Medicines optimization prior to discharge in patients admitted to hospital with heart failure
Abstract
Aims
Approximately half of patients with heart failure and a reduced ejection fraction (HeFREF) are discharged from hospital on triple therapy [angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs), beta-blockers (BBs), and mineralocorticoid receptor antagonists (MRAs)]. We investigated what proportion of patients are on optimal doses prior to discharge and how many might be eligible for initiation of sacubitril–valsartan or sodium-glucose co-transporter-2 inhibitors (SGLT2Is).
Methods and results
Between 2012 and 2017, 1277 patients admitted with suspected heart failure were enrolled at a single hospital serving a local community around Kingston upon Hull, UK. Eligibility for sacubitril–valsartan or SGLT2I was based on entry criteria for the PIONEER-HF, DAPA-HF, and EMPEROR-Reduced trials. Four hundred fifty-five patients had HeFREF with complete data on renal function, heart rate, and systolic blood pressure (SBP) prior to discharge. Eighty-three per cent of patients were taking an ACE-I or ARB, 85% a BB, and 63% an MRA at discharge. More than 60% of patients were eligible for sacubitril–valsartan and >70% for SGLT2I. Among those not already receiving a prescription, 37%, 28%, and 49% were eligible to start ACE-I or ARB, BB, and MRA, respectively. Low SBP (≤105 mmHg) was the most frequent explanation for failure to initiate or up-titrate therapy.
Conclusions
Most patients admitted for heart failure are eligible for initiation of life-prolonging medications prior to discharge. A hospital admission may be a common missed opportunity to improve treatment for patients with HeFREF.
Introduction
The four main disease-modifying medicines for heart failure (HF) and a reduced ejection fraction (HeFREF) exert symptomatic and prognostic benefits that may be observed within a few weeks of initiation.1-4 The benefit of each of these medicines is incremental to, and independent of, the benefit of the others.1 Thus, the old model of initiating and titrating the dose of agents sequentially, only initiating a new class of agent if the response to weeks or months of the previous treatment was insufficient, is flawed.5 The recent European Society of Cardiology (ESC) HF guidelines recommend that patients admitted to hospital have medicines ‘optimized’ prior to discharge.6 In practice, this means that HF specialists should attempt to initiate and up-titrate quadruple therapy, when appropriate, prior to discharge.7, 8
There are many unknowns about the practicalities of initiating and optimizing the dose of medication prior to discharge in patients with HeFREF. For example, in England and Wales, only half of patients with HeFREF admitted to hospital are discharged on a trio of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs), beta-blockers, or mineralocorticoid receptor antagonists (MRAs)9: the reasons for this are poorly understood, and the proportion of patients eligible for initiation or up-titration of these medications is unknown. The proportion of hospitalized patients who might be eligible for initiation of either sacubitril–valsartan or sodium-glucose co-transporter-2 inhibitor (SGLT2I) prior to discharge is also unknown. Polypharmacy is a growing problem for patients with HeFREF,10 and medicines optimization should also involve stopping treatments that are not beneficial, even if they are harmless. The proportion of patients that could have some medicines stopped is also unknown.
We set out to address these uncertainties in patients with HeFREF who were admitted to a hospital serving the population around Kingston upon Hull, UK.
Methods
Patient population
OPERA-HF was a prospective observational study of adults, aged >18 years, admitted with a primary diagnosis of suspected HF to a hospital serving a local community of ~500 000 people in Kingston upon Hull and East Riding of Yorkshire, UK. Other eligibility criteria included treatment with a loop diuretic and at least one of the following as evidence of cardiac dysfunction: left ventricular ejection fraction (LVEF) < 40%, left atrial diameter > 4 cm, or N-terminal pro-B-type natriuretic peptide (NT-proBNP) > 400 ng/L for those in sinus rhythm or >1200 ng/L for those in atrial fibrillation (AF). Variable NT-proBNP cut-offs were used to ensure we enrolled patients whose primary problem was HF and not lone AF (the majority of whom have NT-proBNP levels > 400 ng/L). Patients who were unable or unwilling to give informed consent were not included in the study. Ethical approval was awarded by the South Yorkshire Research Ethics Committee (REC ref.: 12/YH/0344). Patients were enrolled over 50 months between October 2012 and January 2017. The study was conducted in accordance with the Declaration of Helsinki.
Data on demographics, symptoms, bed-side observations, blood results, echocardiography, and 12-lead electrocardiograms were collected on admission and blood results and bed-side observations on the day of, or just prior to, discharge.
HeFREF was defined as an LVEF < 40% or at least moderate left ventricular systolic dysfunction by semi-quantitative visual estimation. Only patients who were alive at discharge with complete data on renal function, systolic blood pressure (SBP), and heart rate (HR) prior to discharge were included in this analysis. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula.
Patients who were discharged on a combination of morphine or diamorphine, midazolam, hyoscine butylbromide, and haloperidol11 or who were not prescribed any of ACE-I or ARB, beta-blocker, or MRA and who died within 30 days of discharge were considered to have been discharged for end-of-life care and were excluded from eligibility analyses. Patients in whom the HF admission was deemed to be due to severe valve disease were also excluded from the eligibility analysis.
Polypharmacy was defined as prescription of ≥5 medication types per day not including ACE-I, ARB, beta-blocker, MRA, loop diuretics, or, for patients in AF, oral anticoagulants. Medications that could be stopped were (i) those with randomized controlled trial data demonstrating absence of benefit for HF with reduced ejection fraction (amlodipine,12 statins,13 and oral iron14) and (ii) the potential to cause harm based on the American Heart Association list of medicines that have a detrimental effect in patients with HF. These include non-steroidal anti-inflammatory drugs, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, doxazosin, and citalopram.15
Eligibility for sacubitril–valsartan, dapagliflozin, and empagliflozin was derived from the PIONEER-HF,16 DAPA-HF,17 and EMPEROR-Reduced18 trial entry criteria, respectively. Patients who were taking ACE-I or ARB, beta-blocker, or MRA were split into three groups (Steps 1–3) based on stages of up-titration derived from the ESC HF guidelines (Supporting Information, Table S1).6 Eligibility for initiation or up-titration of ACE-I, ARB, beta-blocker, or MRA was based on two different sets of criteria: (i) advice on when to be cautious outlined in the supplementary data appendix for the ESC HF guidelines 2021 (do not initiate or up-titrate ACE-I, ARB, or MRA if SBP < 90 mmHg, potassium > 5 mmol/L, eGFR < 30 mL/min/1.73 m2, or serum creatinine concentrations > 221 mmol/L; and do not initiate or up-titrate beta-blocker if HR < 50 b.p.m. or SBP < 90 mmHg)19; and (ii) more conservative criteria using a higher SBP cut-off of <105 mmHg and less aggressive HR cut-offs (<70 b.p.m. for those in sinus rhythm and <80 b.p.m. for those in AF). Loop diuretic dose was defined as furosemide equivalents where 40 mg of furosemide ≈ 1 mg of bumetanide.
Outcome data were collected from the hospital's electronic records. Outcomes assessed were all-cause mortality, and all-cause mortality or first readmission for HF during the first year from discharge. Deaths during a first readmission were counted as deaths in the first-event composite outcome.
Statistical analysis
eGFR was calculated using the Modification of Diet in Renal Disease formula. Worsening renal function (WRF) was defined as an increase in serum creatinine of >26.5 μmol/L during admission.20 Categorical data are presented as percentages, and continuous data are presented as median (25th and 75th centiles). Chi-squared tests were used to compare categorical variables. Independent samples t-test and one-way analysis of variance were used to compare normally distributed continuous variables. The Mann–Whitney U and Kruskal–Wallis tests were used to compare non-normally distributed continuous variables. Associations between variables and outcome were assessed using Kaplan–Meier curves and a Cox proportional hazard regression model, which included a small number of variables to avoid statistical overfitting chosen a priori (age, sex, eGFR, haemoglobin, and log[NT-proBNP]).
Results
Of 1277 patients enrolled, 244 did not have a final diagnosis of HF, and 39 died during admission. Of the 994 remaining patients, 548 (55%) had HeFREF, 455 of whom had complete data on renal function, SBP, and HR (Supporting Information, Figure S1) and comprised the population for this analysis.
The proportion of patients prescribed an ACE-I or ARB, a beta-blocker, an MRA, or triple therapy prior to discharge were 83%, 85%, 63%, and 49%, respectively. Almost all patients (95%) were discharged on a loop diuretic, 73% of whom were discharged on doses ≥ 80 mg of furosemide or equivalent per day. Oral anticoagulants, mainly warfarin, were prescribed for 43% of patients, most of whom had AF. Other commonly prescribed medications included statins (44%), proton pump inhibitors (39%), regular analgesia (37%), aspirin (30%), regular inhalers (25%), and hypoglycaemic medications (25%) (Figure 1). Most patients did not have an implantable device on admission [N = 393 (86%)]. Of those who did, 7% patients had a simple pacemaker, 4% had an implantable cardioverter defibrillator (ICD), 1% had a cardiac resynchronization therapy (CRT) pacemaker, and 2% had a CRT–ICD.

Prescribing patterns by age, sex, and renal function
Older patients were less likely to be taking disease-modifying medication. Women were less likely to be taking an MRA or higher dose loop diuretic (≥80 mg/day of furosemide equivalents) and more likely to be taking regular analgesia than men. Patients with an eGFR < 60 mL/min/1.73 m2 were less likely to be taking an MRA and more likely to be taking a thiazide in addition to a loop diuretic, hypoglycaemic medicines, proton pump inhibitors, oral iron, or xanthine oxidase inhibitor than those with an eGFR ≥ 60 mL/min/1.73 m2. However, there was no difference in the rate of prescription of ACE-I or ARB, or beta-blocker based on renal function (Table 1).
| Variable | All patients | Age | Sex | Renal function | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 455 | Tertile 1 | Tertile 2 | Tertile 3 | P | Male | Female | P | eGFR ≥ 60 | eGFR < 60 | P | |
| N = 152 | N = 152 | N = 151 | N = 301 | N = 154 | N = 222 | N = 233 | |||||
| Age, years | 74 (65–81) | 59 (50–65) | 74 (72–77) | 83 (81–87) | — | 73 (64–80) | 77 (67–82) | 0.20 | 68 (59–78) | 78 (71–83) | <0.001 |
| Male sex, N (%) | 301 (66) | 108 (71) | 105 (69) | 88 (58) | 0.04 | — | — | — | 130 (59) | 171 (73) | 0.001 |
| ACE-I or ARB, N (%) | 379 (83) | 139 (91) | 126 (83) | 114 (76) | 0.001 | 253 (84) | 126 (82) | 0.60 | 191 (86) | 188 (81) | 0.13 |
| Target dose, N (%) | 50 (13) | 29 (21) | 22 (17) | 16 (14) | 0.003 | 48 (19) | 19 (15) | 0.54 | 37 (19) | 30 (16) | 0.21 |
| Beta-blocker, N (%) | 386 (85) | 138 (91) | 130 (86) | 118 (78) | 0.01 | 253 (84) | 133 (86) | 0.58 | 193 (87) | 193 (83) | 0.24 |
| Target dose, N (%) | 54 (14) | 26 (19) | 18 (14) | 11 (9) | 0.01 | 37 (15) | 18 (14) | 0.78 | 29 (15) | 26 (13) | 0.43 |
| MRA, N (%) | 286 (63) | 114 (75) | 99 (65) | 73 (48) | <0.001 | 202 (67) | 84 (55) | 0.01 | 150 (68) | 136 (58) | 0.05 |
| Target dose, N (%) | 4 (1) | 2 (2) | 1 (1) | 1 (1) | 0.20 | 3 (1) | 1 (1) | 0.30 | 2 (1) | 2 (1) | 0.13 |
| Triple therapy, N (%) | 221 (49) | 96 (63) | 80 (53) | 45 (30) | <0.001 | 155 (52) | 66 (43) | 0.09 | 126 (57) | 95 (41) | 0.001 |
| Hydralazine/nitrate, N (%) | 64 (14) | 15 (10) | 21 (14) | 28 (19) | 0.09 | 44 (15) | 20 (13) | 0.67 | 28 (13) | 326 (16) | 0.42 |
| Ivabradine, N (%) | 25 (6) | 13 (9) | 4 (3) | 8 (5) | 0.08 | 16 (95) | 9 (6) | 0.83 | 11 (5) | 14 (6) | 0.68 |
| Digoxin, N (%) | 127 (28) | 48 (32) | 46 (30) | 33 (22) | 0.12 | 88 (29) | 39 (25) | 0.44 | 68 (31) | 59 (25) | 0.21 |
| Loop diuretic, N (%) | 432 (95) | 146 (96) | 146 (96) | 140 (93) | 0.31 | 288 (96) | 144 (94) | 0.37 | 209 (94) | 223 (96) | 0.52 |
| >80 mg/day, N (%) | 333 (77) | 109 (75) | 120 (82) | 104 (74) | 0.21 | 232 (81) | 101 (70) | 0.03 | 156 (75) | 177 (79) | 0.25 |
| Thiazide diuretic, N (%) | 45 (10) | 10 (7) | 22 (15) | 13 (9) | 0.05 | 35 (12) | 10 (7) | 0.10 | 13 (6) | 32 (14) | 0.01 |
| Aspirin, N (%) | 138 (30) | 39 (26) | 46 (30) | 53 (35) | 0.20 | 92 (31) | 46 (30) | 0.91 | 74 (33) | 64 (28) | 0.19 |
| Clopidogrel, N (%) | 83 (18) | 26 (17) | 28 (18) | 29 (19) | 0.89 | 52 (17) | 31 (20) | 0.52 | 37 (17) | 46 (20) | 0.47 |
| Dual antiplatelet, N (%) | 47 (10) | 16 (11) | 13 (9) | 18 (12) | 0.63 | 29 (10) | 18 (12) | 0.52 | 27 (12) | 20 (9) | 0.22 |
| Oral anticoagulant, N (%) | 197 (43) | 67 (44) | 71 (47) | 59 (39) | 0.39 | 136 (45) | 61 (40) | 0.27 | 96 (43) | 101 (43) | 1.00 |
| Warfarin, N (%) | 156 (79) | 54 (81) | 57 (80) | 45 (76) | 0.46 | 106 (78) | 50 (82) | 0.43 | 76 (79) | 80 (79) | 1.00 |
| DOAC, N (%) | 41 (21) | 13 (19) | 14 (20) | 14 (24) | 30 (22) | 11 (18) | 20 (21) | 21 (21) | |||
| Hypoglycaemic agents, N (%) | 112 (25) | 34 (22) | 45 (30) | 33 (22) | 0.22 | 75 (25) | 37 (24) | 0.91 | 41 (19) | 71 (31) | 0.003 |
| >1 anti-diabetic medications, N (%) | 29 (26) | 11 (33) | 8 (18) | 10 (30) | 0.14 | 19 (25) | 10 (27) | 0.91 | 10 (24) | 19 (27) | 0.03 |
| Proton pump inhibitor, N (%) | 179 (39) | 53 (35) | 64 (42) | 62 (41) | 0.38 | 118 (39) | 61 (40) | 1.00 | 71 (32) | 108 (46) | 0.002 |
| Inhalers, N (%) | 112 (25) | 45 (30) | 44 (29) | 23 (15) | 0.01 | 72 (24) | 40 (26) | 0.65 | 61 (28) | 51 (22) | 0.19 |
| Xanthine oxidase inhibitors, N (%) | 31 (7) | 9 (6) | 15 (10) | 7 (5) | 0.17 | 25 (8) | 6 (4) | 0.11 | 6 (3) | 25 (11) | 0.001 |
| Regular analgesia, N (%) | 170 (37) | 60 (40) | 58 (38) | 52 (34) | 0.64 | 98 (33) | 72 (47) | 0.004 | 86 (39) | 84 (36) | 0.56 |
| Anti-depressants, N (%) | 54 (12) | 25 (16) | 15 (10) | 14 (9) | 0.10 | 30 (10) | 24 (16) | 0.09 | 31 (14) | 23 (10) | 0.19 |
| Statin, N (%) | 198 (44) | 55 (36) | 81 (53) | 62 (41) | 0.01 | 129 (43) | 69 (45) | 0.69 | 94 (42) | 104 (45) | 0.64 |
| Amlodipine, N (%) | 12 (3) | 5 (3) | 3 (2) | 4 (3) | 0.77 | 6 (2) | 6 (4) | 0.23 | 6 (3) | 6 (3) | 1.00 |
| Oral iron, N (%) | 39 (9) | 12 (8) | 11 (7) | 16 (11) | 0.54 | 22 (7) | 17 (11) | 0.22 | 12 (5) | 27 (12) | 0.02 |
| Patients with at least one HF exacerbating medicine, N (%) | 23 (5) | 6 (4) | 7 (5) | 10 (7) | 0.54 | 12 (4) | 11 (7) | 0.18 | 7 (3) | 16 (7) | 0.09 |
| Patients with at least one medicine that could be stopped, N (%) | 231 (51) | 66 (43) | 86 (57) | 79 (52) | 0.06 | 147 (49) | 84 (55) | 0.28 | 106 (48) | 125 (54) | 0.22 |
| Medications per daya | 6 (3–9) | 5 (3–8) | 7 (5–9) | 6 (4–8) | 0.06 | 6 (3–8) | 6 (4–9) | 0.45 | 5 (3–8) | 6 (4–9) | 0.07 |
| Polypharmacy, N (%)a | 310 (68) | 92 (61) | 116 (76) | 102 (68) | 0.01 | 201 (67) | 109 (71) | 0.40 | 144 (65) | 166 (71) | 0.16 |
| Eligible for sacubitril–valsartan, N (%)b | 281 (62) | 90 (59) | 90 (59) | 101 (67) | 0.28 | 177 (59) | 104 (68) | 0.07 | 151 (68) | 130 (56) | 0.01 |
| Eligible for dapagliflozin, N (%)c | 345 (76) | 113 (74) | 112 (74) | 120 (80) | 0.44 | 220 (73) | 125 (81) | 0.06 | 186 (84) | 159 (68) | <0.001 |
| Eligible for empagliflozin, N (%)d | 323 (71) | 104 (68) | 107 (70) | 112 (75) | 0.53 | 203 (67) | 120 (78) | 0.02 | 167 (75) | 156 (67) | 0.06 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HF, heart failure; MRA, mineralocorticoid receptor antagonist.
- Shaded cells indicate P ≤ 0.01.
- a Not including ACE-I or ARB, beta-blocker, MRA, loop diuretic, or oral anticoagulation for those in atrial fibrillation.
- b Derived from entry criteria for the PIONEER-HF trial.
- c Derived from the entry criteria for the DAPA-HF trial.
- d Derived from the entry criteria for the EMPEROR-Reduced trial.
Despite great variation of medications prescribed on admission, most patients prescribed an ACE-I or ARB prior to discharge were taking either enalapril or ramipril, and the most patients prescribed a beta-blocker were taking either carvedilol or bisoprolol (Supporting Information, Figure S2).
Eligibility for sacubitril–valsartan or sodium-glucose co-transporter-2 inhibitor
Sixty-two per cent, 76%, and 71% of patients were potentially eligible to start on sacubitril–valsartan, dapagliflozin, and empagliflozin prior to discharge, respectively (Table 1). There was no difference in eligibility based on age, but women were more likely than men to be eligible for empagliflozin (Table 2). The most common reason for ineligibility was low SBP followed by low eGFR (Figure 2). No patient would have failed eligibility for trial inclusion based on their plasma NT-proBNP being insufficiently elevated.
| Variable | ARNI | Dapagliflozin | Empagliflozin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eligible | Ineligible | P | Eligible | Ineligible | P | Eligible | Ineligible | P | |
| N = 281 | N = 174 | N = 345 | N = 110 | N = 323 | N = 132 | ||||
| Demographics and observations | |||||||||
| Age, years | 74 (65–81) | 73 (63–79) | 0.07 | 75 (65–81) | 74 (63–80) | 0.35 | 75 (66–81) | 74 (63–80) | 0.10 |
| Male sex, N (%) | 177 (63) | 124 (71) | 0.08 | 220 (64) | 81 (74) | 0.06 | 203 (63) | 98 (74) | 0.02 |
| New HF diagnosis, N (%) | 77 (27) | 62 (36) | 0.08 | 99 (29) | 40 (36) | 0.15 | 89 (28) | 50 (39) | 0.03 |
| AF, N (%) | 110 (39) | 66 (38) | 0.34 | 137 (40) | 39 (36) | 0.73 | 120 (37) | 56 (42) | 0.58 |
| IHD, N (%) | 110 (39) | 72 (41) | 0.69 | 133 (39) | 49 (45) | 0.27 | 130 (40) | 52 (39) | 0.92 |
| Hypertension, N (%) | 155 (55) | 81 (47) | 0.08 | 191 (55) | 45 (41) | 0.01 | 183 (57) | 53 (40) | 0.002 |
| Diabetes, N (%) | 88 (31) | 63 (36) | 0.31 | 115 (33) | 36 (33) | 1.00 | 107 (33) | 44 (33) | 1.00 |
| CKD, N (%) | 44 (16) | 45 (26) | 0.01 | 51 (15) | 38 (35) | <0.001 | 58 (18) | 31 (24) | 0.19 |
| COPD, N (%) | 71 (25) | 44 (25) | 1.00 | 87 (25) | 28 (26) | 1.00 | 79 (25) | 36 (27) | 0.55 |
| LVEF < 30%, N (%) | 117 (42) | 92 (53) | 0.02 | 146 (42) | 63 (57) | 0.01 | 129 (40) | 80 (61) | <0.001 |
| Systolic BP, mmHg | 114 (107–127) | 97 (91–113) | <0.001 | 112 (105–127) | 93 (90–103) | <0.001 | 114 (106–127) | 94 (90–99) | <0.001 |
| Heart rate, b.p.m. | 73 (65–82) | 75 (68–84) | 0.16 | 73 (65–83) | 75 (67–82) | 0.47 | 73 (65–82) | 75 (68–84) | 0.18 |
| Blood results | |||||||||
| NT-proBNP, ng/L | 6275 (3751–11 443) | 5561 (1330–12 370) | 0.01 | 5561 (2774–10 458) | 7484 (3316–15 809) | 0.02 | 5915 (3424–10 717) | 6305 (1915–14 228) | 0.59 |
| Potassium, mmol/L | 4.2 (3.9–4.6) | 4.3 (3.9–4.7) | 0.18 | 4.2 (3.9–4.6) | 4.2 (4.0–4.7) | 0.24 | 4.2 (3.9–4.6) | 4.2 (3.9–4.6) | 0.15 |
| eGFR, mL/min/1.73 m2 | 62 (46–82) | 51 (33–71) | <0.001 | 62 (46–81) | 45 (27–66) | <0.001 | 61 (44–81) | 53 (39–72) | 0.01 |
| Inpatient WRF, N (%) | 33 (12) | 41 (24) | 0.002 | 45 (13) | 29 (26) | 0.002 | 48 (15) | 26 (20) | 0.21 |
| Medications on discharge | |||||||||
| ACE-I or ARB, N (%) | 234 (83) | 145 (83) | 1.00 | 289 (84) | 90 (82) | 0.66 | 271 (84) | 108 (82) | 0.58 |
| Beta-blocker, N (%) | 239 (85) | 147 (85) | 0.89 | 293 (85) | 93 (85) | 1.00 | 274 (85) | 112 (85) | 1.00 |
| MRA, N (%) | 187 (67) | 99 (56) | 0.05 | 221 (64) | 65 (59) | 0.37 | 208 (65) | 78 (59) | 0.29 |
| Triple therapy, N (%) | 142 (51) | 79 (45) | 0.29 | 169 (49) | 52 (47) | 0.83 | 158 (49) | 63 (48) | 0.84 |
| Loop diuretic, N (%) | 268 (95) | 164 (94) | 0.66 | 329 (95) | 103 (94) | 0.46 | 308 (95) | 124 (94) | 0.64 |
| ≥80 mg/day, N (%) | 204 (76) | 129 (79) | 0.48 | 253 (77) | 80 (78) | 0.79 | 238 (77) | 95 (77) | 1.00 |
| Outcome | |||||||||
| 1 year all-cause mortality, N (%) | 48 (17) | 57 (33) | <0.001 | 65 (19) | 40 (36) | <0.001 | 62 (19) | 43 (33) | 0.003 |
| 1 year all-cause mortality or HF readmission, N (%) | 126 (45) | 98 (56) | 0.02 | 160 (46) | 64 (58) | 0.04 | 154 (48) | 70 (53) | 0.30 |
- ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BP, blood pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; WRF, worsening renal function.
- Shaded cells indicate P ≤ 0.01.
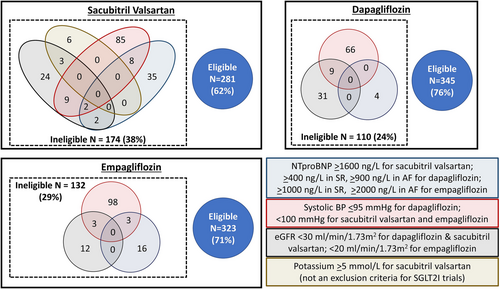
Patients eligible for sacubitril–valsartan or dapagliflozin were less likely to have an LVEF < 30% or inpatient WRF than those deemed ineligible. Patients eligible for empagliflozin were more likely to have a diagnosis of HF prior to admission and less likely to have an LVEF < 30%, but there was no difference in the proportion of patients with WRF. Patients eligible for either class of medication had lower all-cause mortality rates in the following year than those deemed ineligible.
Medicines optimization
Using ESC guideline criteria, ACE-I or ARB could have been initiated in approximately half of patients not taking these medicines; using our more conservative criteria, this fell to about one-third. Using ESC guideline criteria, beta-blockers could have been initiated in most patients, but only one in four using our more conservative cut-offs. MRA could have been initiated in approximately half of patients regardless of which criteria were applied (Table 3 and Supporting Information, Figure S3A–C). Renal dysfunction, hyperkalaemia, cough, lung disease, and gynaecomastia were not common reasons for non-prescription of medications (Table 3).
| Reason | ACE-I or ARB | Beta-blocker | MRA | |||||
|---|---|---|---|---|---|---|---|---|
| S0 ➔ S1 | S1 ➔ S2 | S2 ➔ S3 | S0 ➔ S1 | S1 ➔ S2 | S2 ➔ S3 | S0 ➔ S1 | S1 ➔ S2 | |
| N = 76 | N = 175 | N = 154 | N = 69 | N = 211 | N = 121 | N = 169 | N = 282 | |
| SBP ≤ 90 mmHg, N (%)a | 3 (4) | 13 (7) | 8 (5) | 6 (9) | 9 (4) | 7 (6) | 9 (5) | 14 (5) |
| SBP ≤ 105 mmHg, N (%)b | 25 (33) | 70 (40) | 56 (36) | 23 (33) | 74 (35) | 51 (42) | 60 (36) | 100 (36) |
| eGFR ≤ 30 mL/min/1.73 m2 or Cr ≥ 221 mmol/La/b | 10 (12) | 15 (9) | 13 (8) | NA | NA | NA | 25 (15) | 14 (5) |
| Potassium > 5.0 mmol/La/b | 7 (9) | 18 (10) | 9 (6) | NA | NA | NA | 14 (8) | 18 (6) |
| Heart rate ≤ 50 b.p.m.a | NA | NA | NA | 0 | 2 (1) | 1 (1) | NA | NA |
| Heart rate of ≤70 b.p.m. in SR or ≤80 b.p.m. in AF, N (%)b | NA | NA | NA | 37 (54) | 109 (52) | 64 (53) | NA | NA |
| Severe valvular heart disease | 7 (9) | 8 (5) | 2 (1) | 3 (4) | 10 (5) | 4 (3) | 6 (4) | 11 (4) |
| Palliative | 8 (11) | NA | NA | 7 (10) | NA | NA | 6 (4) | NA |
| Documented intolerance or allergy in the medical notes, N (%) | 20 (26) | 14 (20) | 5 (3) | |||||
| Hyperkalaemia, N | 1 | NA | 0 | |||||
| Hypotension, N | 1 | 0 | 0 | |||||
| Renal dysfunction, N | 3 | NA | 4 | |||||
| Cough, N | 1 | NA | NA | |||||
| Angio-oedema, N | 0 | NA | NA | |||||
| Bradycardia, N | NA | 4 | NA | |||||
| Lung disease, N | NA | 3 | NA | |||||
| Gynaecomastia, N | NA | NA | 1 | |||||
| Non-specified allergy, N | 13 | 7 | 0 | |||||
| Eligible to start or titrate treatment by Criteria A, N (%) | 36 (47) | 131 (75) | 123 (80) | 55 (80) | 190 (90) | 109 (90) | 115 (68) | 230 (82) |
| Eligible to start or titrate treatment by Criteria B, N (%) | 28 (37) | 82 (47) | 83 (54) | 19 (28) | 52 (25) | 26 (22) | 82 (49) | 156 (55) |
- ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; Cr, creatinine; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; HF, heart failure; MRA, mineralocorticoid receptor antagonist; NA, not applicable; S0, not taking the treatment; S1–3, steps of titration as per Supporting Information, Table S1 derived from the ESC HF guidelines; SBP, systolic blood pressure; SR, sinus rhythm.
- a Criteria derived from the ESC HF guidelines.
- b Criteria based on higher SBP and heart rate cut-offs.
From the total population, we identified 299 patients (66%) who should have had an ACE-I or ARB either initiated or up-titrated and 358 patients (79%) who should have had a beta-blocker initiated or up-titrated (Supporting Information, Figure S4). Using higher SBP and HR cut-offs, the proportion of patients in whom an ACE-I or ARB, or beta-blocker might have been initiated or up-titrated decreased to 45% and 24%, respectively (Table 3 and Supporting Information, Figures S3A–C and S5A,B).
Almost all patients (96%) were taking ≥5 medications. If ACE-I or ARB, beta-blocker, MRA, loop diuretic, and oral anticoagulation were subtracted, the proportion taking ≥5 medications fell to 68%, but 51% were taking medications either with no known benefit or with potential harm (Table 1). Although only a minority of patients was taking a medicine that might exacerbate HF (N = 23), even after adjusting for age, sex, renal function, haemoglobin, and NT-proBNP, these patients were at two-fold higher risk of 1 year all-cause mortality or HF hospitalization [hazard ratio = 2.05 (95% confidence interval 1.22–3.45); χ2 = 7; P = 0.007] compared with those not taking such medication.
Outcome
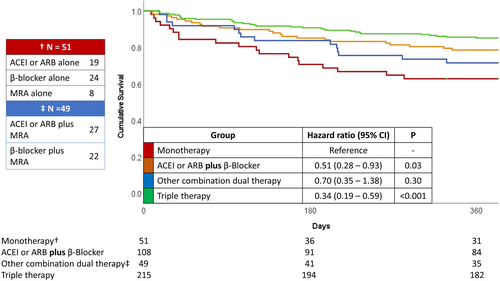
All patients had at least 1 year follow-up during which time, 106 patients died (23%), and a further 118 were readmitted with HF (Supporting Information, Table S2). Patients prescribed an ACE-I or ARB prior to discharge were approximately half as likely to experience either endpoint, independent of age, sex, renal function, haemoglobin, log[NT-ProBNP], and other medication prescriptions (Supporting Information, Table S3). Findings were similar for beta-blockers with a similar trend for MRAs. Compared with those taking only a single agent, those taking a combination of ACE-I or ARB plus beta-blocker had a lower risk of death during the first year following discharge [hazard ratio = 0.51 (0.28–0.93); P = 0.03]. Those taking triple therapy were at even lower risk [hazard ratio = 0.34 (0.19–0.59); P < 0.001] (Figure 3).
Discussion
- >50% of patients would have been eligible to start sacubitril–valsartan and/or an SGLT2I prior to discharge;
- an ACE-I or ARB, beta-blocker, or MRA could have been started in about a third of patients who were not taking these agents at discharge; and
- ~50% of patients were taking a medication that either was of no prognostic benefit or had the potential to cause harm.
Inpatient initiation and titration: can it be done?
New therapies
We found that over half of patients would be eligible to start sacubitril–valsartan based on entry criteria for the PIONEER-HF trial.16 The PIONEER-HF and TRANSITION trials have demonstrated the feasibility and safety of initiation of sacubitril–valsartan prior to discharge in patients with HeFREF.16, 21 Not all patients tolerated higher doses of sacubitril–valsartan, but this failure was not associated with less survival benefit compared with enalapril.22
It is more difficult to infer the proportion of patients who would have been eligible for SGLT2Is: although we found a similar proportion of patients eligible for dapagliflozin or empagliflozin, patients were not enrolled as inpatients in the DAPA-HF or EMPEROR-Reduced trials. The SOLOIST-WHF study [sotagliflozin initiated in patients with HF (regardless of LVEF) and diabetes prior to or just after discharge vs. placebo] found no difference in the rate of discontinuation of sotagliflozin compared with placebo,23 suggesting that initiating SGLT2I prior to discharge is safe.24 Although we used the entry criteria for the EMPEROR-Reduced trial, the EMPULSE trial of empagliflozin vs. placebo in haemodynamically stable inpatients used similar renal function and blood pressure entry criteria and showed benefit.25, 26
However, in the United Kingdom, the National Institute for Health and Care Excellence guidelines, last updated in 2018, only recommend either sacubitril–valsartan or dapagliflozin for patients with HF who have persistent symptoms despite treatment with a stable dose of ACE-I or ARB plus beta-blocker or MRA (i.e. not those currently admitted to, or recently discharged from, hospital).27, 28 Future iterations may reflect the data on safety of initiating either sacubitril–valsartan or SGLT2I as an inpatient, as well as the pragmatism of doing so during hospital admission.
One-year outcomes were worse for those not eligible for treatment with either sacubitril–valsartan or an SGLT2I. A substantial proportion of patients at high risk of adverse outcome might not be eligible for these newer treatments based on the trial evidence so far. This may change as more evidence and experience is generated.
Conventional therapies
Initiation of conventional HF therapy during admission is routine practice: we found that about half of patients discharged on an ACE-I or ARB, or beta-blocker had not been prescribed these medications prior to admission. While only a small minority were not taking an ACE-I or ARB, or beta-blocker at discharge, over one-third of patients were not taking an MRA, about half of whom had no contraindications. This deficit in MRA prescribing is similar to that reported in the UK National Heart Failure Audit, and European and American registry data.9, 29, 30 In addition to developing new therapies, investment in implementation research is required to ensure that patients benefit from innovations in care.
Inpatient initiation and titration: should it be done?
Of those who survive to discharge, one in four patients will be readmitted and one in eight will die within a month of admission.9, 31 Quadruple therapy can reduce the risk of cardiovascular death or HF hospitalization by 62% compared with treatment with ACE-I or ARB plus beta-blocker alone,1 and the time to onset of benefit of several HF medications is known to be <1 month.2-4 As a result, guidelines recommend that patients are seen within 2 weeks of discharge by an HF specialist,6 but fewer than half of care providers can meet this target.9 HF specialist nurses are ideally placed to assess patients soon after discharge, but their availability is highly variable.32 Many patients remain on the doses of medication on which they were discharged until they are readmitted.33 Hospital admission is thus a vital opportunity to start (and perhaps up-titrate) HF medications.
Clinical inertia in the face of apparent clinical stability is often cited as a reason for failure to prescribe or up-titrate life-prolonging medications in outpatients with HeFREF.34 Similar mechanisms may be at play for inpatients. Access to specialist care is another important factor: patients under the care of a cardiologist are far more likely to be discharged on triple therapy than those who do not see an HF specialist as an inpatient.9 Ageism may also play a role; the proportion of patients prescribed life-prolonging medication decreases with advancing age.9, 29, 30
Unwillingness to interfere with the medication regimen of a patient with HF is understandable; those who survive to discharge have often been treated with high doses of intravenous diuretic for more than a week.9, 30 Neither the patient nor clinician wishes the hospital stay to be longer than necessary: each day in hospital with HF costs the National Health Service in the United Kingdom ~£450,35 and nosocomial infection (including COVID) is a common and often fatal complication.36 There is a difficult balance to strike.
It is unrealistic, and potentially dangerous, to attempt to initiate and up-titrate all medications to target doses during an inpatient stay7, 8; such a strategy has never been tested in a prospective trial. However, SGLT2Is do not require titration, and spironolactone potentially only requires one increment in dose from 25 mg/day. Titration of other disease-modifying agents is more complex. Hospital admission may be the best opportunity for initiation and pragmatic initiation and up-titration of quadruple therapy, while also providing a chance to assess tolerability. The selection and timing of device therapies also needs to be considered.6
Discontinuation and polypharmacy
Polypharmacy, and its consequences (such as non-adherence),37 is an increasing problem for patients with HeFREF and those who care for them. Even excluding useful polypharmacy (medications that have prognostic or symptomatic benefit for HF or related conditions), we found that over half of patients met the definition for polypharmacy (≥5 medication types per day).10 Furthermore, we found that ~50% of patients were taking medications with no proven benefit or that were potentially harmful. We cannot infer that it would have been appropriate to stop such medications in all patients, but our data suggest that there is scope for discontinuing some as part of ‘medicines optimization’ prior to discharge.
Limitations
Although our cohort was enrolled prospectively, the limitations of post hoc analyses apply, and we cannot exclude confounding factors. For example, although we had data on some medications given during admission such as intravenous diuretics, nitrates, and inotropic agents, we did not have data on dose or duration of loop diuretics. Although we did not have comprehensive data on symptoms or signs of congestion on either admission or discharge to include in the final analysis, we assume that very few patients were discharged with a substantial amount of congestion as this is against guideline recommendations.6
Our data are from a single centre; thus, our conclusions might not be generalizable, particularly outside the United Kingdom. However, our data are similar to those published by the National Heart Failure Audit9 and in registries elsewhere in Europe and beyond.29, 30
We also lacked more detailed echocardiographic assessment beyond classification of the severity of left ventricular systolic dysfunction. Treatments such as MRA, sacubitril–valsartan, and SGLT2I have only been investigated in patients with an LVEF ≤ 35%, but our population matched the definition of HeFREF in the most recent ESC HF guidelines, which advocate treatment with each of those medications for patients with LVEF < 40%.6
Finally, although observational studies can only demonstrate associations, failure to prescribe guideline-recommended therapies indicates a poor outcome, either because the medications are contraindicated or because someone has neglected to prescribe them.
Conclusions
More than half of patients with HeFREF may be eligible to start on sacubitril–valsartan or an SGLT2I prior to discharge following an admission with HF. A large proportion of patients not prescribed life-prolonging treatments prior to discharge appeared to have no contraindication to their use.
Acknowledgements
None.
Conflict of interest
I.S., S.C.P., and J.M.R. are employed by Philips Research. J.G.F.C., A.L.C., and S.K. have received departmental research support from Philips. J.J.C., O.I.B., P.P., K.D., and J.B. have no conflict of interest to declare.
Funding
J.G.F.C. is supported by a British Heart Foundation Centre of Research Excellence (grant number RE/18/6/34217).




