Previous cardiovascular injury is a prerequisite for immune checkpoint inhibitor-associated lethal myocarditis in mice
The present work was performed in Tecnologico de Monterrey, Hospital Zambrano Hellion, TecSalud.
Abstract
Aims
Immune checkpoint inhibitors (ICIs) are antineoplastic drugs designed to activate the immune system's response against cancer cells. Evidence suggests that they may lead to immune-related adverse events, particularly when combined (e.g., anti-CTLA-4 plus anti-PD-1), sometimes resulting in severe conditions such as myocarditis. We aimed to investigate whether a previously sustained cardiac injury, such as pathological remodelling due to hypertension, is a prerequisite for ICI therapy-induced myocarditis.
Methods
We evaluated the cardiotoxicity of ICIs in a hypertension (HTN) mouse model (C57BL/6). Weekly doses were administered up to day 21 after the first administration. Our analysis encompassed the following parameters: (i) survival and cardiac pathological remodelling, (ii) cardiac function assessed using pressure-volume (PV)-loops, with brain natriuretic peptide (BNP) serving as a marker of haemodynamic dysfunction and (iii) cardiac inflammation (cytokine levels, infiltration, and cardiac antigen autoantibodies).
Results
After the first administration of ICI combined therapy, the treated HTN group showed a 30% increased mortality (P = 0.0002) and earlier signs of hypertrophy and pathological remodelling compared with the untreated HTN group. BNP (P = 0.01) and TNF-α (<0.0001) increased 2.5- and 1.7-fold, respectively, in the treated group, while IL-6 (P = 0.8336) remained unchanged. Myocarditis only developed in the HTN group treated with ICIs on day 21 (score >3), characterised by T cell infiltration and increased cardiac antigen antibodies (86% showed a titre of 1:160). The control group treated with ICI was unaffected in any evaluated feature.
Conclusions
Our findings indicate that pre-existing sustained cardiac damage is a necessary condition for ICI-induced myocarditis.
Introduction
Immune checkpoint inhibitors (ICIs) are a form of cancer immunotherapy. They activate T cells to target and destroy cancer cells by targeting immune checkpoint molecules, such as cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1) and its ligand PD-L1. Although ICIs are effective against cancer, they can lead to immune-related adverse events, including cardiotoxicities, such as myocarditis.1 Yet the exact mechanisms behind these cardiotoxicities remain largely unknown.2 With increasing ICI use, it is crucial to study ICI-induced cardiotoxicity to prevent cardiac immune-related adverse events.1
PD-1 and CTLA-4 are receptors on T cells. They interact with ligands such as PD-L1/L2 and B7–1/2 as coinhibitory signals. These signals prevent excessive T cell activation by self-antigens and repeated antigen recognition in major histocompatibility complex (MHC) molecules, thereby averting autoimmune and excessive immune responses.3 CTLA-4 primarily functions in lymphoid organs during initial naïve T cell activation, while PD-1 regulates activated T cells in peripheral tissues.3 During chronic stimulation, autoreactive T cells may be released and activated. However, the PD-1 axis induces T-cell exhaustion. This state, characterised by reduced function and proliferation, limits autoimmune responses and further damage. It occurs due to PD-1 ligand upregulation by immune and other cells, including cardiomyocytes. These processes help regulate the immune response in cardiac tissue, preventing autoimmunity.3-5 Whereas tumour cells activate this process by expressing PD-1 and CTLA-4 ligands, ICI therapy releases this ‘brake’, activating antitumor immunity.3
We hypothesize that removing this immunomodulatory ‘brake’ might promote heart dysfunction when cardiac antigens are present, ultimately resulting in cardiac inflammation and myocarditis. We base our hypothesis on the following evidence: (1) chronic inflammation releases cardiac self-antigens,6, 7 (2) cardiomyocytes express PD-L1 when damaged,4, 8 (3) limiting T cell activation in the heart by the PD-1/PDL-1 axis prevents myocarditis,8, 9 (4) autoimmune cardiomyopathy and myocarditis resulting from the absence of PD-1 are mediated by autoreactive T cells and autoantibodies to cardiac antigens, and they can occur without previous induced damage in immunocompromised models,6, 9-11 and (5) hypertensive patients who received combined ICI therapy developed fulminant myocarditis after one dose.12 Considering these outcomes, we used a mouse model of hypertension-derived cardiac injury.
This study reveals a critical finding: previously sustained heart damage is a prerequisite for ICI-related myocarditis. We demonstrate that a single dose of combined ICIs (anti-CTLA-4 and anti-PD-1) reduced survival, worsened heart hypertrophy and inflammation, and impaired cardiac function in the presence of sustained cardiac damage. In surviving animals, inflammation progressed with heart-infiltrating cells, including T cells, with increased autoantibody production against heart proteins due to the loss of immunotolerance induced by ICIs. Healthy mice receiving ICIs showed no adverse effects. (Figure 1).
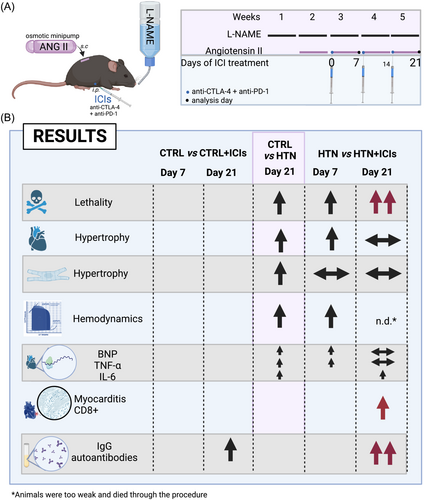
Methods
Three-month-old C57BL/6 male mice (n = 80 total) were randomly assigned using an Excel spreadsheet to generate the following groups: control (CTRL), CTRL with ICIs (+ICIs), HTN, and HTN + ICIs. To establish the HTN model, mice were administered L-NAME (300 mg/L) and NaCl (10 g/L) through their drinking water and angiotensin II (AngII, 0.7 mg/kg/day) by subcutaneous micro-osmotic pump implantation (Alzet, no. 1004).13 After a week (day 0 of the study), ICI treatment commenced with a weekly intraperitoneal administration of 200 μg of anti-CTLA-4 plus 200 μg of anti-PD-1 [InVivoMAb: anti-mouse PD-1 (CD279) and CTLA-4 (CD152), BioXCell, Lebanon, NH 03766 USA] in the CTRL + ICIs and HTN + ICIs groups for up to three doses. CTRL and HTN mice received an intraperitoneal injection with the vehicle InVivoPure dilution buffer (ip0070; Bio X Cell). The measurements were analysed on days 7 and/or 21 of ICI treatment (Figure 1). The animal protocol was approved by the animal use and care committee of Tecnológico de Monterrey Medical School (ID 2019–016). On day 7 after the ICI treatment, the pressure-volume (PV)-loop measurements were performed with an open-heart configuration using a 1.2F PV catheter with the ADV500 PV measurement system (Transonic Science, NY, USA) under sevoflurane anaesthesia (3.5% with 500 mL/min of oxygen). PV-loops were recorded at 1000 Hz, and following steady-state measurement, the inferior cava vein was transiently occluded to record end-diastolic and end-systolic PV relationships upon different preloads. On days 7 and 21, the hearts were excised, weighed, and dissected transversally. Then, the apical section of the heart (about 1 mm thick) was frozen at −80°C before isolating RNA for gene expression analysis using TRIzol reagent (15596026, Invitrogen). The cDNA was reverse-transcribed from 1 μg of total RNA using the SensiFAST cDNA Synthesis Kit (BIO-65053, Bioline). The qPCR reaction for BNP, TNF-α and IL-6 gene expression was performed with a SensiFAST SYBR Lo-ROX Kit (BIO-94020, Bioline) in a QuantStudio 3 RT PCR System (Thermo Fisher Scientific). Data were analysed using the 2−ΔΔCt method to estimate each gene's mRNA expression. The primer sets for GAPDH forward ACCCAGAAGACTGTGGATGG and reverse ACACATTGGGGGTAGGAACA; BNP forward GCTGTAACGGTGAGCACCTA and reverse TGACCCCTCTGATGGAAGGT; TNF-α forward CCAGTGTGGGAAGCTGTCTT and reverse AAGCAAAAGAGGAGGCAACA; IL-6 forward TCCAGTTGCCTTCTTGGGAC and reverse GTGTAATTAAGCCTCCGACTTG. Next, another three heart sections (about 2 mm thick) were fixed in 4% (wt/vol) paraformaldehyde for haematoxylin–eosin staining and immunohistochemistry assays. The cardiomyocyte area (microphotography of the papillary muscles was taken at 10×) was determined only in cells with complete visible cytoplasm and central nuclei (at least 50 cells per mouse were measured). To assess myocarditis, at least five photos encompassing the entire transversal section of the tissue were evaluated for each sample. The percentage of the infiltrated area was calculated using the myocarditis score.14 All measures were performed using AxioVision software. To elucidate the presence of CD3 T cells, 4-μm thick sections were incubated overnight at 4°C with anti-CD3 antibody (1:250, Dako®, M7254). The Envision® detection system and 3,3-′diaminobenzidine (DAB) were applied. The slides were mounted with resin and counterstained with Harris's haematoxylin. Digital high-resolution images were obtained with a Nikon Microscope Eclipse 50i with an image analysis system from the NIS-elements software (Digital Sight dDS-2Mu). Two blinded morphology specialists performed the morphological analysis. Finally, IgG antibody quantification against cardiac antigens was conducted using indirect ELISA. Microtiter plate wells were coated with cardiac antigens, using 1 μg of heart tissue (total soluble proteins). Subsequently, they were incubated with serial dilutions of mouse serum at ratios of 1:40, 1:80, or 1:160. The antibody–antigen reaction was detected using anti-IgG-horseradish peroxidase (Biorad, 1721066). Absorbance was measured at 450 nm.
Results
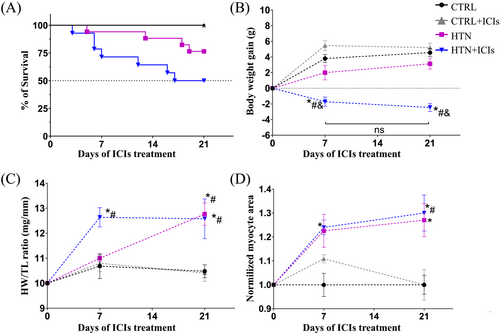
Dual ICI therapy increased mortality in mice, with a statistically significant (P = 0.0002) decrease of 30% in survival, as shown by the Kaplan–Meier curves in the HTN + ICIs group, compared with the HTN group (Figure 2). Well-established clinical features of the HTN model include a mild reduction in weight gain (P = 0.3467 vs. CTRL; Figure 2) and significant cardiac hypertrophy (P = 0.0012 vs. CTRL; Figure 2). ICI treatment accelerated and exacerbated these macroscopic changes, as indicated by the significant weight loss (P = 0.0019 vs. HTN; Figure 2) and earlier heart weight gain probably associated with oedema in the HTN + ICIs group (P = 0.054 vs. CTRL; Figure 2). By contrast, the progression of the myocyte area was not altered by ICI administration (P = 0.9 vs. HTN; Figure 2). Notably, mortality and pathological remodelling were not affected by ICI administration in the control animals. These findings show that the ICI treatment only accelerated and exacerbated pre-existing damage in the HTN model in immunocompetent conditions.
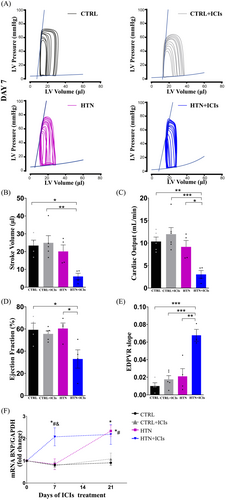
Next, cardiac haemodynamics were assessed to evaluate the functional impact of the ICIs (Figure 3). Seven days after the first ICI dose, the stroke volume, cardiac output, and ejection fraction (Figure 3, respectively) were significantly reduced (70%, 67% and 45%, respectively) compared with the HTN group, while the end-diastolic pressure–volume relationship (EDPVR) increased 3.5-fold (Figure 3). Moreover, at this point in the model (second week of Ang II), the HTN group did not show changes in any of the evaluated parameters. These data indicate that the use of ICIs accelerated the progression of HTN, leading to increased ventricular chamber stiffness and hypertrophy (Figure 2), but this occurred only when a subjacent cardiac insult was present. Notably, at this model stage, the HTN group did not exhibit significant measurable changes compared with the controls, even at the gene expression level based on BNP levels. The expression of BNP aligned with the observed remodelling and functional alterations in both the CTRL and HTN groups, consistent with the haemodynamic changes (Figure 3).
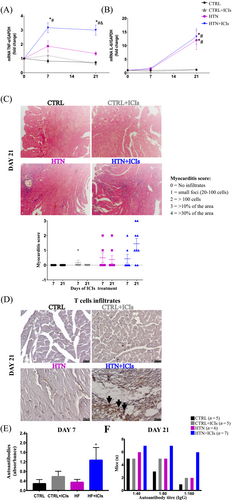
As a result of the haemodynamic stress, inflammation markers were also modulated, indicated by the gene expression in the cardiac tissue of the cytokines TNFα and IL-6 (Figure 4). After 7 days of ICI treatment, TNF-α significantly increased in the HTN + ICIs group compared with the HTN group. Similarly, IL-6 was not affected by ICI treatment, showing the same expression pattern as the HTN group. These data indicate that the observed early damage was associated with increased inflammatory pathways. Indeed, on day 21, we found that the exacerbated inflammatory process on day 7 evolved into myocarditis, as assessed by cell infiltration based on the myocarditis score14 (Figure 4) and T cell phenotyping (Figure 4). In addition to T cell infiltration, the exacerbated inflammatory process also involved a significant increase in autoantibodies against cardiac antigens. This was observed in all mice in the HTN + ICIs group (Figure 4), with a titre response of 1:160 in 86% of these mice (n = 6) compared with 20% in the CTRL (n = 1) and 40% in the CTRL + ICIs and HTN mice (n = 2) on day 21 (Figure 4). The minimum titre autoantibody response induced by the ICI treatment was 1:80. Importantly, despite the increase in autoantibodies in the CTRL + ICIs group, these mice did not develop cardiac inflammation or dysfunction.
Discussion
Our data indicate that dual therapy with ICIs, anti-CTL-4 and anti-PD-1, induced mortality by exacerbating pre-existing cardiac damage in immunocompetent conditions, likely by the loss of cardiac tolerance, which resulted in cardiac autoreactivity. The accelerated cardiac remodelling and dysfunction triggered by ICIs in the HTN model led to myocarditis with T-cell infiltration and increased circulating cardiac autoantibodies. These data strongly suggest that autoreactive T cells become activated due to cardiac antigen release caused by sustained cardiac damage and the breakdown of the immune checkpoint pathway by ICI therapy.
PD-1 and CTLA-4 are important coinhibitory signals expressed in T cells to prevent autoimmunity and excessive immune reactions.3 Knockout or transgenic mice from these coinhibitory signals have demonstrated associations between myocarditis, cardiomyopathy, and elevated IgG autoantibodies.9, 10, 15, 16 Additionally, these models have shown activated T-cell infiltration in response to cardiac antigens.9, 11
However, it is crucial to underline that the existing data have not definitively established the direct role of ICIs in wild type immunocompetent strain mice and that C57BL/6 mice have been described as myocarditis-resistant mice.11, 17, 18 Therefore, even PD-1-deficient mice do not develop spontaneous cardiomyopathies without additional modification.10, 11 Notably, our own findings recapitulate the clinical and pathological hallmarks of ICI-associated myocarditis19 as observed in patients treated with dual therapy, in whom hypertension appears to be the only discernible cardiovascular risk factor.12 Furthermore, we confirmed the mechanisms observed in PD-1 knockout mice, particularly the increased presence of IgG cardiac antibodies and T-cell cardiac infiltration.
Supporting our hypothesis that previous damage is a prerequisite for developing myocarditis, we tested it in immunocompetent conditions. In line with our hypothesis, a recent study conducted in PD-1 knockout mice found that repetitive neurohormonal stress increased mortality, hypertrophy, left ventricular dysfunction, and T-cell infiltration.20 On the other hand, the A/J mice strain, which possesses an MHC haplotype susceptible to myocarditis21 and complement C5 deficiency, developed myocarditis after ICI treatment without any additional stimulus.11 This was accompanied by the infiltration of activated T cells specific to cardiac antigens. Importantly, it should be noted that the development of myocarditis in this scenario requires a multiple-dose regimen involving more than five doses.11 These data indeed suggest that damage is a prerequisite when there is no immunological deficiency or in the absence of HLA haplotypes that confer susceptibility to myocarditis.
In summary, our data indicate that ICI therapy does not evoke fulminant myocarditis or another inflammatory process in healthy conditions in which the immune system is not compromised (immunocompetent mice). By contrast, when a cardiac insult exists, such as hemodynamic changes induced by hypertension, ICIs can be deadly as early as the first dose, as documented in patients.12 Even surviving mice showed myocarditis and increased IgG cardiac autoantibodies, indicating an inflammatory sustain process. Moreover, in this model, we have previously demonstrated that the establishment of HF involves inflammatory cytokine and immunoglobulin deposition dependent on B cell activation.13 With the ICIs worsening this process, the role of B cell activation needs to be addressed.
Some limitations of this study are that we did not conduct a quantitative characterisation of the cellular infiltration within the cardiac tissue, nor did we identify the specific cardiac antigen responsible for inducing the autoreactive response. Although C57BL/6 mouse strain not spontaneously develop myocarditis, previous research has suggested that cardiac myosin (α-MyHC) is a plausible candidate for autoreactivity due to impaired T-cell tolerance. However, this hypothesis still requires further validation and demonstration. Another limitation of this work is that we only evaluated B-type natriuretic peptide (BNP) gene expression, which, until it provides valuable information about cardiac stress and dysfunction, does not directly measure acute cardiac damage. On the other hand, having both troponin and BNP measurements provides a more comprehensive assessment of cardiac health in animal models.
Conclusion and perspectives
In the HTN mouse model, treatment with ICIs significantly increased both mortality and the occurrence of myocarditis. In cases of sustained inflammation in heart tissue, dual ICI therapy can disrupt immune tolerance. This disruption is likely directed against self-cardiac antigens, exacerbating inflammation, fostering T cell infiltration, elevating autoantibodies against cardiac antigens, and ultimately contributing to the development of myocarditis.
Although not all patients with chronic heart tissue inflammation develop myocarditis during ICI therapy, it is vital to identify those at potential risk. Currently, there are no universally accepted guidelines for managing immune-mediated myocarditis resulting from ICI treatment. However, researchers have proposed implementing early screening for patients undergoing ICI therapy, including monitoring cardiac troponin levels, assessing electrocardiograph abnormalities19 or examining circulating immune checkpoints.22
Based on our observations in this preclinical model, we suggest that evaluating the presence of cardiac autoantibodies or cardiac antigens may serve as a potential predictor of cardiac inflammation and the development of myocarditis. Further research is needed to validate these findings in other pathological models and to ascertain the critical role of self-antigens, such as troponin and cardiac myosin. These self-antigens are commonly associated with heart failure of various etiologies23 and are known drivers of cardiac inflammation.6, 11
Acknowledgements
The authors wish to thank José Luis Maravillas-Montero, Marion E. G. Brunck, and Arvind Bhimaraj for the critical discussion and ideas, as well as the Cardiovascular and Metabolomics Research Group and Institute Obesity Research (I031-IOR004-C6-T2-T) and to CONACYT grant numbers: A1-S-23901, A1-S-43883 to GGR and 740975 and 769256 to NRI.
Conflict of interest
None declared.




