Body mass index, frailty, and outcomes in heart failure with preserved ejection fraction
Abstract
Aims
Relationship between body mass index (BMI), frailty, and clinical adverse events remains unclear in patients with heart failure (HF) with preserved ejection fraction (HFpEF) in different patient populations. We aimed to compare the association of BMI, frailty, and clinical adverse events between a US cohort from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) study and a Chinese cohort from the Heart Failure Registry of Patient Outcomes (HERO) study.
Methods and results
We used data of 1715 participants enrolled from America in the TOPCAT study and 1487 patients with HFpEF in the Chinese registry study, the HERO. We evaluated the relationship between BMI and frailty using multivariate restricted cubic spline logistic regression. Association between frailty and BMI categories and primary outcomes including HF hospitalization, aborted sudden death, and cardiovascular death, all-cause mortality, and HF hospitalization were analysed by Cox proportional hazards models. The patients' mean age was 72 ± 11 years for both study populations, with 50% and 46% female for the TOPCAT study and the HERO study, respectively. Patients in the TOPCAT study had a higher mean BMI (33.9 vs. 24 kg/m2), with 72.3% vs. 52.9% defined as moderately to severely frail (frailty index > 0.3). In the TOPCAT study, risk of frailty rose as BMI increased, but not in the HERO study. Patients with frailty were at significant higher risk for the primary composite outcomes [hazard ratio (HR) 1.84 (95% confidence interval: 1.46–2.32)], all-cause mortality [HR 1.73 (1.34–2.25)], and HF hospitalization [HR 1.83 (1.40–2.40)] in the TOPCAT study. The corresponding numbers in the HERO study were 1.26 (1.01–1.57), 2.21 (1.45–3.35), and 1.15 (0.81–1.37), respectively. The association of frailty with clinical outcomes did not vary with BMI categories in the two studies.
Conclusions
BMI distribution and association between BMI and frailty risk were different between the two study populations. Frailty was associated with clinical adverse events and this association was consistent across different BMI categories in both studies.
Introduction
Frailty is an emerging global health burden that is characterized by a decline in homeostatic reserves across multiple physiological systems and an increase in vulnerability to endogenous and exogenous stressors.1-3 Patients with frailty are at increased risk of hospitalization, falls, institutional placement, and functional decline.4, 5
Heart failure (HF) is intimately linked with frailty. HF and frailty share common pathophysiological mechanisms, including dysregulation of neurohormonal, metabolic, inflammatory, and immunologic pathways.6-9 Patients with HF are predisposed to developing frailty, and frailty increases the risk of HF progression and adverse clinical outcomes. Approximately half of patients with HF are frail, but estimates range widely from 15% to 89% depending on the criterion used.8 Up to 60–90% of patients with HF with preserved ejection fraction (HFpEF) were identified as frail10; the rate is higher than that in patients with HF with reduced ejection fraction. HFpEF is particularly relevant to frailty as both conditions can be considered as a systemic ‘geriatric’ syndrome with aging and multimorbidity at the central of the pathogenesis.11
Obesity is a prevalent condition among patients with HFpEF. The relationship between body mass index (BMI) and frailty has not been well studied in this patient population. Obesity was reported to be associated with frailty,12 due to higher levels of systemic inflammation and insulin resistance, while other studies have not found comparable results.13-15 It is not known whether different study populations account for the inconsistence in the association between obesity and frailty in patients with HFpEF.
In this study, we aimed (i) to investigate the relationship between BMI and frailty in HFpEF patients from two different ethnic groups: patients from the Americas regions enrolled into the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial and Chinese patients from the Heart Failure Registry of Patient Outcomes (HERO) study, and (2) to quantify the association of frailty and clinical outcomes in patients with HFpEF by BMI categories.
Methods
Data of TOPCAT study
The TOPCAT study was a multicentre, randomized controlled trial that evaluated the efficacy of spironolactone in patients with HFpEF. The mean follow-up was 3.3 years. In this study, 3445 participants with symptomatic HF and a left ventricular ejection fraction (LVEF) ≥ 45% were randomized in a 1:1 ratio to either spironolactone or placebo. The design and primary findings have been published.16, 17 We obtained the data from the Biologic Specimen and Data Repository Information Coordinating Center of the US National Heart, Lung, and Blood Institute via an approved proposal. We used subjects enrolled in the Americas regions (the United States, Canada, Brazil, and Argentina, N = 1767) and excluded patients with BMI ≤ 18.5 (N = 8) owing to spare data and patients with missing frailty index (FI) items ≥ 20% (N = 44). A total of 1715 patients from the TOPCAT study were included in the current analysis.
Data of HERO study
The HERO study was a prospective, longitudinal, seasonally rotating, multicentre registry study of patients hospitalized with HF. Those who signed a consent form were followed up for 12 months. All patients admitted in the randomly assigned observational window with a primary diagnosis of HF at 73 hospitals in Henan Province, China, from November 2017 to November 2018 were registered in the study. The study design and patient characteristics have been described elsewhere.18 Among 5620 registered patients, 4497 patients discharged alive and consented to follow-up. We excluded patients with missing FI items ≥ 20% (N = 678), patients with missing data on height or weight (N = 514), patients with BMI ≤ 18.5 (N = 137), and patients with an ejection fraction < 45% or unknown (N = 1681). A total of 1487 patients from the HERO study were included in the current analysis.
Frailty index
In both datasets, frailty was evaluated using an FI, which is created through a standard protocol.19 We applied a modified FI (BMI was excluded) developed in a post hoc analysis of the TOPCAT study using 39 items20 for participators of the TOPCAT study. A comparable FI was constructed for patients enrolled into the HERO study, with the main variation being the evaluation of quality of life (QoL), as the Minnesota Satisfaction Questionnaire, instead of the Kansas Heart Failure Questionnaire, which was applied in the TOPCAT study, was used to measure QoL in the HERO study. A comprehensive description of variables included in the FI along with the scoring system in the two databases is available in Supporting Information, Tables S1 and S2. FI was calculated using the recommended approach, that is, the sum of the deficits divided by a total number of non-missing deficits assessed.21, 22 FI scores ranged between 0 and 1, with the lowest score corresponding to the least frail while the highest score the frailest. An FI < 0.2 is generally accepted as non-frail, 0.2–0.3 mild frail, 0.3–0.4 moderate frail, and >0.4 severe frail.23, 24 We used 0.3 as the cut point and classified patients into non-frail or mildly frail group (FI ≤ 0.3) and moderate to severe frailty group (FI > 0.3).
Outcome measure
We focused on outcomes happened during the 1 year follow-up period in the HERO study and the 3 year follow-up period in the TOPCAT study. The primary outcome was consistent with the TOPCAT study's primary composite endpoint, which was composed of HF hospitalization, aborted sudden death, and cardiovascular death. The second outcomes included all-cause mortality and HF hospitalization. More detailed information regarding outcome evaluation was previously reported.16-18
Statistical analysis
Participant characteristics were presented as mean ± standard deviation (SD) for continuous variables or as frequency (percentage) for binary or categorical variables. Continuous variables were compared using Student's t-test, and categorical variables were compared using χ2 tests. Distribution of BMI and FI in the two different study populations was plotted by density plot. The adjusted associations between BMI as a continuous variable and risk of moderate to severe frailty were plotted using multivariable restricted cubic spline models with four knots, using logistic regression to adjust age, sex, New York Heart Association (NYHA) classification, smoking status, BNP/N-terminal pro-brain natriuretic peptide, and diuretic. There is evidence that these variables have an effect on frailty.25-27 This model permits appreciation of differential effects of BMI on frailty through the spectrum of BMI measurements and so enables more accurate characterization the BMI–frailty relationship.
Based on the different BMI criteria of Asians [normal weight (18.5–22.9), overweight (23–27.9), class 1 obesity (28–32.9), and class 2 or greater obesity (≥33.0)] and Americans (18.5–24.9, 25–29.9, 30–34.9, and≥35.0 for corresponding classification) defined by World Health Organization, patients in the TOPCAT study and the HERO study were divided into four categories: normal weight, overweight, class 1 obesity, and class 2 obesity.28, 29 The association between FI groups and clinical outcomes based on different BMI categories were evaluated using Cox proportional hazards models. Hazard ratios (HRs) and the related 95% confidence intervals (CIs) were used to present outcomes risk. Statistical significance was considered as a P < 0.05, and all reported probability tests were two-tailed. All analyses were performed using R (Version 4.2.1) and RStudio software.
Results
Baseline characteristics
Patients enrolled into the TOPCAT study were comparable with those registered into the HERO study in age (72 years in both studies), sex (50% and 46% female, respectively), LVEF (58% and 57.9%, respectively), and creatinine level (mean 1.17 and 1.18 μmol/L, respectively) but were significantly different in other characteristics (Table 1). Hypertension, diabetes, stroke, and myocardial infarction were more prevalent in the TOPCAT study participants (90% vs. 50.9%, 44.8% vs. 20.6%, 9.0% vs. 16.7%, and 20.5% vs. 11.2%, respectively), but fewer patients in the TOPCAT study were current smoker (6.7% vs. 22.8%). Patients in the HERO study had a 10 mmHg higher systolic and diastolic blood pressure (138 vs. 128 mmHg, and 81 vs. 71 mmHg, respectively) and were less likely taking diuretics, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and beta-blockers (52.1% vs. 88.9%, 43.4% vs. 78.9%, and 49.7% vs. 78.6%, respectively). Despite the fact that the HERO study included more individuals with severe clinical symptoms (100% in NYHA III/IV, vs. 35% in NYHA III in the TOPCAT study), a higher proportion of patients in the TOPCAT study were moderately to severely frail (72.3% vs. 52.9%).
| TOPCAT (n = 1715) | HERO (n = 1487) | P value | |
|---|---|---|---|
| Age (years), mean ± SD | 72 ± 11 | 72 ± 11 | 0.935 |
| Female, N (%) | 857 (50.0) | 803 (46.0) | <0.001 |
| Current smoker (%) | 114 (6.7) | 339 (22.8) | <0.001 |
| NYHA class III/IV | 600 (35.0) | 1487 (100) | <0.01 |
| LVEF | 58.0 ± 7.7 | 57.9 ± 8.0 | 0.638 |
| Systolic blood pressure (mmHg) | 128 ± 16 | 138 ± 25 | <0.001 |
| Diastolic blood pressure (mmHg) | 71 ± 11 | 81 ± 15 | <0.001 |
| History of diseases | |||
| Hypertension (%) | 1543 (90.0) | 757 (50.9) | <0.001 |
| Diabetes (%) | 768 (44.8) | 306 (20.6) | <0.001 |
| History of myocardial infarction (%) | 351 (20.5) | 166 (11.2) | <0.001 |
| History of stroke | 154 (9.0) | 248 (16.7) | <0.001 |
| Laboratory | |||
| BNP | 481.35 (1098.13) | — | — |
| NT-proBNP, pg/mL | — | 3868.3 (5597.2) | — |
| Serum potassium (mmol/L) | 4.19 ± 0.43 | 4.25 ± 3.76 | 0.526 |
| Serum creatinine (μmol/L) | 1.17 ± 0.34 | 1.18 ± 3.44 | 0.86 |
| Albumin (g/dL) | 3.98 ± 1.62 | 3.85 ± 0.6 | 0.006 |
| Haemoglobin (g/dL) | 12.9 ± 1.7 | 12.2 ± 2.2 | <0.001 |
| Medications, n (%) | |||
| Diuretics | 1524 (88.9) | 774 (52.1) | <0.001 |
| ACE-I/ARB | 1353 (78.9) | 646 (43.4) | <0.001 |
| Beta-blocker | 1348 (78.6) | 739 (49.7) | <0.001 |
- ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HERO, Heart Failure Registry of Patient Outcomes; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
- Obesity was defined as body mass index of ≥30 for the TOPCAT population and ≥28 for the HERO population.
Relationship between body mass index and frailty
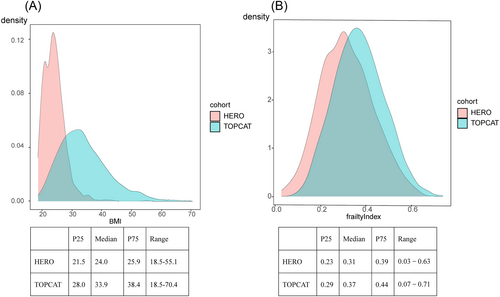
Figure 1 shows the density plots of BMI (Figure 1) and the FI (Figure 1) for participants in the TOPCAT study and participants in the HERO study, respectively. Distribution of BMI was heavily right shifted for the TOPCAT study population (Figure 1). In contrast, the FI distributions were essentially overlapping for these two populations (Figure 1). Patients in the TOPCAT study had a much higher mean BMI (33.9 vs. 24) compared with those in the HERO study, with 12.0% vs. 41.3% defined as normal weight, 23.3% vs. 45.3% defined as overweight, 26.5% vs. 11.5% defined as class 1 obesity, and 38.1% vs. 2% defined as class 2 obesity. Patients in the TOPCAT study also had a higher mean FI (0.37 vs. 0.31), with 72.3% vs. 52.9% defined as moderate to severe frail.
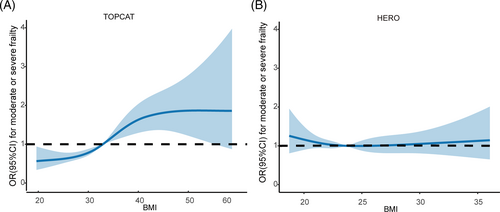
Adjusted odds ratios (ORs) of moderate to severe frailty along the continuum of BMI are illustrated in Figure 2. For patients in the TOPCAT, the lowest frailty OR was seen below BMI of 31 kg/m2, with higher ORs in the higher BMI regions (Figure 2). Adjusted OR for moderate to severe frailty was 1.05 (95% CI: 1.03–1.07) with 1 SD higher BMI. Compared with normal weight, class 1 obesity [adjusted OR 1.49 (95% CI: 1.04–2.13)] and class 2 obesity [adjusted OR 2.42 (95% CI: 1.66–3.52)] were associated with a significant higher risk of moderate to severe frailty. In contrast, in the HERO study, the ORs of frailty did not vary much with the spectrum of recorded BMI (Figure 2). The association between continuous BMI and risk of moderate to severe frailty was not significant [adjusted OR 1.00 (95% CI: 0.97–1.03)].
Impact of body mass index and frailty on outcomes
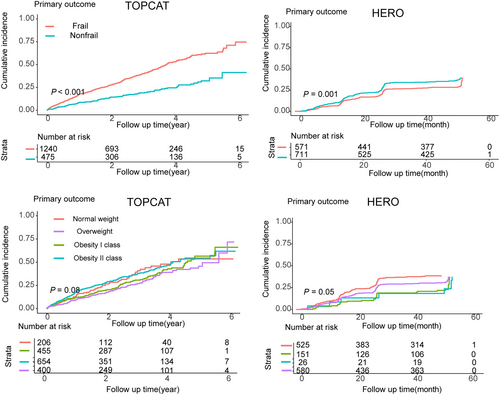
Figure 3 displays Kaplan–Meier curves for the primary outcomes stratified by BMI and FI in the analysis. Patients with moderate to severe frailty (FI > 0.3) were at significant higher risk for the primary composite outcomes [HR 1.84 (95% CI: 1.46–2.32)], all-cause mortality [HR 1.73 (1.34–2.25)], and HF hospitalization [HR 1.83 (1.40–2.40)] compared with others in the population of TOPCAT study (Table 2). Similar results were observed except for HF hospitalization in the HERO study [HRs 1.26 (1.01–1.57), 2.21 (1.45–3.35), and 1.15 (0.81–1.37) for primary composite outcomes, all-cause mortality, and HF hospitalization, respectively]. Overweight and class 1 obesity were associated with significant lower risk of all-cause mortality [HRs 0.63 (0.45–0.86) and 0.60 (0.44–0.84), respectively], but not other outcomes in the TOPCAT study. Similar results were observed in the HERO study [HR 0.65 (0.45–0.95) for overweight for all-cause mortality] (Table 2).
| TOPCAT | P value | HERO | P value | ||
|---|---|---|---|---|---|
| Reference (FI ≤ 0.3) | 1 | 1 | |||
| FI > 0.3 | Primary outcomes | 1.84 (1.46–2.32) | <0.001 | 1.26 (1.01–1.57) | 0.04 |
| All-cause mortality | 1.73 (1.34–2.25) | <0.001 | 2.21 (1.45–3.35) | <0.001 | |
| HF hospitalization | 1.83 (1.40–2.40) | <0.001 | 1.15 (0.81–1.37) | 0.72 | |
| Reference (normal weight) | 1 | 1 | |||
| Overweight | Primary outcomes | 0.85 (0.62–1.17) | 0.32 | 0.94 (0.75–1.17) | 0.57 |
| All-cause mortality | 0.63 (0.45–0.86) | 0.004 | 0.65 (0.45–0.95) | 0.03 | |
| HF hospitalization | 0.94 (0.64–1.38) | 0.74 | 0.97 (0.75–1.27) | 0.84 | |
| Class 1 obesity | Primary outcomes | 1.02 (0.75–1.39) | 0.89 | 0.95 (0.70–1.36) | 0.77 |
| All-cause mortality | 0.60 (0.44–0.84) | 0.002 | 1.01 (0.54–1.89) | 0.97 | |
| HF hospitalization | 1.25 (0.86–1.81) | 0.24 | 0.71 (0.47–1.08) | 0.11 | |
| Class 2 obesity | Primary outcomes | 1.13 (0.83–1.53) | 0.44 | 0.57 (0.23–1.39) | 0.22 |
| All-cause mortality | 0.78 (0.55–1.06) | 0.22 | 0.45 (0.06–3.24) | 0.43 | |
| HF hospitalization | 1.34 (0.93–1.95) | 0.12 | 1.35 (0.48–3.79) | 0.57 |
- FI, frailty index; HERO, Heart Failure Registry of Patient Outcomes; HF, heart failure; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
- Adjusted for age, sex, New York Heart Association, smoking status, BNP/N-terminal pro-brain natriuretic peptide, and diuretic.
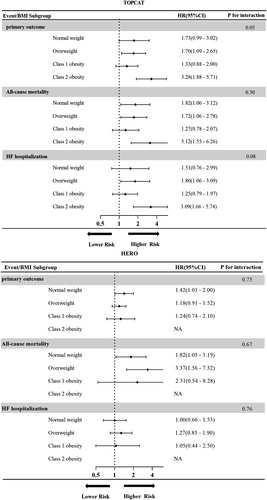
Figure 4 shows the relationship between frailty and outcome events based on different BMI categories. The association of moderate to severe frailty with clinical adverse outcomes was similar in different BMI categories except numerically higher HRs for those with class 2 obesity in the TOPCAT study (P for interaction all > 0.05). The association of moderate to severe frailty with clinical outcomes did not vary with BMI categories either in the HERO study (P for interaction all > 0.05).
Discussion
To the best of our knowledge, this is the first study to compare profiles of frail and BMI in patients with HFpEF in various population regions. Patients enrolled into the TOPCAT study had much higher BMI and comparable level of frailty, compared with those enrolled from the HERO study. The risk of moderate to severe frailty increased with the spectrum of recorded BMI in the TOPCAT study while did not differ significantly in the HERO study. In the TOPCAT study, moderate to severe frailty was associated with a two-fold higher risk of clinical adverse outcomes and this was consistent across different BMI categories. Similar trend was observed in the HERO study. Overweight and obesity were associated with lower risk of all-cause mortality in both the TOPCAT study and the HERO study.
Numerous studies have investigated the association between BMI and frailty, while few studies have focused on the relationship between BMI and frailty in patients with HFpEF. Understanding the relationship between BMI and frailty is important as it provides insights into the impact of weight status on the development and progression of frailty, which is crucial for patient outcomes. In community-dwelling older adults, it has been observed that the relationship between BMI and frailty is U-shaped curve both in Asians and in Caucasians.30, 31 In the TOPCAT trial, the risk of frailty increased with increasing BMI throughout a wide BMI spectrum, from 20 to >50 kg/m2. Contrarily, likelihood of frailty was not linked with BMI over 18.5 to >35 kg/m2 in the equally frail HERO study population. This suggests that frailty in HFpEF patients was extremely heterogeneous32 and that frailty can affect patients of different ethnic backgrounds in different ways.33 One such example is that Asians are more likely than Caucasians to develop diabetes at lower BMI thresholds.34 This highlights the importance of body composition rather than just BMI. Individuals with normal BMI but low muscle mass may still be susceptible to frailty due to inadequate muscle strength and functionality. This is a similar process to the high prevalence of diabetes in the less obese Asian population.
Numerous studies have demonstrated that patients with HF who are frail are more likely to experience negative clinical outcomes35 and that those who are obese have lower mortality rates from all causes and from cardiovascular disease.36 But few studies have focused on the relationship between clinical adverse outcomes and frailty based on different BMI categories in patients with HFpEF. Our finding showed that the association of frailty with clinical outcomes did not vary with BMI categories in the two studies. In the TOPCAT study, although there was no statistically significant heterogeneity by BMI categories in the relative risk increase for clinical outcomes with moderate to severe frailty, there was a clear trend that severe obesity (class 2) exacerbates the adverse effect of frailty on patients' outcomes. This finding is consistent with studies showing that frail liver transplant candidates with class 2 or greater obesity having a more than three-fold higher risk of wait-list mortality compared with other candidates and patients with higher BMI were more likely to have adverse clinical outcomes.37, 38
This study also highlights again the limited representativeness of Asian populations in clinical trials poses a significant challenge to the generalizability of clinical trial results.39 Due to different genetic, environmental, and cultural variables, patients may respond differently to treatment.40, 41 This underrepresentation inhibits our understanding of how interventions and treatments may specifically affect Asians. Moreover, clinical trials typically impose strict eligibility criteria, resulting in a more homogenous study population. Conversely, registry studies encompass a broader range of individuals, capturing real-world data that include patients with comorbidities or characteristics that may have been excluded from clinical trials.42 When compared with participants in the HERO study, patients in the TOPCAT study had a higher prevalence of comorbid conditions (hypertension, diabetes, stroke, and myocardial infarction), took more medications, and had a higher percentage of frail patients. The different characteristics of the TOPCAT study and the HERO study and different results in the association between BMI and frailty probably due to the two different study populations came from randomized controlled trial and real world, respectively. The observed differences in the relationship between BMI and FI likely reflect differences in the population in different regions and differences between registry study and randomized controlled trial.
Strengths and limitations
We analysed the data from two sizable cohorts that had strict, well-controlled protocols. To our knowledge, this is the first study of describing relationships of frailty and BMI in different regions and association between obesity, frailty, and outcomes in patients with HFpEF. But when interpreting the findings, some aspects of this study should be taken into consideration. First, we did not explore the outcome results for patients with class 2 obesity accounting for a small proportion in the HERO study. Second, we excluded underweight patients and therefore were unable to provide a wider range of explanations for the association between BMI and frailty. Third, as with any observational study, there was the potential for residual and unmeasured confounding factors.
Conclusions
Our results suggest that BMI distribution and association between BMI and frailty risk were different between the TOPCAT study and the HERO study. Frailty was associated with clinical adverse events and this association was consistent across different BMI categories in the TOPCAT study and the HERO study.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was supported by the National Key Research and Development Program of China, Grant/Award Number: 2020YFC2004803; Capital's Funds for Health Improvement and Research, Grant/Award Number: 2022-2-2067; and the Beijing Municipal Science and Technology Commission, Grant/Award Number: D171100006817001.




