Estimating the clinical and budgetary impact of using angiotensin receptor neprilysin inhibitor as first line therapy in patients with HFrEF
Nicklas Bergh and Krister Lindmark are joint first authors. Martin Cowie contributed as last author.
Abstract
Aims
Recent updates of international treatment guidelines for heart failure with reduced ejection fraction (HFrEF) differ regarding the use of angiotensin receptor neprilysin inhibitor (ARNI) as first-line treatment. The American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) 2022 guidelines gives ARNI a Class IA recommendation for HFrEF patients while the European Society of Cardiology's guidelines are less clear when ARNI could be considered as first line treatment option in de novo patients. This study aimed to model the clinical and budgetary outcomes of implementing these guidelines, comparing conservative ARNI prescription patterns with less conservative in Sweden and in the United Kingdom.
Methods and results
A health economic model was developed to compare different treatment patterns for HFrEF. Incident cohorts were included on an annual basis and followed over 10 years. The model included treatment specific all-cause mortality and hospitalization rates, as well as drug acquisition, monitoring, and hospitalization costs. Increasing the use of ARNI could lead to about 7000–12 300 life years gained and 2600–4600 hospitalizations prevented in Sweden. These health benefits come with an additional cost of 112–195 million euros. Similar results were estimated for the United Kingdom, albeit on a larger population.
Conclusions
Increasing the proportion of patients receiving ARNI instead of angiotensin converting enzyme inhibitors as first-line treatment of HFrEF will lead to a considerable number of additional life years gained and prevented hospitalizations but with additional cost in terms of health care expenditure in Sweden and in the United Kingdom.
Introduction
A ‘four-pillar approach’ of rapid introduction of four classes of heart failure (HF) disease modifying drugs is recommended in the treatment of heart failure with reduced ejection fraction (HFrEF), in the latest updated European Society of Cardiology Heart Failure Guidelines (ESC guidelines)1 as well as in the updated guidelines from the American College of Cardiology and American Heart Association (ACC guidelines).2 This rapid parallel introduction replaces the previous recommendation of a sequential introduction of different classes of therapy over a period of many weeks or even months. In these updated guidelines, renin-angiotensin system inhibitors [angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), or the angiotensin receptor neprilysin inhibitor (ARNI)] are used in combination with beta-blockers (BB), mineralocorticoid receptor antagonists (MRA) and sodium-glucose transporter type 2 inhibitors (SGLT2i).1
There are subtle differences between the ESC guidelines and the ACC guidelines. In the ACC guidelines, ARNI is given a first-line Class IA for patients with HFrEF and New York Heart Association (NYHA) Class II–III, with ACEi only ‘when ARNI is not feasible’, and ARB only when ACEi are not tolerated because of cough or angio-oedema.2 A switch from ACEi or ARB to ARNI is a Class IB recommendation for those with chronic symptomatic HFrEF and NYHA Class II or III, where these drug classes have been tolerated. In contrast, the ESC guidelines recommend ACEi or ARNI as first line use for all patients with HFrEF to reduce the risk of HF hospitalization and death where ACEi has Class 1A and ARNI Class 1B recommendation, and that an ACEi (or ARB) is replaced by an ARNI in ambulatory patients with HFrEF, who remain symptomatic despite optimal treatment. Additionally, another recommendation is made for an ARNI ‘to be considered’ (Class IIbB recommendation) first line for patients who are ACEi/ARB naïve, that is, de novo HF. The ESC guidelines do not specify under what circumstances ARNI can be considered first line, more than stating that ARNI is safe to initiate and may be an option before discharge in recently hospitalized but stable patients with HFrEF. The interpretation and implementation of the recommendations for the use of ARNI in first-line treatment may vary considerably across Europe.
To our knowledge, no economic studies assessing the impact of implementing HF treatment guidelines have been published. In this study, the clinical and budgetary outcomes of variable ARNI therapy prescription patterns were modelled. A conservative interpretation reflecting current care was compared with a moderate and an assertive interpretation of ARNI as first line therapy for HFrEF. The moderate interpretation is henceforth referred to as ESC21 ‘moderate’, in line with the ESC heart failure treatment guidelines1 and the assertive interpretation as ESC21 ‘assertive’, in line with the recommendations from ACC.2 The base case analysis was based on data from Sweden, where ARNI has been assessed to be cost effective as first line therapy instead of ACEi.3 Nonetheless, ARNI is recommended in Swedish national and local guidelines as a replacement for other renin-angiotensin system inhibitors in patients who remain symptomatic.4 The impact of less conservative interpretations of guidelines with regard to ARNI was also explored in scenario analyses for the United Kingdom.
Methods
Eligible population
The patient population included in the analysis is aligned with the ESC 2021 updated guidelines and approved reimbursement conditions of sacubitril/valsartan in Sweden. It consists of patients diagnosed with HFrEF (ejection fraction, EF ≤ 40%), NYHA Class II–IV, and estimated glomerular filtration rate (eGFR) > 30 L/min/1.73 m2.3, 5 1 Epidemiological inputs used to derive the eligible population are displayed in Table 1.
| Sweden | United Kingdom | |||
|---|---|---|---|---|
| Item | Value | Reference | Value | Reference |
| Total population | 10 452 326 | Statistics Sweden. Value represents total population for the 4th quarter 20216 | 67 595 824 | Office for National Statistics, Principal projection - UK population year 20227 |
| Population growth rate | 0.007 | Statistics Sweden. Value represents geometric mean of population growth between 2016 and 20216 | 0.032 | |
| Incidence of HF (per 100 000 inhabitants) | 330 | Lindmark et al. (2019)8 | 332 | Conrad et al. (2018)9 |
| HF patients with HFrEF | 64.50% | Boman et al. (2021)10 | ||
| HF patients with NYHA II–IV | 83.70% | Savarese et al. (2021)11 | ||
| HF patients with eGFR>30 mL/min | 90.00% | Assumption | ||
| Percentage of patients eligible for treatment | 93.00% | |||
- HF, heart failure; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association.
Description of the simulation model
A decision tree paired with a semi-Markov model was developed using Microsoft Excel® to simulate the budgetary impact and health outcomes of implementing new treatment guidelines for heart failure. The model included two health states (alive and dead), with the alive health state being partitioned into eight treatment combination states based on the possible treatments included in the analysis: ACEi/ARB or ARNI + BB, with MRA and SGLT2i as possible additional treatments.
The model simulation begins with initiating the newly diagnosed patient population on treatment across the eight treatment combinations described by the decision tree (Figure 1). Patients subsequently enter a semi-Markov model used to reflect treatment pathways and mortality over the modelled time horizon (Figure 1). Real-world treatment pathways reflected in the model included switching from ACEi/ARB to ARNI and treatment discontinuation of ARNI, MRA, and SGLT2i, represented by the arrows in Figure 1.
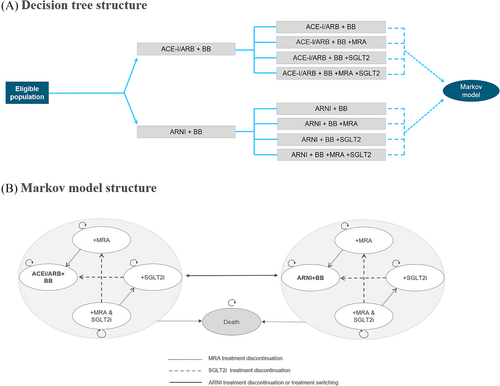
The model cycle length was 1 year with the first cycle accounting for both treatment initiating (decision tree) and treatment switching, discontinuation and mortality (semi-Markov model). The analysis was conducted over a 10-year time horizon to adequately capture differences in both clinical and budgetary outcomes amongst treatment guidelines. Each year, a new cohort of incident patients enters the model such that 10 cohorts in total are included in the analysis. The analysis covers all newly diagnosed heart failure patients over this time-period. Patients diagnosed prior to 2022 were not included in the analysis, as they were assumed to having received their first-line treatment prior to the updated guidelines.
The model includes health outcomes in terms of life years and hospitalizations, with risk of mortality and hospitalization being treatment specific (see section Risks of death and hospitalization), and costs in terms of drug acquisition costs, monitoring costs and hospitalization costs (see Resource use and costs section). All outcomes were half-cycle corrected to account for the fact that events and transitions could occur at any point in the cycle. Analyses were performed from a health care system perspective.
The ‘conservative’, ‘moderate’, and ‘assertive’ ESC21 guideline treatment distributions
The key difference between the comparator ‘ESC21 conservative’, (reflecting current care) and interventions (‘ESC21 moderate’ and ‘ESC21 assertive’ interpretation of ARNI indications) is the proportion of patients treated with ACEi/ARB (+BB) versus ARNI (+BB). The analysis assumes that in the conservative scenario 20% of Swedish patients with HFrEF receive ARNI (+BB). For the ESC21 scenarios, an increase in ARNI (+BB) prescriptions is expected (60% for ESC21 moderate, 90% for ESC21 assertive scenarios) due to ARNI becoming a first line treatment option for a larger proportion of patients. In contrast, the prescription rates of the other foundational treatments are not expected to differ between comparator and interventions (20% with no add on treatment, 80% with add on treatment with MRA and SGLT2i), as clinical practice and guidelines are aligned on the importance of use of all four drug classes for HF treatment (ACEi/ARB/ARNI, BB, MRA, and SGLT2i).1, 2
Additionally, the analysis included estimates of annual treatment switching rates from ACEi/ARB to ARNI (6.2%) and treatment discontinuation rates for ARNI (18.9%) and MRA (35.1%), which were retrieved from Swedish nationwide registers.12 It was assumed that all patients would receive treatment with either ACEi/ARB or be switched to ARNI. Moreover, an estimate for an annual discontinuation rate for SGLT2i (7%) was sourced from the DAPA-HF study (original rate of 10.5% in 18.2 months was transformed into a 12-month value).13 The aforementioned discontinuation rates were applied during the year after diagnosis. For subsequent years, discontinuations rates were assumed to be 10% of those during the first year to account for patients stabilizing on treatment.14
Risks of death and hospitalization
Risks of death and hospitalization for patients treated for HF were based on the overall survival and HF hospitalizations of patients treated with ACEi/ARB + BB and the relative clinical efficacy of adding or switching disease modifying treatments to treatment with ACEi/ARB + BB.
Overall survival for HF patients treated with ACEi/ARB + BB were derived from the Swedish Heart Failure Registry (SwedeHF) covering patients with a hospital or an outpatient visit index registration between 1 April 2016 and 31 December 202015, 16 (see Data S1 for details). The annual hospitalization rate for ACEi/ARB + BB treated patients (13.6%) was sourced from the PARADIGM-HF study (using the all-cause hospitalization rate).5
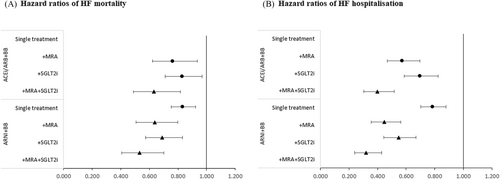
Relative clinical efficacy for survival and risk of hospitalizations were derived from Vaduganathan et al. where they estimated the treatment effects of comprehensive disease-modifying pharmacological therapy (ARNI, BB, MRA, and SGLT2i) versus conventional ‘basic’ therapy (ACEi/ARB + BB).17 Based on these estimates, the hazard ratio (HR) for comprehensive therapy was imputed in the Vaduganathan et al. study,17 using the product of the respective treatment effects (ARNI, MRA, and SGLT2i). The same method was applied to derive the HRs of the combination regiments formed by adding MRA and SGLT2i to ARNI+BB, as well as adding MRA and SGLT2i in combination to ACEi/ARB + BB. The full set of relative treatment effects is displayed in Figure 2A,B, where estimates for the indirect treatment comparison (ITC) is displayed as circles and imputed HRs as triangles. The HRs are also presented in Data S1. A mortality adjustment was included in the model ensuring that the risk of mortality across treatment combinations was never lower than in the general population.18, 19
Resource use and costs
Unit costs for drug acquisition, hospitalization, and patient monitoring are reported in Table 2. The analyses are based on official list prices, not including any potential confidential discounts. The drug acquisition costs were sourced from the Swedish Dental and Pharmaceutical Benefits Agency (TLV) price list,20 while target doses are taken from ESC 2021 guidelines and assumed to be equal for all treatment arms.1 A country-specific price reduction based on results reported by Kavanos et al.21 (see Data S1) was applied after patent expiry (59.4% during the first year and 70.6% during the second year) in the model's base case for both sacubitril/valsartan (ARNI) and dapagliflozin (SGLT2i),21 as generic competitors enter the market (assumed to be in 2025 for dapagliflozin and 2026 for sacubitril/valsartan). The cost of a HF hospitalization event (6133 EUR, see Table 2) was calculated using the Nordic diagnosis-related group grouper (NORD DRG) Somatic weight list for 2022.22
| Sweden (base case) | United Kingdom | |||||||
|---|---|---|---|---|---|---|---|---|
| Package cost (SEK) | Annual treatment cost (EUR) | Description | Reference | Package cost (GBP) | Annual treatment cost (EUR) | Description | Reference | |
| ACEi (enalapril) | 62.69 | 64 | 10 mg, 100 tablets | TLV price list. ATC C09AA02 | 5.35 | 244 | 10 mg, 28 tablets | NICE British National Formulary. Available at: bnf.nice.org.uk/medicinal-forms/drug-name.html |
| ARB (candesartan) | 83.81 | 28 | 32 mg, 100 tablets | TLV price list. ATC C09CA06 | 1.69 | 26 | 32 mg, 28 tablets | |
| BB (bisoprolol) | 63.65 | 22 | 10 mg, 100 tablets | TLV price list. ATC C07AB07 | 0.65 | 10 | 10 mg, 28 tablets | |
| ARNI (sacubitril/valsartan) | 3687.65 | 1492 | 103/97 mg, 168 tablets | TLV price list. ATC C09DX04 | 91.56 | 1394 | 103/97 mg, 56 tablets | |
| MRA (spironolactone) | 136.34 | 46 | 50 mg, 100 tablets | TLV price list. ATC C03DA01 | 17.78 | 76 | 50 mg, 100 tablets | |
| SGLT2i (dapagliflozin) | 1259.03 | 437 | 10 mg, 98 tablets | TLV price list. ATC A10BK01. | 36.59 | 557 | 10 mg, 28 tablets | |
| Unit cost (SEK) | Unit cost (EUR) | Description | Reference | Unit cost (GBP) | Unit cost (EUR) | Description | Reference | |
|---|---|---|---|---|---|---|---|---|
| Monitoring visit | 2160 | 201 | Unit cost for outpatient visit with a general physician | Södra sjukvårdsregionen price list (2022). Code BLÄK0113 | 39.04 | 46 | Unit cost for outpatient visit with a general physician | NICE Technology Appraisal 388: Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. Page 88. Original cost: 33 GBP. Price was inflated using the Bank of England's inflation calculator (latest available values are for 2021). |
| Hospitalization cost | 65 900 | 6133 | Unit cost for HF hospitalization event | Socialstyrelsen. Prospektiva sjukhusvikter, NordDRG 2022. Average price for codes E47A, E47C, and E47E. | 2114 | 2467 | Unit cost for HF hospitalization event | Health Trust Reference Costs 2019–20. Weighted average cost of codes EB03A, EB03B, EB03C EB03D, EB03E. Original cost: 2061 GBP. Price was inflated using the Bank of England's inflation calculator (latest available values are for 2021). |
- Exchange rate by the European Central Bank (see Resource use and costs section). Drug costs were retrieved in June 2022.
- ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; BB, beta-blockers; ARNI, angiotensin receptor neprilysin inhibitors; eGFR, estimated glomerular filtration rate; HF, heart failure; HErEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; SEK, Swedish kronor; SGLT2i, sodium glucose co-transporter 2 inhibitors; TLV, Tandvårds & läkemedelsförmånsverket; VAT, value added tax.
The unit cost for regular monitoring visits was obtained from the Swedish Southern Regions' price list 2022 (201 EUR, see Table 2). The monitoring costs are applied at each cycle up to patients' death. It was assumed that only one annual monitoring visit per patient was needed, regardless of treatment option. No additional cost for switching was included as it was assumed to be done during the regular monitoring visits.23
The analyses use country-specific values for the United Kingdom, including epidemiological and population data input values, drug and visit costs and price reductions due to patent expiry. For the ease of comparison, unit costs were converted to Euros using the conversion rate from the European Central Bank (as of 6 July 2022) of 1 SEK = EUR 0.09307 and 1 GBP = 1.1672 EUR.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were performed (see Data S1). In addition, a number of scenario analyses were undertaken. Firstly, the sensitivity to the relative clinical efficacy data was explored, and secondly, sensitivity to the estimated survival of ACEi/ARB + BB treated patients on which the relative treatment effects were applied. The combined efficacy of ARNI, MRA and SGLT2i was derived from an ITC and might have been overestimated as an additive effect of treatments was assumed (i.e., multiplicative hazard ratios), while it is possible that the benefits of the treatment combination regimes might be attenuated because of overlapping mechanistic pathways. Imputed HRs for mortality and hospitalizations were therefore derived assuming a (one standard error) lower treatment effect. Next, relative clinical efficacy on all-cause mortality was derived from an alternative systematic review and network meta-analysis performed by Tromp el al.,24 comparing the aggregate treatment benefit of pharmacological therapy for HFrEF patients, analysing data from 75 relevant clinical trials including more than 95 000 patients. Survival of ACEi/ARB + BB treated patients from Vaduganathan et al., was explored as well as extrapolating the Swedish RWE survival based on an exponential distribution instead of the Weibull distribution.17 An annual hospitalization rate of 30% was assumed to account for that hospitalization events might be more frequent in a non-clinical trial setting. Lastly, a 3% discount rate on cost and outcomes and a 5-year time horizon were applied.
Results
The base case results of the health and budgetary impact analysis for Sweden are presented in Table 3.
| Base case | Scenario analysis | |||
|---|---|---|---|---|
| Sweden | United Kingdom | |||
| ESC21 moderate | ESC21 assertive | ESC21 moderate | ESC21 assertive | |
| Budget impact in EUR (incremental, undiscounted): | ||||
| Overall cost over 10 years | 111 856 496 | 195 266 300 | 765 091 004 | 1 338 450 921 |
| Acquisition costs | 126 493 682 | 221 008 273 | 808 909 672 | 1 415 475 107 |
| Monitoring costs | 1 411 334 | 2 470 416 | 2 210 939 | 3 870 103 |
| Hospitalization costs | −16 048 519 | −28 212 389 | −46 029 607 | −80 894 288 |
| Health outcomes (incremental, undiscounted): | ||||
| LYG | 7020 | 12 289 | 48 520 | 84 931 |
| Hospitalizations | −2617 | −4600 | −18 655 | −32 784 |
- All analyses are against ESC21 Conservative. Exchange rate by the European Central Bank (see Resource use and costs section).
- EUR, Euro; LYG, life years gained.
For an eligible population of 160 863 patients over 10 years, the incremental life years gained of implementing new treatment guidelines (compared to the conventional situation informed by ESC21 conservative) is indicated to be 7020 years in the ESC21 moderate scenario and 12 289 years in the ESC21 assertive scenario. Additionally, heart failure hospitalizations can be avoided (−2617 in ESC21 moderate scenario and −4600 in ESC21 assertive scenario). These health benefits come with an additional overall cost of 112 million EUR for ESC21 moderate and 195 million EUR for ESC21 assertive scenarios. These incremental costs are mainly driven by acquisition costs, while monitoring costs explain only a small part of the cost increase. The cost per treated patient-year is 1079 EUR, 1227 EUR and 1335 EUR for ESC21 conservative, moderate and assertive, respectively (see Data S1).
Sensitivity analysis
The results of the sensitivity analyses are presented in this section.
Probabilistic and deterministic sensitivity analysis
The results from the DSA are presented in Figure 3 for the ESC21 ‘moderate’ scenario as tornado diagrams for incremental life-years and budget impact, displaying the 10 most influential parameters (similar results are generated running a DSA on ESC21 ‘assertive’ scenario, see Data S1). In terms of incremental life-years, the most influential parameters were the mortality hazard ratios for ACEi/ARB + BB (varied across the 95% confidence interval), for ARNI + BB with MRA + SGLT2i, and with SGLT2i as add-on treatments. For incremental costs, the most influential parameters were the price reduction at loss of exclusivity for ARNI and SGLT2i, the eligible population size and the proportion on ARNI.
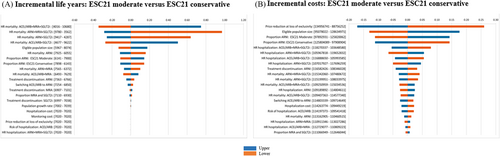
The results from the PSA are presented in Data S1. Accounting for the joint uncertainty in the parameters, the mean incremental life-years from the PSA were marginally higher than the base case results (7020 vs. 7157 for ESC21 ‘moderate’ and 12 289 vs. 12 632 for ESC21 ‘assertive’). The mean incremental costs increased by 35% (150 vs. 112 million EUR for ESC21 ‘moderate’ and 264 vs.195 million EUR for ESC21 ‘assertive’), mainly attributed to uncertainty around the price reduction at loss of exclusivity for ARNI and SGLT2i. Furthermore, the scatter plots display a clear positive correlation between incremental life-years and costs, which is expected since patient living longer will consume drugs over a longer time, increasing life-time acquisition costs, being the key cost driver.
Scenario analysis
The largest impact on the results was associated with using a 5-year (rather than 10-year) time horizon. For the clinical inputs, the largest impact on survival was generated by basing survival of ACEi/ARB and BB treated patients from the analyses of Vaduganathan et al.17 rather than using Swedish RWE survival, with incremental life years decreasing by about 25% in both scenarios (see Table 4). Incremental overall cost was stable across the scenarios explored. However, by increasing the base line risk of hospitalization to 30%, the overall incremental cost was reduced by 16% due to further avoided hospitalizations.
| ESC21 moderate (incremental against ESC21 conservative) | ESC21 assertive (incremental against ESC21 conservative) | |||||
|---|---|---|---|---|---|---|
| LYG | Hospitalizations | Overall cost (EUR) | LYG | Hospitalizations | Overall cost (EUR) | |
| Base case | 7020 | −2617 | 111 856 496 | 12 289 | −4600 | 195 266 300 |
| Alternative mortality HRs Vaduganhatan et al. (2020) | 5459 | −2721 | 109 009 168 | 9547 | −4783 | 190 286 665 |
| Alternative hospitalization HRs Vaduganhatan et al. (2020) | 7020 | −2098 | 115 035 815 | 12 289 | −3686 | 200 870 887 |
| Mortality HRs derived from Tromp et al. (2022) | 6798 | −2614 | 110 827 305 | 11 862 | −4596 | 193 438 508 |
| ACEi/ARB and BB survival from Vaduganhatan et al. (2020) | 5179 | −2970 | 114 505 639 | 9066 | −5221 | 199 851 829 |
| Extrapolating survival of ACEi/ARB and BB using exponential distribution | 7121 | −2581 | 111 646 860 | 12 465 | −4536 | 194 926 114 |
| ACEi/ARB and BB hospitalization risk 30% | 7020 | −5516 | 94 075 098 | 12 289 | −9695 | 164 013 700 |
| 3% discount rate of costs and outcomes | 5798 | −2617 | 99 465 778 | 10 149 | −4600 | 173 680 892 |
| Five-year time horizon | 1314 | −939 | 69 476 253 | 2300 | −1642 | 121 583 442 |
- ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; BB, beta-blockers; ESC, European Society of Cardiology; EUR, Euro; HR, hazards ratio; LYG, life years gained.
Results from the United Kingdom
Applying the same modelling and using data sources from the United Kingdom (see Table 1 and Data S1) with an eligible HFrEF population of about 1 173 000 patients over 10 years, the incremental life years of implementing a less conservative interpretations of the European 2021 HFrEF treatment guidelines is indicated to be 48 520 in ESC moderate scenario and 84 931 in ESC assertive scenario. These interventions also prevent heart failure hospitalizations −18 655 in ESC21 moderate and −32 784 in ESC21 assertive. These health benefits come with an incremental overall cost of 765 million EUR for ESC21 moderate and 1.3 billion EUR for ESC21 assertive. As for Sweden, the incremental costs are mainly driven by acquisition costs. The cost in the United Kingdom per treated patient-year is estimated to be 766 EUR, 913 EUR and 1021 EUR for ESC conservative, moderate and assertive, respectively.
Discussion
This study modelled the clinical and budgetary impact of variable ARNI therapy prescription patterns from a health care system perspective in two European countries based on a ‘four-pillar approach’, comparing a conservative interpretation of the updated ESC 2021 treatment guidelines with less conservative interpretations regarding ARNI as first line therapy for HFrEF. The results demonstrate that increasing the proportion of patients receiving ARNI, rather than ACEi/ARB, would imply a considerable number of additional life years gained and prevented hospitalizations. This comes with the cost of increased health care expenditure and is in line with previous analyses that have demonstrated cost effectiveness but not cost savings of replacing ACEi with ARNI for the treatment of HFrEF.20 The results of this study can be seen as a supplement to the results of the clinical studies and provide reference values for policy makers.
For ease of cross-country comparability, the undiscounted cost per life-year gained applying a moderate or assertive interpretation of ESC guidelines over the 10-year time horizon following 10 patient cohorts were 15 900 EUR (Sweden) and 15 800 EUR (United Kingdom).
Overall, the expected additional health benefits in relation to the expected additional costs demonstrate that an increased proportion of patients receiving ARNI would imply high value for money and a rational allocation of resources. In contrast, a restricted use of ARNI would imply substantial loss in population health benefits. These results are aligned with the findings of prior cost-effectiveness analyses comparing ARNI against ACEi.25-27 Given the indicative cost-effectiveness results, the impact of the increased use of ARNI in the first line is likely to be generalizable to other countries with similar health care systems, which should be explored in further research. These findings should be interpreted carefully, as this study is not a traditional cost-effectiveness analysis that models one cohort of patients over a life-time horizon.
Although the treatment guidelines in the United States and Europe are based on the same evidence, they arrive at slightly different recommendations regarding the use of ARNI as first line treatment, with a more cautious recommendation in the European guidelines. One potential explanation for this could be the variation in resources available across different healthcare funding settings and geographies, which may have persuaded the authors of the European guidelines to provide flexibility and not to mandate the use of ARNI as first line. The downside of such flexibility is that patients may lose out from the incremental benefit of innovation. More discussion of clinical impact, value for money and shared decision making is urgently required to ensure optimal allocation of resources and innovations.
Strengths and limitations
As for all health economic models, simplifying assumptions were applied. Treatment strategies were operationalized by the proportion of patients treated with ACEi/ARB + BB versus ARNI+BB. The implementation in clinical practice is likely more complex. However, this simplification allows for a transparent analysis with a clear interpretation.
Given variations in healthcare systems, reimbursement mechanisms, and healthcare costs, there are limitations to generalizing the budgetary impact results across European countries. Factors like pricing negotiations, generic drug availability, and healthcare resource availability impact overall budgetary implications, factors important to consider when extending the findings of this study beyond the scope of our investigation.
In the analysis, the health benefit was estimated in terms of life years gained. However, heart failure is also associated with a significant decrease in quality of life. Consequently, in health technology assessments, treatment with ARNI has been associated not only with increased survival compared to ACEi, but also improvements in quality of life. It is therefore likely that less conservative interpretations of the guidelines will also have a benefit on patient quality of life and thus also quality-adjusted life year estimates.
The use of Swedish RWE to inform survival of ACEi/ARB and BB treatment patients, on which the relative treatment effects of disease modifying treatments was applied, strengthens the analysis. The results were also robust to a set of sensitivity analyses exploring a plausible range of clinical assumptions.
Conclusions
Implementing and adhering to less conservative interpretations of the ESC HFrEF treatment guideline, with an increased proportion of patients receiving ARNI instead of ACEi/ARB, in Sweden and the United Kingdom, is associated with additional health benefits but comes with an additional cost. Our results indicate higher value for money by using ARNI instead of ACEi/ARB as first line therapy based on a ‘four-pillar approach’, and that discussion around guideline adherence and interpretation is warranted to ensure optimal allocation of health care resources.
Acknowledgements
The authors would like to acknowledge Julio Sosa (MD, MSc), Jonas Lindblom (PhD), and Marcus Hultberg (MSc) from Parexel International for their contribution to this research.
Conflict of interest
O. Kaeck is employee of Novartis and G. Lanne was employee of Novartis by the time of the Manuscript. Royalties have been paid for preparatory work to Niklas Bergh and Martin Cowie. J. Lissdaniels has received consultancy fees from Novartis.
Funding
This project was funded by Novartis Pharmaceuticals.
References
- 1 In the United Kingdom, reimbursement is restricted to LVEF ≤ 35%.




