Dose titration of sacubitril/valsartan for heart failure with reduced ejection fraction: a real-world study
Chen Wang and Zongwei Lin contributed equally to this work.
Abstract
Aims
The study aims to explore the real-world titration patterns of sacubitril/valsartan in a chronic heart failure (HF) follow-up management system and the effect on the recovery of ventricular remodelling and cardiac function in China.
Methods and results
This is a single-centre, observational study of 153 adult outpatients with HF and reduced ejection fraction who were managed in the chronic HF follow-up management system and prescribed with sacubitril/valsartan from August 2017 to August 2021 in China. All patients tried to titrated sacubitril/valsartan to the tolerant dose during follow-up. The primary outcome was the proportion of patients who reached and maintained the target dose of sacubitril/valsartan. The main secondary outcomes were the changes in left atrium diameter, left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF) from baseline to 12 months. Among the patients, 69.3% were male, with a median age of 49 years. The baseline systolic blood pressure (SBP) was 117.6 ± 18.3 mmHg before starting the treatment of sacubitril/valsartan. Benefiting from the management system, 117 (76.5%) patients achieved the target dose of sacubitril/valsartan, and the median time to reach the target dose was 3 (IQR 1–5) months. Advanced age and lower SBP may be predictors of failure to reach the target dose. Compared with baseline, standard treatment resulted in a pronounced improvement in left ventricular geometry and cardiac function. The patients showed a significant increase in LVEF [28 (IQR 21–34) % vs. 42 (IQR 37.0–54.3) %, P < 0.001], with a great reduction in left atrium diameter [45 (IQR 40.3–51.0) mm vs. 41 (IQR 37.0–45.3) mm, P < 0.001] and LVEDD [65 (IQR 60.0–70.3) mm vs. 55 (IQR 52–62) mm, P < 0.001] during 12 month follow-up. Of patients, 36.5% had a LVEF ≥50%, 54.1% had LVEF >40%, and 81.1% experienced an increase in LVEF of ≥10%. After 12 month follow-up, the proportion of patients with New York Heart Association classification I or II increased from 41.8% to 96.4%. Additionally, there was a significant improvement in N-terminal pro-B-type natriuretic peptide (P < 0.001). At Month 12, 50% of patients achieved the target dose of beta-blockers. No serious adverse events caused by sacubitril/valsartan were observed during the follow-up.
Conclusions
Optimising HF follow-up management was essential and effective in a real-world clinical setting; the majority could reach the target dose of sacubitril/valsartan within the management system and achieve a remarkable improvement in cardiac function and ventricular remodelling.
Introduction
Chronic heart failure (HF) is a global health and economic burden associated with significant morbidity and mortality.1 Nowadays, treatment of chronic HF has witnessed major changes. Sacubitril/valsartan, the first angiotensin II receptor blocker-neprilysin inhibitor (ARNI), can act on the renin–angiotensin system and neutral endopeptidase system simultaneously.2 Based on the evidence of Prospective comparison of ARNI with angiotensin-converting enzyme inhibitor (ACEI) to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF),3 updated HF guidelines provided class of recommendation I, level of evidence B to replace ACEI with sacubitril/valsartan in patients who remain symptomatic. The guidelines also recommend uptitrating to the target dose or maximally tolerated dose.4, 5
Unfortunately, the target dose of sacubitril/valsartan in clinics is often lower than what is recommended in HF guidelines, with the proportion of patients achieving the target dose being far below 50%.6-8 A recent observational study conducted in China observed that only 31 (31%) patients achieved the target dose.6 Additionally, the target dose of sacubitril/valsartan was achieved in 30% of patients in a 2021 multinational study of 29 546 patients.7 In a large cohort study of 26 191 patients with HF in Germany in 2019, only 21% of the patients reached the target dose.8 There are many reasons for the low proportion of patients who reached target dose, such as poor adherence of patients to the treatment and insufficient understanding of HF guidelines by clinicians. Practical chronic HF follow-up management can be an important strategy to address the multiple reasons for the low target dose rate of sacubitril/valsartan. Although the updated European Society of Cardiology (ESC) HF guideline4 has put more weight in HF management, it is still relatively understudied. Follow-up management for HF patients to achieve optimized dosing of medical therapy has been less studied in clinical practice.
Herein, we developed a chronic HF follow-up management system tailored to Chinese patients, aiming to increase the proportion of patients who achieve the target dose of sacubitril/valsartan and improve the recovery of ventricular remodelling and cardiac function.
Methods
Study design
This was a single-centre, observational study of adult inpatients and outpatients (aged ≥18 years) with HF with reduced ejection fraction (HFrEF) [left ventricular ejection fraction (LVEF) ≤ 40% by echocardiography before initiation of sacubitril/valsartan administration] and New York Heart Association (NYHA) class II–IV. All patients consecutively enrolled in the study were treated with sacubitril/valsartan between August 2017 and August 2021 at Qilu Hospital (Shandong Province, China). Patients with congenital heart disease were excluded from this study. Other exclusion criteria were as the follows: a known contraindication to sacubitril/valsartan, ACEI, angiotensin receptor blocker (ARB), or beta-blocker; estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 at screening; currently in the titration period; lost to follow-up; and less than 1 month treatment with sacubitril/valsartan.
Other therapies for optimal treatment under ESC and American College of Cardiology (AHA) guidelines in 2016,5, 9 including beta-blockers and mineralocorticoid receptor antagonists (MRAs), are recommended in the protocol. The guidelines recommended dosage of sacubitril/valsartan and beta-blockers were shown in supplemental Table S1.
The study protocol was approved by the ethics committee of the Qilu Hospital of Shandong University, and the participants' clinical data were retrospectively reviewed. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Chronic heart failure follow-up management system
All patients enrolled in our study were managed using the chronic HF follow-up management system during hospitalization and after discharge. To improve dose titration in patients with HF, we combined several types of interventions, which include (i) HF team: the main members of the team are specialist HF cardiologists and doctors' assistants. Cardiologists are hospital-based and specialized in providing optimized therapeutic plans and suggestions. Doctors' assistants are responsible for patient reminders and the appointment of clinic visits and checks. (ii) Patient education: we provided patients with the fundamental knowledge of HF, the use of medications, self-care management, and self-monitoring (including measurements of blood pressure and heart rate by themselves). We educated patients via patient education which was implemented in a variety of formats, such as offline lectures, online learning sessions, and healthcare brochures. (iii) Follow-up: The patients' phone numbers, addresses, and WeChat numbers were registered in our management system in order to be followed-up. The frequency of follow-up visits for the patients in the titration period was once per 2–4 weeks. Patients in the titration period were recommended to receive follow-up in our HF clinics, with blood pressure, heart rate, N-terminal pro-B-type natriuretic peptide (NT-proBNP), electrolyte, and renal function tested. For patients who were inconvenient for offline visits in HF clinics, we recommended them to receive blood tests in local clinics and medicine therapy adjustment online once per 2–4 weeks. If patients forgot the visit time, we would make a phone call once per 2 weeks to ask for specific reason and clinical status to make corresponding adjustment of follow-up. Notably, self-test blood pressure, heart rate, weight, fluid intake and output, and exercise tolerance were stored in the HF management brochures per day. The HF cardiologists adjusted the dose of medications according to family blood pressure and heart rate instead of those in the office. For patients already uptitrated to the target dose or stable HF patients who were on optimal medical therapy, follow-up was adjusted to once per 3–6 months. During this period, the patients were required to be followed-up in HF clinics, with echocardiography tested to evaluate cardiac function and ventricular remodelling, with Holter monitored to evaluate the heart rate and arrhythmia, and with blood tested (i.e. liver and renal function, electrolyte, and NT-proBNP).
Data collection
The following data were collected at enrolment and during follow-up: patient demographics, clinical characteristics, medical history, laboratory results, echocardiographic images, use of HF medications and devices, and patient health status. After the initiation of sacubitril/valsartan, patients underwent two-dimensional echocardiography every 3–6 months during follow-up. The parameters collected included the left atrium (LA) diameter, left ventricular end-diastolic diameter (LVEDD), and LVEF. All echocardiographic measurements were calculated as the mean values of five cardiac cycles. Intra-observer variability of echocardiographic measurements is shown in Figure S1. Echocardiographic images were acquired using diagnostic cardiac sonography per the American Society of Echocardiography guidelines.10 Notably, the echocardiographic images of majority of the patients were assessed by the same cardiac sonographer.
Definition
Complete recovery of LVEF was defined as LVEF ≥50%. If patients showed an increase in LVEF of ≥10% but <50%, these patients were considered as partial recovery of LVEF. LVEF less than the baseline was defined as worsening of LVEF. Stable LVEF was defined as an increase in LVEF of <10%.
Study endpoints
The primary outcome assessed was the proportion of patients who reached and maintained the target dose of 200 mg twice daily (b.i.d.) at the time of censoring. The secondary outcomes were the changes from baseline to 12 months in (i) NT-proBNP; (ii) ventricular remodelling, assessed by LA diameter, LVEDD, and LVEF; (iii) cardiac function, assessed by the rate of recovery of LVEF; (iv) NYHA functional class; (v) use of other HF medications; (vi) unplanned HF readmissions; and (vii) treatment-related adverse events (Table S2).
Statistical analysis
SPSS Statistics software (version 19.0; Chicago, IL, USA) was used for all the statistical analyses. Quantitative data were reported as mean ± standard deviation if normally distributed and medians with interquartile ranges if skewed. Categorical variables were reported as percentages (%). The patients were stratified according to the dose of sacubitril/valsartan (target dose vs. low target dose). Continuous variables were compared between the target dose group and the lower than target dose group using Student's t-test and Mann–Whitney rank-sum test. Comparisons of parameters between baseline and 3–12 months after first dose of sacubitril/valsartan were calculated using a paired-samples t-test or Wilcoxon signed-rank test. Categorical variables were compared using Pearson's χ2 test or Fisher's exact test. Multivariate logistic regression analyses were performed to identify the clinical baseline factors influencing the failure to reach the target dose of sacubitril/valsartan at the time of data censoring and were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Candidate predictors were identified from clinical characteristics at baseline, with P < 0.1 in a univariate analysis for inclusion. Intra-observer variability of cardiac ultrasound measurements was examined using Bland–Altman plots by one observer for 20 patients at baseline and 3 months in a random order. The observer was blinded to the previous measurements. The differences between the two measurements were plotted against the averages of the two measurements and 95% CI limits of agreement were computed. Statistical significance was defined as a two-sided P < 0.05.
Results
Patients' characteristics
Between 1 August 2017and 31 August 2021, 233 patients with HFrEF who were enrolled in the follow-up system were prescribed sacubitril/valsartan. Of these, 40 patients who were in the titration period, 29 patients who were lost to follow-up, and 11 patients with other causes were excluded (Figure 1). Hence, data from 153 patients (median age, 49 years; 69.3% male) was analysed in our study. Unplanned readmission for HF occurred in 21 patients (13.7%) during follow-up. The baseline characteristics of the study population are shown in Tables 1 and S3. Only 23.5% had ischaemic cardiomyopathy, whereas 40.5% had dilated cardiomyopathy. A higher proportion of patients were NYHA Class III (43.8%), followed by Class II (41.8%). Systolic blood pressure (SBP) was 117.6 ± 18.3 mmHg, and diastolic blood pressure (DBP) was 74.5 ± 14.6 mmHg. The median LVEF was 28 (IQR 21–34) %, and LVEDD was 65.4 ± 8.5 mm. Before starting sacubitril/valsartan, 57 (37.3%) patients were treated with either an ACEI or ARB.
| Total (n = 153) | < Target dose of sacubitril/valsartan (n = 36) | Target dose of sacubitril/valsartan (n = 117) | P value | |
|---|---|---|---|---|
| Age (years) | 49 (35–61) | 61.5 (48.0–67.0) | 44.0 (33.0–57.5) | <0.001 |
| Male | 106 (69.3%) | 21 (58.3%) | 85 (72.6%) | 0.103 |
| Ischaemic aetiology | 36 (23.5%) | 13 (36.1%) | 23 (19.7%) | 0.042 |
| Medical history | ||||
| Hypertension | 48 (31.4%) | 9 (25.0%) | 39 (33.3%) | 0.346 |
| Diabetes mellitus | 32 (20.9%) | 8 (22.2%) | 24 (20.5%) | 0.825 |
| OMI | 22 (14.4%) | 10 (27.8%) | 12 (10.3%) | 0.009 |
| Previous PCI/CABG | 24 (15.7%) | 10 (27.8%) | 14 (12.0%) | 0.023 |
| Stroke/TIA | 6 (3.9%) | 0 | 6 | — |
| Atrial fibrillation | 23 (15.0%) | 9 (25.0%) | 14 (12.0%) | 0.056 |
| CKD | 18 (11.8%) | 6 (16.7%) | 12 (10.3%) | 0.297 |
| Previous HF hospitalization | 124 (81.0%) | 28 (77.8%) | 96 (82.1%) | 0.567 |
| NYHA class | ||||
| I | 0 | 0 | 0 | — |
| II | 64 (41.8%) | 19 (52.8%) | 45 (38.5%) | 0.128 |
| II | 67 (43.8%) | 13 (36.1%) | 54 (46.2%) | 0.288 |
| IV | 22 (14.4%) | 4 (11.1%) | 18 (15.4%) | 0.523 |
| Vital sign and laboratory findings | ||||
| SBP (mmHg) | 117.6 ± 18.3 | 112.2 ± 20.0 | 119.2 ± 17.6 | 0.044 |
| DBP (mmHg) | 74.5 ± 14.6 | 67.6 ± 17.2 | 76.6 ± 13.0 | <0.001 |
| Heart rate (beats/min) | 78 (68–89) | 72.0 (66.5–83.8) | 80 (68–91) | 0.052 |
| NT-proBNP (pg/mL)a | 1604.5 (455.9–3532.5) | 2724.5 (748.5–5032.5) | 1379.0 (395.6–3340.5) | 0.081 |
| Echocardiographic parameters | ||||
| LA diameter (mm)b | 45.9 ± 8.0 | 46.1 ± 9.5 | 45.8 ± 7.6 | 0.843 |
| LVEDD (mm)c | 65.4 ± 8.9 | 61.4 ± 6.5 | 66.6 ± 9.2 | 0.002 |
| LVEF (%) | 28 (21–34) | 30 (25–36) | 27 (20–33) | 0.051 |
| Previous HF management | ||||
| Beta blocker | 76 (49.7%) | 22 (61.1%) | 54 (46.2%) | 0.117 |
| ACEI/ARB | 57 (37.3%) | 17 (47.2%) | 40 (34.2%) | 0.157 |
| MRA | 65 (42.5%) | 20 (55.6%) | 45 (38.5%) | 0.070 |
| Loop diuretics | 49 (32.0%) | 16 (44.4%) | 33 (28.2%) | 0.068 |
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DBP, diastolic blood pressure; HF, heart failure; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptides; NYHA, New York Heart Association; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TIA, transient ischemic attack.
- a A total of 25 patients were lack of the data of NT-proBNP.
- b A total of 11 patients lacked the data of LA diameter.
- c There were 3 patients that lacked the data of LVEDD.
Of the 153 patients, 36 did not reach 200 mg b.i.d. at the time of censoring. Patients who failed to achieve the target dose of sacubitril/valsartan tended to have advanced age (61.5 [IQR 48.0–67.0] vs. 44.0 [IQR 33.0–57.5], P < 0.001), ischaemic cardiomyopathy (36.1% vs. 19.7%, P = 0.042), previous percutaneous coronary intervention or coronary artery bypass grafting treatment (27.8% vs. 12.0%, P = 0.023), lower SBP (112.2 ± 20.0 mmHg vs. 119.2 ± 17.6 mmHg, P = 0.044), and lower DBP (67.6 ± 17.2 mmHg vs. 76.6 ± 13.0 mmHg, P < 0.001), but smaller LVEDD (61.4 ± 6.5 mm vs. 66.6 ± 9.2 mm, P = 0.002), compared with patients who achieved the target dose.
Multivariate analysis demonstrated that predictors of failure to reach the target dose were advanced age (OR 1.061, 95% CI 1.024–1.100; P = 0.001) and lower SBP (OR 0.971, 95% CI 0.945–0.998; P = 0.034) (Table 2).
| Variable | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age (years) | 1.059 (1.028–1.091) | < 0.001 | 1.061 (1.024–1.100) | 0.001 |
| SBP at baseline (mmHg) | 0.977 (0.955–1.000) | 0.046 | 0.971 (0.945–0.998) | 0.034 |
| Medical history of HP | 0.667 (0.286–1.555) | 0.348 | — | — |
| Medical history of AF | 2.452 (0.959–6.269) | 0.061 | 1.081 (0.364–3.209) | 0.889 |
| Medical history of CKD | 1.750 (0.606–5.054) | 0.301 | — | — |
| Ischaemic HF aetiology | 2.310 (1.019–5.239) | 0.045 | 1.243 (0.469–3.294) | 0.662 |
| Prior use of ACEI/ARB | 1.722 (0.807–3.674) | 0.160 | — | — |
| Initial dose of sacubitril/valsartan (mg) | 0.993 (0.986–1.000) | 0.047 | 0.994 (0.987–1.001) | 0.092 |
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; HP, hypertension; SBP, systolic blood pressure.
Dose titration
The target dose of sacubitril/valsartan was reached in 76.5% of the patients at the time of censoring. Notably, 81.7% of patients reached 200 mg b.i.d. during the follow-up, but 6.4% of these patients whose titration dropped from 200 mg b.i.d. to a lower dose, which was maximum tolerated dose at the censored time. The median time to achieve the target dose was 3 (IQR 1–5) months. A total of 43.1% of the patients were prescribed a 50 mg b.i.d. dose as the initial dose (Table S4). More than 50% of the patients reached 200 mg b.i.d. after 3 months, while 67.6% achieved the target dose at 6 months of follow-up. The proportion of patients who achieved the target dose had increased to 69.9% at 12 month follow-up visit (Figure 2).

Changes in left atrium diameter, left ventricular end-diastolic diameter, and left ventricular ejection fraction
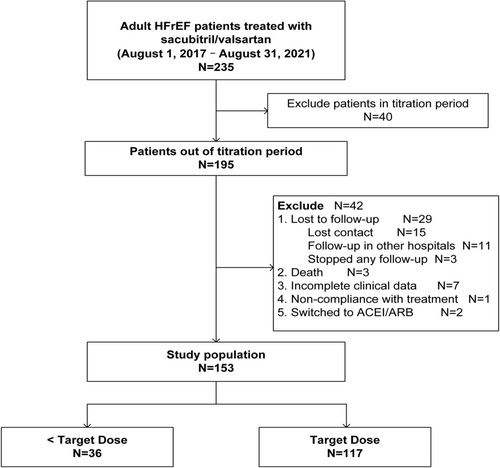
Sacubitril/valsartan resulted in a significant increase in LVEF at Month 3 (37 [IQR 30.8–45.0] %), Month 6 (40 [IQR 34.0–51.5] %), Month 9 (40.5 [IQR 33.8–54.3] %), and Month 12 (42 [IQR 37.0–54.3] %) of follow-up, compared with baseline (28 [IQR 21–34] %) (all P < 0.001). To evaluate the continuity of improvement during 12 month follow-up, we analysed the changes in LVEF every 3 months. The LVEF at Month 6 was higher than that at Month 3 (40 [IQR 34.0–51.5] % vs. 37 [IQR 30.8–45.0] %, P < 0.001). Compared with Month 6, the LVEF trended to increase at Month 9 (40 [IQR 34.0–51.5] % vs. 40.5 [IQR 33.8–54.3] %, P = 0.175), but with no statistical significance. There was statistically significant again between Months 12 and 9 (42 [IQR 37.0–54.3] % vs. 40.5 [IQR 33.8–54.3] %, P < 0.001) (Figure 3).
Reductions in LA diameter were found at Month 3 (42 [IQR 36.5–47.0] mm), Month 6 (41 [IQR 37–45] mm), Month 9 (40 [IQR 36–46] mm), and Month 12 (41 [IQR 37.0–45.3] mm) during the follow-up period, compared with baseline (45 [IQR 40.3–51.0] mm) (all P < 0.001). However, there were no differences in LA diameter between Months 3 and 6 (P = 0.226), Months 6 and 9 (P = 0.818), and Months 9 and 12 (P = 0.483) (Figure 3).
Moreover, as shown in Figure 3, a significant decrease in LVEDD was observed at Month 3 (61 [IQR 55.5–68.0] mm), Month 6 (58 [IQR 53.5–63.5] mm), Month 9 (58 [IQR 54.0–64.3] mm), and Month 12 (55 [IQR 52–62] mm) during the follow-up period, compared with baseline (65 [IQR 60.0–70.3] mm) (all P < 0.001). LVEDD was smaller at Month 6 than that at Month 3 (P = 0.002). There was no significant change between Months 6 and 9 (P = 0.121), with similar change between Months 9 and 12 (P = 0.098).
The percentage of patients with complete recovery and partial recovery of LVEF increased to 36.5% and 44.6%, respectively, at Month 12 visit (Figure 3). LVEF >40% was observed in up to 54.1% of the patients at Month 12 (Figure 3). Meanwhile, we found that the proportion of patients with worsening LVEF decreased during the 12 months of follow-up. And there was a decreasing trend in the percentage of stable LVEF during follow-up (Figure 3).
Change in New York Heart Association class
The proportion of patients with NYHA Class I notably increased to 28.9% after 12 months of follow-up. Overall, the proportion of patients with NYHA Class II was still the highest, which was more than 60% during the follow-up period, while the proportion of patients with NYHA Class III or IV decreased from 58.2% to 3.6% (Figure S2A). Additionally, the proportion of patients with improvements in NYHA class increased during the follow-up period (Figure S2B).
Other changes under treatment with sacubitril/valsartan
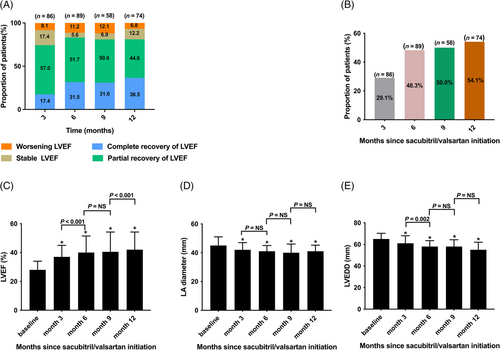
Analyses of changes in NT-proBNP levels demonstrated a significant decrease at 3, 6, 9, and 12 months compared with those in baseline (all P < 0.001) (Figure 4). Additionally, a significant increase in eGFR level at 6 months (92.7 [IQR 76.3–116.4] mL/min/1.73 m2 vs. 91.0 [IQR 70.4–110.8] mL/min/1.73 m2, P = 0.024) was observed compared with that at baseline, whereas no significant change was found at 3, 9, and 12 months (all P > 0.05) (Figure 4). Compared with those at baseline, significant decreases in SBP (Figure 4) and DBP (Figure 4) were observed at 3, 6, 9, and 12 months (P < 0.001).
Concomitant medication
Overall, the proportion of patients who achieved the target dose of beta-blockers increased during the follow-up, with more than half of the patients reaching the target dose at as early as 6 month visit. The proportion of patients who achieved the target dose was 56.2% and 53.0% at months 9 and 12, respectively. After the 12 month treatment, only 26.5% of the patients received a dose <50% of the target dose (Figure 2). A reducing trend of the use of MRA, loop diuretics, digoxin, and ivabradine during the 12 months of follow-up was observed (Figure S3).
Adverse events
One-tenth of the patients experienced symptoms of hypotension or SBP less than 90 mmHg. Furthermore, 3 (2.0%) patients reported hyperkalaemia, 4 (3.4%) patients reported worsening renal function, and no patient reported intolerable cough or angioedema in our study (Table S2).
Discussion
In this real-world observational study, 76.5% of study patients achieved the target dose of sacubitril/valsartan at the time of censoring with the help of our chronic HF follow-up management system. In addition, optimized HF follow-up management and target-dose therapy contributed to a significant improvement in cardiac function and rehospitalization.
The prognostic benefit associated with target dose therapy of sacubitril/valsartan has been demonstrated in previous studies,11, 12 but the proportion of the target dose was low in clinical practice. So far, fewer real-world studies have reported a similar high rate of achieving the target dose of sacubitril/valsartan as in our study.6-8, 13-15 A retrospective study using electronic medical records from Germany showed that only 14% of 1263 patients reached the target dose.14 A recent multicentre, retrospective study enrolled 983 patients in China reported that only 1.6% of patients achieved the target dose of sacubitril/valsartan within 6 months.15 Another retrospective study in America reported that 17% of 200 patients with HFrEF reached the target dose of sacubitril/valsartan within 4 months.13 Asides sacubitril/valsartan, a low proportion of target doses was also observed in other cornerstone medications for HF patients like beta-blockers, ACEI, and ARB. Change the Management of Patients With Heart Failure (CHAMP-HF) reported that the rate of achieving the target dose of renin–angiotensin system inhibitors and beta-blockers were 8.5% and 18.4%, respectively, in 2588 US patients with HFrEF.16 In a multinational study in North America and Europe, 68 172 new users of HFrEF medications were enrolled and the target dose achievement were 15%, 10%, 12%, and 30% in ACEI, ARB, beta-blockers, and ARNI, respectively.7 Underdosage of guideline-recommended medical therapies in HFrEF remains highly prevalent, and clinical inertia was a main barrier to achieving optimal dosing of HF therapies.
Clinical inertia is defined as ‘recognition of the lack of treatment intensification, but failure to take action in patients to achieve evidence-based goals’17 Patient-related, physician-related, and system-related factors may be the main reasons.18 First, poor adherence to medical therapies is the most important patient factor. Previous data clearly showed that high adherence to sacubitril/valsartan was associated with significantly lower rates of all-cause deaths and all-cause rehospitalization.19 However, poor adherence of patients with HF was common in the real world. Lack of knowledge of their condition, misunderstanding of HF conceptions, and being unconvinced of the efficacy of the medications might be the primary explanations for patients' poor adherence to guideline-recommended medication; hence, patient education is crucial.17 Second, compared with patient factors, it is easier to ignore physician factors, which include overrating the quality of the care they deliver, lack of awareness of the goals of HF care, lack of familiarity with the guidelines, and fear of the adverse events.20 Third, system-related factors, which include lack of effective communication and team cooperation, played a key role in clinical inertia. Based on the above problems and the current situation, we established a patient-centred and HF guideline-oriented follow-up management system for patients with chronic HF to reduce clinical inertia with some promising approaches. The first approach was to improve patient adherence by intensifying fundamental education of HF (e.g. understanding the causes of HF, the effects and side effects of medications, being aware of the need for follow-up visits, and avoiding random drug withdrawal) and providing information of self-care management (e.g. correct measurement of heart rate and blood pressure and management of activity tolerance and body weight). The second approach was to strengthen the education of HF physicians, such as training on HF guidelines, sharing cases, and regularly learning about the latest HF hotspots. The objective is to improve the proactivity of doctors in titrating medications with patience. The third approach was ensuring rigorous and efficient follow-up, which included providing patient reminders for clinical visits through networks or telephones, different follow-up patterns according to different visit distances, and a designated person responsible for outpatient appointments and examinations. All these efforts might have contributed to the high proportion of target dose achievement in sacubitril/valsartan, as well as beta-blockers in our study.
As is well known, a high proportion of target dose achievement of sacubitril/valsartan contributes to more clinical benefits of reduced mortality and rehospitalization. Cardiac remodelling is pivotal to the progression of HFrEF,21 but few studies have investigated the relationship between reversed cardiac remodelling and the target dose of sacubitril/valsartan. Definitive data regarding the impact of ARNI therapy on remodelling parameters are now available from two prospective trials, PROVE-HF (Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure)22 and EVALUATE-HF (Study of Effects of Sacubitril/Valsartan vs. Enalapril on Aortic Stiffness in Patients with Mild to Moderate HF with Reduced Ejection Fraction).23 Both studies demonstrated marked improvements in biomarkers and echocardiographic parameters of reverse cardiac remodelling in HFrEF. Much of the observed clinical benefit of sacubitril/valsartan therapy in patients with HFrEF is likely related to significant reverse cardiac remodelling with high target dose rates (65–83%) of the medication.22, 23 The 12 week target dose rate in the TITRATION trial reached to 76%, in which the participants had experienced the run-in period.24 Significantly, the above studies with high target doses are RCTs, but most studies are far below 50% in the real world.6-8, 13, 14 In our real-world study, the recovery of LVEF of ≥10% and reverse cardiac remodelling was achieved in 80% of the participants, among whom 77% reached the target dose of sacubitril/valsartan. The well-established benefits of sacubitril/valsartan for cardiac reverse remodelling are attributed to the high target dose rates. Therefore, optimising the management and treatment in a strict follow-up management system is key to increasing the target dose rates of sacubitril/valsartan.
In the ASIAN-HF registry, the guideline-recommended dose was achieved in only 13% of patients for beta-blockers.25 However, the proportion of target dose achievement for beta-blockers was high during follow-up in our management system, with over 50% of patients receiving the target dose within 6 months. In addition, the titration speed of beta-blockers was almost consistent with that of sacubitril/valsartan. Consequently, due to the improvement in cardiac function and symptoms, the rate of use of other supporting medications (loop diuretics, digoxin, and ivabradine) decreased significantly. Importantly, we still asked patients whose cardiac function had already fully recovered to visit HF clinics every 6 months to receive regular monitoring in our follow-up management system. Notably, Compared with PARADIGM-HF study,3 patients enrolled in our study were much younger, which was mainly caused by the low proportion of ischaemic HF aetiology26 and the ethnic characteristics of HF patients in Asia-Pacific region.27
According to the 2016 ESC guideline,9 MRA received a Class I recommendation for symptomatic patients with LVEF ≤35%. However, no consensus or clear recommendations in guidelines is available for decision of MRA treatment withdrawal in patients with clinical remission and improved LVEF. In the present study, four-fifth of patients discontinued the MRA treatment due to the achievement of complete recovery in LVEF and stable clinical status, with only one-fifth of patients who withdrew MRA because of adverse events. More trials are needed to evaluate the effects of MRA withdrawal on LV remodelling and cardiac function in the future.
In the PARADIGM-HF trial,3 sacubitril/valsartan was found to reduce the rate of decrease in the eGFR, compared with enalapril. Several studies have observed favourable renal effects of sacubitril/valsartan in HFrEF patients in the real world.28-30 In a study of 54 outpatients, patients treated with sacubitril/valsartan showed a significant improvement in eGFR after 1 year follow-up, compared with historical controls who received optimal medical therapy (P < 0.001 for improvement).29 Another study of 108 HFrEF patients followed for 1 year found that treatment with sacubitril/valsartan improved eGFR compared with the historical control arm (P < 0.05).28 In line with the previous observational studies, we found a transient increase in eGFR at 6 month follow-up. However, no differences in eGFR were found during follow-up at other time points. More data supporting benefits of sacubitril/valsartan in renal function are urgently needed in the future.
This study had some limitations. First, as an observational analysis, some data were inevitably unavailable, which explained the absence of some clinical data during follow-up. Second, this was a single-centre study with small sample size and short-term follow-up, which may reduce the generalizability of the findings. Third, our study did not analyse the influence of socioeconomic aspects on dose titration because sacubitril/valsartan was not on the national medical insurance reimbursement list by 31 August 2021. This economic situation may affect the choice of high-dose treatment.
In conclusion, optimising HF follow-up management was essential and effective in a real-world clinical setting; the majority reached the target dose of sacubitril/valsartan within the management system and achieved a remarkable improvement in cardiac function and ventricular remodelling.
Acknowledgements
We thank all the investigators and patients who participated in this project.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81873516, 81900444), National Key Research and Development Program of China (2017YFC1308303, 2021YFF0501404), Natural Science Foundation of Shandong Province (ZR2019PH030, ZR2019BH052, ZR2020QH007), Clinical Research Center of Shandong University (2020SDUCRCA009), and China International Medical Foundation (Z-2019-42-1908-2).




