Factors influencing left ventricular thrombus resolution and its significance on clinical outcomes
Abstract
Aims
A left ventricular thrombus (LVT) is not uncommon in patients with impaired LV systolic function. However, the treatment strategy for LVT has not yet been fully established. We aimed to identify the factors influencing LVT resolution and the significance of LVT resolution on clinical outcomes.
Methods
We retrospectively investigated patients diagnosed with LVT with left ventricular ejection fraction (LVEF) < 50% on transthoracic echocardiography from January 2010 to July 2021 in a single tertiary centre. LVT resolution was monitored through serial follow-up transthoracic echocardiography. The primary clinical outcome was a composite of all-cause death, stroke, transient ischaemic attack, and arterial thromboembolic events. LVT recurrence was also evaluated in patients with LVT resolution.
Results
There were 212 patients diagnosed with LVT (mean age, 60.5 ± 14.0 years; male, 82.5%). The mean LVEF was 33.1 ± 10.9%, and 71.7% of patients were diagnosed with ischaemic cardiomyopathy. Most patients were treated with vitamin K antagonists (86.7%), and 28 patients (13.2%) were treated with direct oral anticoagulants or low molecular weight heparin. LVT resolution was observed in 179 patients (84.4%). LVEF improvement failure within 6 months was a significant factor hindering LVT resolution (hazard ratio, HR: 0.52, 95% confidence interval, CI: 0.31–0.85, P = 0.010). During a median 4.0 years of follow-up (interquartile range, IQR: 1.9 to 7.3 years), 32 patients (15.1%) experienced primary outcomes (18 all-cause deaths, 15 strokes, and 3 arterial thromboembolisms) and 20 patients (11.2%) experienced LVT recurrence after LVT resolution. LVT resolution was independently associated with a lower risk for primary outcomes (HR: 0.45, 95% CI: 0.21–0.98, P = 0.045). In the patients with resolved LVT, discontinuation or duration of anticoagulation after resolution were not significant predictors for LVT recurrence, but LVEF improvement failure at LVT resolution was associated with a significantly higher risk of LVT recurrence (HR: 3.10, 95% CI: 1.23–7.78, P = 0.016).
Conclusions
This study suggests that LVT resolution is an important predictor for favourable clinical outcomes. LVEF improvement failure interfered with LVT resolution and appeared to be a crucial factor for LVT recurrence. After LVT resolution, continuation of anticoagulation did not seem to impact LVT recurrence and the prognosis.
1 Introduction
Left ventricular thrombus (LVT) is a relatively common complication in patients with impaired left ventricular (LV) systolic function and is a potential risk factor for thromboembolic events such as stroke.1, 2 Although many studies on LVT have investigated patients with acute myocardial infarction, it is considered an important complication even in non-ischaemic cardiomyopathy.3-5
Anticoagulation therapy is used for LVT resolution and to reduce the thromboembolic risk in patients. However, in some patients, LVT persists even after anticoagulation therapy, carrying a high risk of thromboembolism.6 Generally, a vitamin K antagonist (VKA) is recommended as anticoagulation therapy for LVT. Recently, direct oral anticoagulation (DOAC) has been developed and studies are in progress, but the evidence for its use remains limited.7, 8 Some acute myocardial infarction treatment guidelines recommend 3–6 months of anticoagulation therapy for LVT; however, the appropriate duration has not yet been fully investigated.9-11 Furthermore, the duration of anticoagulation following LVT resolution and the clinical course following cessation of anticoagulation remain unclear. Thromboembolic complications occur frequently in patients with chronic heart failure (HF),12 and some of these events are likely to be LVT related.13 However, unless LVT is observed on imaging studies, routine anticoagulant therapy is not recommended in patients with chronic HF; therefore, the risk of LVT may be overlooked.
Given these, we aimed to investigate the factors affecting LVT resolution and recurrence, the impact of LVT on prognosis, and the appropriate anticoagulant treatment strategy in patients with left ventricular systolic dysfunction.
2 Methods
2.1 Study design and population
Between January 2010 and July 2021, data from patients with LVT and LV ejection fraction (LVEF) < 50% on transthoracic echocardiography (TTE) at Severance Cardiovascular Hospital were retrospectively collected. LVT was verified through image review in all cases. Cases wherein LVT was unclear or where there was lack of evidence of LVT on additional imaging tests (i.e., contrast transthoracic echocardiography and cardiac computed tomography) were excluded. Patients without follow-up echocardiography after the first diagnosis or those with previously diagnosed LVT who were receiving anticoagulation therapy at the time of enrolment were also excluded. In addition, patients who underwent percutaneous cardiopulmonary support, surgical thrombectomy, surgical anterior ventricular endocardial restoration, heart transplantation, and left ventricular assist device implantation as well as those with confirmed in-hospital death were excluded during the follow-up period. This study was approved by the Institutional Review Board of the Yonsei University Health System (approval number: 4-2022-0806), and the investigation conforms with the principles outlined in the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective study design.
2.2 Baseline and data collection
LVT resolution was examined through serial follow-up TTE. Echocardiographic parameters were collected from TTE on the first identified LVT, first confirmed LVT resolution, 6 months after diagnosis, and at final follow-up. However, when LVT recurrence was confirmed or re-resolution was performed in serial follow-up TTE, these parameters were additionally collected. LV improvement failure was defined as a decrease in LVEF on subsequent TTE compared with the first TTE. Patients were divided into two groups according to LVT resolution. Additionally, we compared the patients with ischaemic aetiology and those without ischaemic aetiology. All laboratory and clinical information were collected from electronic medical records when the echocardiographic parameters were collected. Particularly, information on the prescription date, prescription duration, and type of antiplatelet or anticoagulation therapy was reviewed through electronic medical records.
2.3 Clinical outcomes
Clinical outcomes including all-cause death, ischaemic stroke, transient ischaemic attack (TIA), and arterial thromboembolic events were investigated from electronic medical records. Thrombus resolution was defined as complete LVT resolution in the follow-up TTE. Persistent LVT was defined as cases in which LVT disappearance was not confirmed in a series of subsequent TTEs and LVT was observed in the final TTE. Ischaemic stroke or TIA was defined as a cerebral infarction that was confirmed through imaging studies such as computed tomography scan or cerebrovascular magnetic resonance imaging. Arterial thromboembolism was defined as a coronary or peripheral arterial embolism (excluding ischaemic stroke or TIA) that was confirmed through imaging studies. LVT recurrence was defined as confirmed resolution and re-observation of the LVT in subsequent TTEs. The primary outcome was defined as a composite of all-cause death, ischaemic stroke, TIA, and arterial thromboembolic events.
2.4 Statistical analysis
Continuous variables are expressed as mean ± standard deviation and compared using Student's t-test. Categorical variables are expressed as frequencies (%) and compared using the χ2 test or Fisher's exact test. LVEF at baseline (diagnosis) and after 6 months were compared using a paired t-test. Kaplan–Meier survival analyses were used to compare clinical outcomes, and differences were compared using log-rank tests. Multivariable Cox proportional hazard ratio analyses were used to identify significant factors for LVT resolution and clinical outcome. To identify the factors for LVT resolution, in addition to variables with a P-value <0.1 in univariable analysis, factors affecting LVT resolution were also selected for multivariable analysis.14 To determine the association between LVT resolution and clinical outcome, three multivariate models were used. Model 1 was adjusted for age and sex, and model 2 was additionally adjusted for baseline LVEF and aetiology of HF. Model 3 was adjusted by adding the combination of anticoagulation and antiplatelet therapy and duration of anticoagulation therapy to variables of model 2. A two-sided P-value of <0.05 was considered statistically significant. Statistical analyses were conducted using the R software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Clinical characteristics of the study population
Among 470 patients with first documented LVT on TTE, 212 were analysed after the exclusion criteria were applied (Figure S1). Baseline characteristics of the study population are described in Table 1. Patients comprised mostly men (n = 175, 82.5%) with ischaemic heart disease (71.7%) and the mean LVEF was 33.1 ± 10.9%. A total of 18.9% (n = 40) of patients experienced acute coronary syndrome (ACS) at the time of LVT diagnosis. Most patients (86.7%) were treated with VKA, and only 13.2% were treated with DOACs or low molecular weight heparin.
| Total (n = 212) | Persistent thrombus (n = 33) | Thrombus resolution (n = 179) | P-value | |
|---|---|---|---|---|
| Age, years | 60.5 ± 14.0 | 64.6 ± 9.9 | 59.8 ± 14.5 | 0.021 |
| Male sex, n (%) | 175 (82.5%) | 29 (87.9%) | 146 (81.6%) | 0.530 |
| Body surface area, m2 | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | 0.305 |
| Body mass index, kg/m2 | 24.3 ± 3.7 | 23.4 ± 3.1 | 24.5 ± 3.8 | 0.114 |
| Hypertension, n (%) | 114 (53.8%) | 16 (48.5%) | 98 (54.7%) | 0.636 |
| Diabetes mellitus, n (%) | 69 (32.5%) | 11 (33.3%) | 58 (32.4%) | 1.000 |
| Chronic kidney disease, n (%) | 15 (7.1%) | 1 (3.0%) | 14 (7.8%) | 0.537 |
| Atrial fibrillation/atrial flutter, n (%) | 35 (17.0%) | 6 (19.4%) | 29 (16.6%) | 0.904 |
| Malignancy, n (%) | 29 (13.7%) | 6 (18.2%) | 23 (12.8%) | 0.587 |
| Prior stroke or transient ischaemic attack, n (%) | 49 (23.1%) | 10 (30.3%) | 39 (21.8%) | 0.400 |
| Prior coronary artery bypass graft, n (%) | 16 (7.5%) | 3 (9.1%) | 13 (7.3%) | 0.995 |
| Prior coronary stenting, n (%) | 67 (31.6%) | 14 (42.4%) | 53 (29.6%) | 0.211 |
| Ischaemic cardiomyopathy, n (%) | 152 (71.7%) | 29 (87.9%) | 123 (68.7%) | 0.042 |
| Stress cardiomyopathy, n (%) | 7 (3.3%) | 0 (0.0%) | 7 (3.9%) | 0.532 |
| Dilated cardiomyopathy, n (%) | 49 (23.1%) | 4 (12.1%) | 45 (25.1%) | 0.160 |
| Hypertrophic cardiomyopathy, n (%) | 4 (1.9%) | 0 (0.0%) | 4(2.2%) | 0.864 |
| STEMI/NSTEMI/Recent MI, n (%) | 40 (18.9%) | 5 (15.2%) | 35 (19.6%) | 0.725 |
| Revascularization | 0.664 | |||
| Coronary stenting, n (%) | 45 (21.2%) | 6 (18.2%) | 39 (21.8%) | |
| Coronary artery bypass graft, n (%) | 12 (5.7%) | 1 (3.0%) | 11 (6.1%) | |
| Newly developed RWMA, n (%) | 116 (54.7%) | 13 (39.4%) | 103 (57.5%) | 0.083 |
| RWMA, n (%) | 1.000 | |||
| LAD territory, n (%) | 112 (64.7%) | 21 (65.6%) | 91 (64.5%) | |
| No LAD territory, n (%) | 61 (35.3%) | 11 (34.4%) | 50 (35.5%) | |
| Global hypokinesia of LV, n (%) | 40 (18.9%) | 3 (9.1%) | 37 (20.7%) | 0.187 |
| Aneurysm of LV apex, n (%) | 26 (12.3%) | 5 (15.2%) | 21 (11.7%) | 0.794 |
| LVEF at LVT diagnosis, % | 33.1 ± 10.9 | 35.2 ± 11.0 | 32.7 ± 10.9 | 0.228 |
| LVEDD at LVT diagnosis, mm | 58.2 ± 8.9 | 55.9 ± 8.9 | 58.6 ± 8.9 | 0.111 |
| Anticoagulation | ||||
| VKA | 182 (86.7%) | 30 (90.9%) | 152 (85.9%) | 0.525 |
| DOAC | 23 (10.8%) | 2 (6.1%) | 21 (11.9%) | 0.511 |
| LMWH | 5 (2.4%) | 1(3.0%) | 4 (2.3%) | 1.000 |
| Anticoagulation alone | 40 (18.9%) | 5 (15.2%) | 35 (19.6%) | 0.725 |
| DAPT alone | 1 (0.5%) | 0 (0.0%) | 1 (0.5%) | 1.000 |
| Anticoagulation + DAPT | ||||
| VKA + DAPT | 60 (28.3%) | 8 (24.2%) | 52 (29.1%) | 0.724 |
| DOAC + DAPT | 5 (2.4%) | 0 (0.0%) | 5 (2.8%) | 0.728 |
| Anticoagulation+MAPT | ||||
| VKA + Aspirin | 48 (22.6%) | 8 (24.2%) | 40 (22.3%) | 0.990 |
| DOAC + Aspirin | 7 (3.3%) | 1 (3.0%) | 6 (3.4%) | 1.000 |
| VKA + Clopidogrel | 43 (20.3%) | 10 (30.3%) | 33 (18.4%) | 0.186 |
| DOAC + Clopidogrel | 8 (3.8%) | 1 (3.0%) | 7 (3.9%) | 1.000 |
- Values are mean (standard deviation), n (%, percentage).
- DAPT, dual antiplatelet agent; DOAC, direct oral anticoagulation; LAD, left anterior descending artery; LMWH, low molecular weight heparin; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVT, left ventricular thrombus; MAPT, mono antiplatelet agent; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; RWMA, regional wall motion abnormality; STEMI, ST elevation myocardial infarction; VKA, vitamin K antagonist.
3.2 Left ventricular thrombus resolution
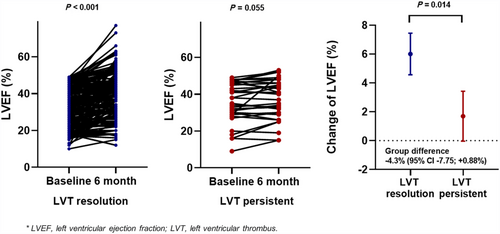
LVT resolution was observed in 179 (84.4%) patients. The median duration from diagnosis to resolution of LVT was 108 days (interquartile range, IQR: 57.5–237 days). These patients were younger and had a lower prevalence of ischaemic cardiomyopathy, but there were no differences in the presence of ACS or the type of anticoagulation therapy compared with patients with persistent LVT (Table 1). Among patients with LVT resolution, 37.4% continued anticoagulants even after LVT resolution. Anticoagulation therapy was discontinued in 12.4% of patients with persistent LVT because of bleeding events. There were no differences in LVEF at diagnosis (P = 0.228) between the LVT resolution and persistent LVT groups. However, in the LVT resolution group, LVEF significantly improved within the 6-month follow-up (baseline vs. 6-month, 32.7 ± 10.9% vs. 38.7 ± 12.1%; P < 0.001), but not in the persistent LVT group (baseline vs. 6-month, 35.2 ± 11.0% vs. 36.9 ± 11.8%; P = 0.055). The change in LVEF from baseline to the 6-month follow-up significantly differed between the two groups (group difference: −4.3%; 95% CI, −7.75 to +0.88, P = 0.014) (Figure 1). In both the univariable and multivariable models, LVEF improvement failure within the 6-month follow up (hazard ratio, HR, 0.52; 95% confidence interval, CI, 0.31–0.85; P = 0.010) was an independent factor for impaired LVT resolution (Table 2, Table S1).
| Total (n = 212) | Hazard ratio [95% confidence interval] | P-value |
|---|---|---|
| Age | 0.99 [0.98–1.00] | 0.208 |
| Female | 1.55 [0.97–2.48] | 0.069 |
| Atrial fibrillation/atrial flutter | 0.80 [0.49–1.31] | 0.376 |
| Chronic kidney disease | 0.41 [0.15–1.09] | 0.073 |
| Ischaemic cardiomyopathy | 1.27 [0.69–2.32] | 0.439 |
| STEMI/NSTEMI/Recent MI | 0.77 [0.47–1.29] | 0.325 |
| RWMA | 1.21 [0.71–2.08] | 0.478 |
| Aneurysm of LV | 0.66 [0.32–1.37] | 0.268 |
| LVEF at diagnosis | 1.00 [0.98–1.02] | 0.875 |
| LVEF improvementa failure | 0.52 [0.31–0.85] | 0.010 |
| Anticoagulation with antiplatelet therapy | 0.88 [0.67–1.14] | 0.337 |
- LV, left ventricle; LVEF, left ventricular ejection fraction; LVT, left ventricular thrombus; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; RWMA, regional wall motion abnormality.
- a Changes in LVEF between the time of LVT diagnosis and after 6 months.
Additional results for comparing the patients according to HF aetiology are described in Table S2. Although LVEF at baseline was significantly lower in the non-ischaemic HF group (25.9 ± 10.6 vs. 36.0 ± 9.7%, P < 0.001), there was no difference in LVEF after 6 months between the two groups (36.8 ± 13.6 vs. 39.1 ± 11.2%, P = 0.197). The change in LVEF from baseline to 6 months was significantly higher in patients with non-ischaemic HF (10.9 ± 12.2 vs. 3.1 ± 6.8%, P < 0.001). The LVT resolution occurred more in patients with non-ischaemic HF than in those with ischaemic HF (93.3% vs. 80.9%, P = 0.042, Table S2).
3.3 Clinical outcomes in patients with left ventricular thrombus
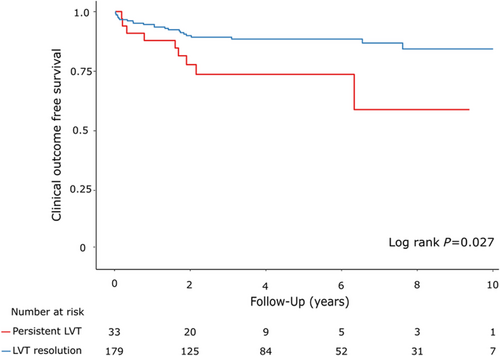
During the follow-up period (median, 4.0 years; IQR, 1.9–7.3 years), 32 patients (15.1%) experienced clinical events (18 all-cause-death, 15 stroke/TIAs, 3 arterial thromboembolisms) (Table 3). The primary clinical outcome occurred in 9 patients (27.3%) in the LVT persistent group and in 23 patients (12.8%) in the LVT resolution group (log rank P = 0.027, Figure 2). LVT resolution was a significant independent factor for the primary clinical outcome (Table 4). Multivariable Cox proportional hazard model adjusted for age and sex showed that LVT resolution is associated with lower risk of the primary outcome (HR, 0.45; 95% CI, 0.21–0.98; P = 0.045). In addition, LVT resolution remained a significant prognostic factor for lower risk of primary outcome after adjusting for baseline LVEF, aetiology of HF, anticoagulation duration, and combination of antiplatelet and anticoagulation therapy (HR, 0.29; 95% CI, 0.12–0.67, P-value = 0.004). Meanwhile, there was no significant difference in clinical outcome according to HF aetiology (Table S3). LVT resolution was also a significant factor associated with lower risk of clinical outcome regardless of aetiology of HF and was the only prognostic factor in patients with non-ischaemic HF (Table S4).
| Total (n = 212) | Persistent thrombus (n = 33) | Thrombus resolution (n = 179) | Log rank P | |
|---|---|---|---|---|
| Primary outcome | ||||
| Composite of all-cause death, ischaemic stroke/TIA, arterial thromboembolism | 32 (15.1) | 9 (27.3) | 23 (12.8) | 0.027 |
| Secondary outcome | ||||
| All-cause death | 18 (8.5) | 5 (15.2) | 13 (7.3) | 0.078 |
| Ischaemic stroke/TIA | 15 (7.1) | 2 (6.1) | 13 (7.3) | 0.827 |
| Arterial thromboembolism | 3 (1.4) | 2 (6.1) | 1 (0.6) | 0.009 |
| Composite of ischaemic stroke/TIA and arterial thromboembolism | 18 (8.5) | 4 (12.1) | 14 (7.8) | 0.396 |
- Values are n (%, percentage).
- LVT, left ventricular thrombus; TIA, transient ischaemic attack.
| Hazard ratio | 95% confidence interval | P-value | |
|---|---|---|---|
| Unadjusted | 0.43 | 0.20–0.93 | 0.032 |
| Model 1 | 0.45 | 0.21–0.98 | 0.045 |
| Model 2 | 0.42 | 0.19–0.92 | 0.030 |
| Model 3 | 0.29 | 0.12–0.67 | 0.004 |
- Model 1: adjusted by age and sex. Model 2: Model 1 + baseline left ventricular ejection fraction and ischaemic cardiomyopathy. Model 3: Model 2 + combination of anticoagulation and antiplatelet therapy and duration of anticoagulation therapy.
- LVT, left ventricular thrombus.
3.4 Clinical outcomes including left ventricular thrombus recurrence after left ventricular thrombus resolution
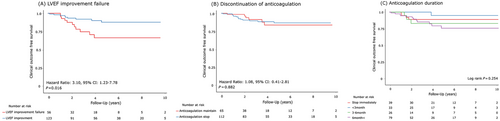
Of the 179 patients with LVT resolution, LVT recurrence occurred in 11.2% (n = 20) of patients after LVT resolution. There was no significant difference in clinical outcomes between the patients with and without LVT recurrence, and a total of three clinical events occurred after LVT recurrence (two all-cause-death and one stroke/TIA) (Table S5). Of the patients with LVT recurrence, 14 patients (70%) experienced LVT recurrence after discontinuing anticoagulation therapy. In patients with resolved LVT, the discontinuation or duration of anticoagulation after resolution were not significant predictors for LVT recurrence, but LVEF improvement failure at the time of LVT resolution was an independent factor for higher risk of LVT recurrence (HR, 3.10; 95% CI, 1.23–7.78; P = 0.016, Figure 3). Similarly, for composite clinical outcomes after LVT resolution, LVEF improvement failure was an independent prognostic factor (Table S6).
4 Discussion
The main findings of this study were as follows: about 85% of the patients experienced LVT resolution through anticoagulation using VKA or DOACs in patients with systolic dysfunction. LVT resolution was an independent predictor for the primary outcome, which was a composite of all-cause death, ischaemic stroke, TIA, and arterial thromboembolism. Improvement failure in LV systolic function was a significant predictor for hindering LVT resolution and crucial factor for LVT recurrence. The duration of anticoagulation after LVT resolution or combination with antiplatelet therapy did not affect occurrences of clinical events.
LVT is common in patients with HF with reduced LVEF (HFrEF). A retrospective single-centre study discovered LVT in 123 (1.3%) of 9485 patients with HFrEF.15 Although the prevalence of LVT is thought to vary depending on the cause of HF, it occurs more frequently in the setting of ischaemic heart disease. LVT was found in 15% of patients with ST-elevation myocardial infarction involving the LV anterior wall16 and is more likely to occur in those with LV aneurysm or severe LV systolic function.17 As LVT is closely related to an increased risk of death and increasing systemic embolic risk, it is important to evaluate for the presence of LVT using imaging tests at the first diagnosis of or follow-up for HF, and initiation of the appropriate treatment for LVT is essential.18
In this study, LVT resolution was an independent predictor of clinical outcomes. LVEF improvement failure was associated with not only impaired LVT resolution but also a higher risk of LVT recurrence. In addition, LVT resolution was an independent predictor of better clinical outcomes. Virchow's triad (blood stasis, hypercoagulability, and tissue injury) is the pathophysiological cornerstone in thrombus formation.5 Conversely, this suggests that restoration of LV systolic function may reduce blood stasis as well as the risk of thrombus formation in the LV chamber. A recent consensus statement recommends anticoagulation therapy to be discontinued when LVEF improves to >35%, assuming that LV thrombosis has resolved.14 In this study, LVT resolution occurred more in patients with non-ischaemic causes of HF than in those with ischaemic causes. One of the reasons for this result may be that LVEF improvement is more pronounced in non-ischaemic HF than in ischaemic HF. This study highlights the importance of treatment for LVEF improvement in patients with LVT.
To resolve LVT, DOAC has replaced VKA and is being actively utilized in clinical practice. The 2013 ACC/AHA STEMI guidelines recommend that LVT in patients with HFrEF with coronary artery disease be treated with VKA and antiplatelet therapy for 3–6 months.9 However, this set of guidelines was developed a long time ago. In addition, the guidelines' recommendations regarding the selection and duration of anticoagulation agents for LVT in non-ischaemic cardiomyopathy are insufficient.14 In the stroke guidelines, VKA is recommended as the primary choice, but if intolerance occurs, the use of DOAC is recommended as an alternative.11 However, according to the results of a recent meta-analysis, there was no significant difference in the efficacy of VKA and DOAC for LVT resolution.19 In the present study, 21 of 23 DOAC users showed LVT resolution, and no clinical events occurred in any DOAC user. Our results may contribute to defining the indications for DOAC use in patients with LVT. The 2022 AHA scientific statement notes that it may be reasonable to consider using DOAC instead of VKA in patients with LVT, and that DOAC may be an attractive option especially for patients who have difficulty maintaining the therapeutic international normalized ratio (INR) range with VKA.14 However, there is also concern that DOAC is inferior to VKA in terms of safety. A retrospective cohort study comparing the use of VKA and off-label DOAC use in 514 patients with LVT demonstrated no differences between VKA and DOAC use in LVT resolution.20 In this study, DOAC use was associated with a higher risk of systemic embolic events than VKA use, after adjusting for several clinical variables. However, the fact that systemic embolic rates began to differ between the two groups at late follow-up raises the possibility that the difference in systemic embolic rates between DOACs and warfarin may be due to causes other than differences in efficacy against LVT. The effect of long-term VKA and DOAC use in HFrEF patients with LVT should be re-evaluated through future randomized control trials. In addition, the effect of antiplatelet therapy on LVT resolution is still unclear. Some studies have shown that it increases bleeding.14 Considering the pathophysiology of LVT formation, the use of anticoagulation instead of antiplatelet therapy is reasonable.5 Our study also showed that combined use of antiplatelet and anticoagulation treatment was not associated with LVT resolution. In patients receiving anticoagulation therapy, antiplatelet therapy does not appear to play a role in LVT resolution.
Guidelines recommend the use of anticoagulants for 3–6 months in the presence of LVT, but there is no clear recommendation on the duration of anticoagulation therapy. A recent review suggested that the duration of anticoagulation therapy should be decided based on individual ischaemic and bleeding risk and LV function improvement.5 In our study, LVT resolved in 84.4% of patients in a median of 108 days from their first diagnosis. This indicates that LVT resolution occurred within 3–6 months in most patients, which is consistent with the duration of LVT anticoagulation therapy suggested by the current guidelines. In addition, although 62.6% of patients with LVT resolution discontinued anticoagulation, there was no difference in the risk of clinical outcomes between the groups who stopped anticoagulation and those who did not (Figure 3 and Table S6). Additionally, there was no difference in the risk of clinical outcomes in the group that continued anticoagulation therapy for more than 6 months after LVT resolution and the group that did not (Figure 3 and Table S6). If LVEF improvement and LVT resolution are demonstrated on imaging, the cessation of anticoagulation should be considered. However, LVT recurrence increases the risk of stroke and is strongly associated with LVEF deterioration and ventricular aneurysm or dyskinesia.21, 22 In patients with these factors, termination of anticoagulant therapy should be carefully determined.
This study had several limitations. First, as a retrospective single-centre study, the number of study subjects was small and the interval or frequency of TTE was different. However, in patients with LVT recurrence, the median number of TTE performed until recurrence was 1 (IQR: 1–2), and in patients without recurrence, the median number of TTE up to the last TTE performed was 2 (IQR: 1–4). There was no significant difference between them (P = 0.195). Adjusting for the duration to the last TTE, there was no difference between patients with LVT recurrence and those without. Second, LVT was evaluated only through TTE. Because a small number of patients underwent cardiac magnetic resonance imaging (CMR) (22.7%, n = 47) or contrast TTE (9.4%, n = 20), there were limitations to the imaging methods we could use to identify LVT in this study. Compared with CMR, the sensitivity and specificity of TTE for detecting LVT in patients with LV anterior wall MI are 70% and 98%, respectively.23 Because of the low sensitivity of TTE, there could be some patients with LVT who may have been excluded from this study. In terms of economic and medical accessibility, we believe that simple TTE is more practical than CMR; therefore, our study results are still of sufficient value. Third, variables affecting LVT resolution such as severity of LV regional wall motion abnormality, LVT location, mobility, and size were not included in this study. In addition, whether the INR was well controlled within the target range in those using warfarin could also have influenced the study results. However, as most of the study participants were followed up, it can be assumed that they received treatment according to the standard of care. In warfarin users, possible challenges in achieving adequate INR levels within the target range might reflect real-world practice.
In conclusion, LVT resolution was an important predictor of better clinical outcomes, and LVEF improvement failure was strongly associated with hindering LVT resolution. After LVT resolution, administration of anticoagulation did not affect LVT recurrence and the prognosis, but LVEF improvement failure at the time of LVT resolution was a crucial factor for LVT recurrence. This study reaffirmed that LVT resolution can be achieved with anticoagulation therapy for an average of 3 months, and suspension of anticoagulation could be considered if LVEF improves at the time of LVT resolution.
Conflict of interest
None declared.
Funding
This work was supported by the National Research Foundation of Korea grant funded by the Korean government (MSIT) (Grant Number: 2020R1C1C1013627).




