.58 Of note, the risk of adverse events with vericiguat was low in the VICTORIA trial, without a higher risk of symptomatic hypotension, syncope, worsening renal function (this study included patients with estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2), or electrolytes disturbances.42 This fact may facilitate the implementation of vericiguat in clinical practice.46
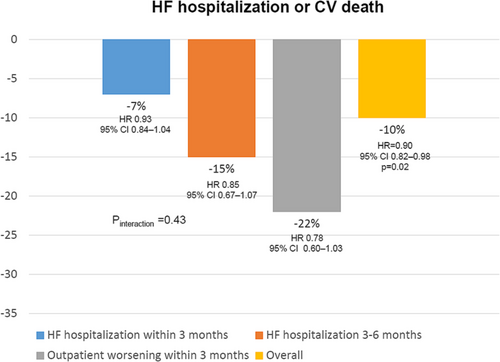
On the other hand, new studies are currently ongoing with vericiguat. Thus, the VICTOR study is a clinical trial that is currently evaluating a lower risk HFrEF population compared with the VICTORIA trial, as it has included patients with symptomatic chronic HFrEF, elevated NT-proBNP levels, and no HF hospitalization in the past 6 months and no intravenous diuretic therapy in the past 3 months before randomization.12 This study will provide significant results that will extend the information about the efficacy of vericiguat to the whole spectrum of patients with HFrEF.
Therefore, all these data indicate that treatment optimization should be performed as earlier as possible, mainly when patients are more vulnerable for new events (HF hospitalization or death). In this context, there is solid evidence that vericiguat provides further benefits added to standard therapy in patients with HFrEF and prior worsening HF.
Conclusions
HF is associated with high morbidity and mortality, particularly in those patients with recent worsening HF. Therefore, the early implementation of those therapies that have demonstrated to modify the clinical course of HF is mandatory. VICTORIA is the larger clinical trial that has particularly focused on patients with recent worsening HF, showing a significant clinical benefit of adding vericiguat to standard therapy in HFrEF. In this context, guidelines state that vericiguat can be considered for treating patients with HFrEF and recent worsening HF in addition to standard therapy to reduce HF burden. Considering the implication of different neurohormonal systems, the severity of the disease, and the very high risk of patients during the vulnerable period, the introduction of vericiguat should not be delayed in this clinical setting. In addition, vericiguat could also be considered in patients with HFrEF taking disease-modifying therapies with a poor clinical evolution.
Lay summary
Patients with a recent worsening HF episode are at high risk of death or HF hospitalization. HF is a complex syndrome in which different neurohormonal systems are implied. Only through a comprehensive approach that considers these pathways, HF burden can be reduced. The cGMP deficiency has a negative direct impact on the pathogenesis of HF. Vericiguat restores the relative cGMP deficiency, which reduces cardiovascular mortality and HF hospitalization.
Acknowledgements
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain) with funding from Bayer Hispania SL.
Conflict of interest
A.O. has received honoraria for lectures from Novartis, Lilly, and AstraZeneca and for participation in advisory boards from Bayer. L.A.-B. has participated in advisory boards from Bayer. P.M. has received honoraria for lectures from Novartis, Bayer, Boehringer-Lilly, and AstraZeneca and for participation in advisory boards from Bayer and Vifor. E.C. has received honoraria for lectures from Novartis, Lilly, Pfizer, Rovi, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, CSL Vifor, and Zambon and for participation in advisory boards from Bayer. A.M.-R. reports research grants, participation in advisory board meetings, or personal fees for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Ferrer, Lilly, Novartis, and Pfizer. M.P.B. reports research grants, participation in advisory board meetings, or personal fees for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, and Pfizer. R.B. has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, Gilead, GSK, Menarini, Pfizer, Rovi, and Vifor and for participation in advisory boards from Bayer. A.B.M.F. has participated in advisory boards from Bayer. G.C. reports personal fees for participation in advisory board meetings from Bayer and personal fees for lectures from Bayer, Novartis, AstraZeneca, Servier, Boehringer Ingelheim, and Lilly. L.F.R. has received honoraria for lectures from Novartis, Bayer, Boehringer-Lilly, AstraZeneca, and Vifor and for participation in advisory boards from Bayer, Novartis, and AstraZeneca.




