Prevalence of transthyretin amyloid cardiomyopathy in pacemaker patients
Abstract
Aims
Transthyretin amyloid cardiomyopathy (ATTR-CM) is characterized by increased wall thickness, diastolic dysfunction and progressive heart failure symptoms. The disease may infiltrate the conduction system leading to conduction disturbances requiring an implantation of permanent cardiac pacemaker (PM), but the extent is unknown. Here, we report the prevalence of ATTR-CM in patients ≥65 years with PM.
Methods and results
In this prospective, cross-sectional single-centre study patients were recruited from our out-patient pacemaker clinic. Eligibility criteria were age above 65 years, permanent cardiac pacemaker and competent to give informed consent. Patients underwent echocardiography at the pacemaker visit and were referred to 99mTc-DPD-scintigraphy (DPD) and blood samples if septum thickness was ≥12 mm, defined as left ventricular hypertrophy (LVH). Fifty eight of the 128 patients had LVH on echocardiography. Eleven patients had a DPD-scintigraphy based diagnosis of ATTR-CM, which represent 19% of patients with LVH and 9% of the total cohort. Patients diagnosed with ATTR-CM had higher concentrations of cardiac biomarkers (P < 0.001), higher E/E′ (P = 0.001), and lower global longitudinal strain (P = 0.003) on echocardiography and more heart failure symptoms (P = 0.001).
Conclusions
The prevalence of ATTR-CM in elderly patients with PM and LVH on echocardiography was 19%.
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is caused by accumulation of misfolded transthyretin in the myocardium. The disease predominantly affects older patients and leads to increased wall thickness, diastolic dysfunction and progressive heart failure symptoms.1, 2 Patients are often symptomatic when diagnosed and most deaths are from cardiac causes.3 Median survival after diagnosis of ATTR-CM has been reported 3.5 years,3 depending on the disease stage at diagnosis.4 The prevalence of ATTR-CM has increased over the last decades,5 possibly due to better and safer diagnostics, but the condition is still underdiagnosed.5 Scintigraphy with a bone mineral tracer has shown excellent sensitivity and specificity in diagnosing ATTR-CM compared with the previous gold standard, myocardial biopsy.6 Light-chain amyloidosis (AL) should simultaneously be ruled out by serum light chains and electrophoresis as scintigraphy not always distinguishes these two forms of cardiac amyloidosis. Amyloid deposits may also infiltrate the conduction system in the myocardium leading to sinus node dysfunction, bundle branch block or atrioventricular (AV) block requiring implantation of a permanent cardiac pacemaker (PM). Today, the disease specific cause requiring implantation of PM remains unknown in most cases. Hypertension- or coronary artery related fibrosis in the conduction system is a plausible mechanism in many cases,7 but the role of ATTR-CM in this clinical setting is unknown. Here we report the prevalence of ATTR-CM with 99mTc-DPD-scintigraphy in patients with PM aged ≥65 years.
Methods
We conducted a prospective, cross-sectional single-centre study between October 2019 and June 2021. Patients were recruited from our out-patient pacemaker clinic at a community hospital. Patients were eligible if they had PM, age above 65 years and were competent to give informed consent, judged by a PM nurse and author 1. Patients requiring emergency PM placement and elective placement were included. Exclusion criterion was permanent stay at a nursing home as these patients often are frail and at the end of their lives in our country. Specific ATTR-CM mutations were not investigated. All patients proved written informed consent. The local and regional Ethics Committee approved the study.
Study design
Patients underwent transthoracic echocardiography at the PM visit by author 1 with supervision from author 2. We measured end-diastolic septum thickness, preferably in parasternal short axis view using M-mode. If septum thickness was ≥12 mm, patients underwent blood samples the same day and were scheduled for 99mTc-DPD-scintigraphy (DPD) within the next month.
Data collection
Medical history was obtained at the PM visit and from electronic hospital records. Hypertension was defined by the use of antihypertensive medications. Blood pressure was measured three times in supine position during the PM interrogation and the average of the last two measurements was reported. Electrocardiographic low voltage was defined as QRS amplitude ≤5 mm in the limb leads. When ventricular paced rhythm was present, previous ECG before the PM implantation was used to examine low-voltage. Previous hospitalization for heart failure and stroke was defined by ICD-10 diagnosis in electronic hospital records, made by the treating physician at the index stay. Aortic stenosis was present when peak velocity ≥4.0 m/s or previous valve replacement. High degree AV-block was defined as Mobitz type II, total AV-block or permanent atrial fibrillation with slow conduction requiring PM implantation. Blood samples included haemoglobin, creatinine, troponin I, n-terminal pro-brain natriuretic peptide (NT-proBNP), thyroid stimulating hormone (TSH), serum electrophoresis, and free light chains. AL-amyloidosis was excluded if kappa/lambda ratio was between 0.3–1.6 and the absence of abnormal monoclonal band at immunofixation of serum. Estimated glomerular filtration rate was calculated with the CKD-EPI formula.8
Echocardiographic imaging
Echocardiographic examinations were performed using Vivid 9 (GE Ultrasound, Horten, Norway). Septum thickness was analysed at time of examination, other recordings were analysed post-hoc by EchoPAC Plug-in version 203 (GE Ultrasound). End diastolic septum thickness was measured in the parasternal view, preferably the short axis view by M-mode. Left atrial volumes were measured using Simpsons method and indexed for body surface area. Other standard measurements of cardiac chamber size and Doppler measurements were in accordance with the European Society of Cardiology and American Society of Echocardiography recommendations.9 Ejection fraction was measured semi-automatically and tracings were manually adjusted to fit the endocardial border. Analysis of left ventricular global longitudinal strain (GLS) was performed from apical 2-, 3-, and 4-chamber views. Frame rate was 70 frames per second. The region of interest was automatically drawn and adjusted to the myocardial thickness and border when necessary. GLS was then calculated by the average of the peak strain values of the 17 segments. Apical sparing was present when the average apical longitudinal strain divided by (average basal- plus average mid-longitudinal strain) was >1.
Scintigraphy protocol
Patients with ≥12 mm diastolic septum thickness were scanned using Siemens Symbia T6 (Siemens Healthcare, Erlangen, Germany) single photon emission computed tomography cameras. The protocol has been described previously.10 Experienced physicians unrelated to the study analysed the images. Tracer uptake was graded in accordance with the visual Perugini score (0–3).11 A diagnosis of ATTR-CM was made when left or both ventricles revealed high uptake (score 2–3).
Statistical analyses
Statistical analyses were performed using SPSS statistics version 19 (IBM Corp). Continuous normally distributed variables were expressed as means ± SD, non-normally distributed variables as median (minimum to maximum due to small sample size) and categorical variables as counts (frequency percentage). Between group differences were analysed with unpaired Student's t-test, Mann–Whitney U or Fisher exact test, as appropriate. Two-sided P-values were used throughout the analyses. Author 1 carried out data analyses with supervision from author 2.
Results
We identified 524 patients from electronic hospital records with PM in our area. After excluding patients <65 years, patients with cognitive failure unable to give informed consent, and patients with permanent stay at nursing home, 170 patients were eligible and invited to participate in the study. Thirty-five patients would not participate, 7 patients withdrew consent, and 128 patients were included in the final analyses. Figure 1 summarizes study design and major findings. In the total cohort the mean age was 79 ± 7.0 years, 28% were female, 62% had hypertension, 32% had coronary artery disease, and 10% had one or more episodes of hospitalization for heart failure. The indication for PM implantation was 32% for sinoatrial (SA) block or tachy-brady syndrome, 56% for high-degree AV block, and 12% had other reasons (unexplained syncope with bifasicular block, sinus bradycardia or type I AV-block with symptoms).
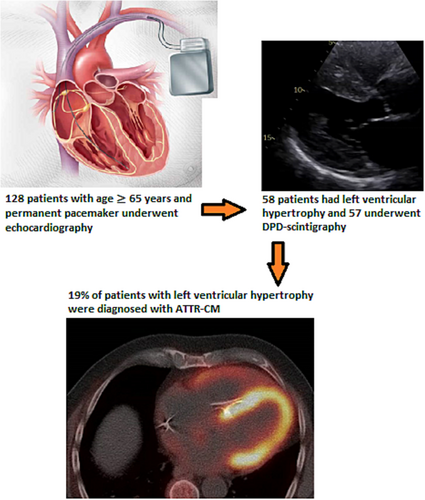
Table 1 shows baseline characteristics in patients with LVH. ATTR-CM patients had more heart failure hospitalizations, were more symptomatic, had higher troponin I and NT-proBNP concentrations, and higher E/E′ and lower GLS on echocardiography. Table 2 shows echocardiographic parameters and laboratory values in patients with LVH, with and without a diagnosis of ATTR-CM. Poor image quality led to missing values for 3 (5%) EF- and 6 (10%) GLS calculations in patients with LVH. None of our patients was diagnosed with AL-amyloidosis; one patient had this disease before entering the study, but no uptake on DPD-scintigraphy. Monoclonal gammopathy of undetermined significance was present in three patients, and a haematologist was consulted when this condition was unknown before study entry. Eight of the 11 patients diagnosed with ATTR-CM had the first PM implantation during the preceding 12 months, but time since implantation had a wide range and was not significantly different between the two groups.
| LVH with ATTR-CM (n = 11) | LVH without ATTR-CM (n = 47) | P-value | |
|---|---|---|---|
| Age, years | 84.3 ± 6.1 | 80.5 ± 7.2 | 0.11 |
| Female gender | 1 (9.1) | 4 (8.5) | 1.0 |
| BMI, kg/m2 | 23.8 ± 1.5 | 27.8 ± 4.3 | 0.89 |
| Systolic blood pressure, mmHg | 129 ± 19 | 143 ± 20 | 0.045 |
| Diastolic blood pressure, mmHg | 71 ± 8.3 | 80 ± 9.0 | 0.002 |
| Low voltage on ECG | 4 (36) | 6 (13) | 0.14 |
| NYHA class | 2.5 ± 0.52 | 1.6 ± 0.61 | 0.001 |
| Hospitalization for HF | 5 (50) | 5 (11) | 0.015 |
| Syncope before PM-implantation | 3 (27) | 13 (28) | |
| Indication for PM implantation | |||
| SA-block or tachy-brady | 1 (9.1) | 12 (26) | 0.43 |
| High degree AV-blocka | 9 (82) | 27 (57) | 0.18 |
| Other | 1 (9.1) | 8 (17) | |
| Time since implantation (months) | 10.7 ± 12.7 | 60.7 ± 50.9 | 0.60 |
| Co-morbidities | |||
| Hypertension | 9 (82) | 30 (64) | 0.31 |
| Coronary artery disease | 6 (55) | 19 (47) | 0.50 |
| Myocardial infarction | 4 (36) | 13 (28) | 0.72 |
| Previous stroke | 2 (18) | 4 (9) | 0.32 |
| Atrial fibrillation | 7 (64) | 20 (43) | 0.32 |
| Aortic stenosis | 1 (9.1) | 4 (8.5) | 1.0 |
| Treatment | |||
| ACEI/ARB | 6 (55) | 26 (55) | 1.0 |
| Beta-blockers | 5 (45) | 14 (30) | 0.48 |
| Loop diuretics | 8 (73) | 8 (17) | 0.001 |
| Aldosterone antagonists | 1 (9.1) | 1 (2.1) | 0.35 |
| Other vasoactive medications | 7 (64) | 18 (38) | 0.18 |
| Anticoagulants | 7 (64) | 25 (53) | 0.74 |
| Antiplateled therapy | 2 (18) | 15 (32) | 0.48 |
| Statin | 6 (55) | 30 (64) | 0.40 |
- Values are presented as mean ± SD or n (%).
- ACEI, angiotensin converting enzyme inhibitors; ARB, aldosterone reseptor blockers; ATTR-CM, transthyretin amyloid cardiomyopathy; AV, atrioventricular; LVH, left ventricular hypertrophy; SA, sinoatrial.
- a Including permanent atrial fibrillation with slow conduction requiring pacemaker implantation.
| LVH with ATTR-CM (n = 11) | LVH without ATTR-CM (n = 47) | P-value | |
|---|---|---|---|
| Echocardiographic parameters | |||
| Septal wall thickness, cm | 1.70 (1.30–2.00) | 1.31 (1.20–1.72) | <0.001 |
| Posterior wall thickness, cm | 1.62 ± 0.35 | 1.30 ± 0.19 | 0.0032 |
| LV end diastolic diameter, cm | 4.60 ± 0.89 | 5.20 ± 0.76 | 0.38 |
| LV ejection fraction, % | 52.3 ± 9.5 | 53.8 ± 8.1 | 0.11 |
| LV global longitudinal strain, % | −12.4 ± 4.1 | −14.4 ± 3.5 | 0.0030 |
| Apical sparing, n (%) | 3 (27) | 1 (2.1) | |
| Left atrial volume index, mL/m2 | 75.8 ± 37.0 | 55.8 ± 32.3 | 0.68 |
| E wave, cm/s | 0.93 ± 0.54 | 0.54 ± 0.12 | 0.080 |
| E/A ratio | 0.71 (0.49–1.04) | 0.69 (0.25–1.51) | 0.52 |
| E wave deceleration time, ms | 290 (200–366) | 320 (197–632) | 0.79 |
| E′ average, m/s | 0.053 ± 0.016 | 0.065 ± 0.020 | 0.055 |
| E/E′ average ratio | 11.8 (8.4–26.6) | 8.7 (6.7–19.7) | 0.001 |
| Aortic valve peak velocity, m/s | 1.62 ± 0.78 | 1.62 ± 0.35 | 0.45 |
| TR peak velocity, m/s | 2.79 ± 0.32 | 2.59 ± 0.38 | 0.51 |
| TAPSE, cm | 1.66 ± 0.41 | 2.03 ± 0.36 | 0.009 |
| Laboratory values | |||
| Haemoglobin, g/dL | 13.8 ± 2.14 | 14.3 ± 1.75 | 0.49 |
| Estimated GFR, mL/min/1.73 m | 46 ± 25 | 58 ± 18 | 0.030 |
| Troponin I, ng/L | 199 (18–259) | 25.8 (<10–193) | <0.001 |
| NT-proBNP, ng/L | 3787 (1604–19 630) | 887 (68–9365) | <0.001 |
| TSH, mIE/L | 1.18 ± 0.74 | 1.66 ± 0.91 | 0.19 |
- Values are presented as mean ± SD or median (minimum-maximum).
- ATTR-CM, transthyretin amyloid cardiomyopathy; LV, left ventricular; LVH, left ventricular hypertrophy; NT-proBNP, n-terminal pro-brain natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TSH, thyroid stimulating hormone.
Discussion
This study is the first to acknowledge the prevalence of ATTR-CM in an elderly PM population. Previous studies have shown high rates of conduction disturbances and bradycardia in patients with ATTR-CM, but these studies were often from tertiary centers including both AL-amyloidosis and ATTR-CM caused by mutations.1, 12, 13 Here we report a 19% prevalence of ATTR-CM in elderly patients with PM and LVH on echocardiography. In the total cohort, the prevalence of ATTR-CM was 9%.
Patients diagnosed with ATTR-CM had signs of higher filling pressures on echocardiography, higher concentrations of cardiac biomarkers and more heart failure symptoms. The prevalence of hypertension and other cardiac co-morbidities was frequent, also amongst patients diagnosed with ATTR-CM. This may be explained by the study population, being elderly patients with LVH. However, our study shows that hypertension often precedes ATTR-CM, and that judging LVH to be caused by previous loading conditions may lead to underdiagnosis of ATTR-CM. When echocardiography is suggestive of LVH in elderly patients, and especially when heart failure symptoms are present, ATTR-CM should be suspected irrespective of a history of hypertension. A newly published position paper underscores this and recommends investigation for cardiac amyloidosis with DPD-scintigraphy when septum ≥12 mm in specific clinical settings in patients >65 years.2
The use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and beta-blockers was high in our LVH cohort, also in patients diagnosed with ATTR-CM. ATTR-CM patients often develop side effects, especially orthostatic hypotension, and these drug classes are often not tolerated over time.14 A diagnosis or exclusion of ATTR-CM by DPD-scintigraphy may thus help the cardiologist to have a personalized approach to medications and follow-up of patients with LVH.
Our findings are in accordance with the prevalence of ATTR-CM in other clinical settings. ATTR-CM was found in 16% of patients scheduled for TAVI15 and in 13% of elderly patients with heart failure hospitalization with preserved ejection fraction.16 A systematic review also reported ATTR-CM in 7% of elderly patients with and initial diagnosis of hypertrophic cardiomyopathy, in 2% of patients undergoing carpal tunnel syndrome surgery and in 1% of patients who underwent DPD-scintigraphy for any non-cardiac reason.17
High degree AV block may be a late presenting complication in ATTR-CM patients,18 possibly reflecting a higher burden of amyloid in the myocardium. Most patients diagnosed with ATTR-CM in our study had their first implantation of PM during the preceding year possibly reflecting a poor prognosis, but this study did not address follow-up data. Other limitations of this study include small sample size, patient recruitment from a single center and selection bias cannot be fully excluded. Other cardiac co-morbidities were frequent amongst patients and our study cannot address if ATTR-CM was the cause for requiring PM. Furthermore, we included only those with septum ≥12 mm and early stages, mainly affecting the conduction system, could have been missed. We did not evaluate disease specific mutations in our study amongst elderly patients.
In conclusion, we report a 19% prevalence of transthyretin amyloid cardiomyopathy in elderly patients with permanent cardiac pacemaker and left ventricular hypertrophy on echocardiography.
Acknowledgements
The authors thank Heidi Nystad and Tommy Sandoy for tremendous work at the pacemaker clinic. We are also grateful for the good service and hospitality from the people working at the Department of Nuclear Medicine in our community hospital, especially Sissel Steien.
Conflict of interest
None declared.
Funding
No funding.




