Effects of angiotensin receptor-neprilysin inhibitor on insulin resistance in patients with heart failure
The work was performed in the Jikei University School of Medicine.
Abstract
Aims
Although the haemodynamic effects of angiotensin receptor-neprilysin inhibitor (ARNI) on patients with heart failure have been demonstrated, the effect on glucose metabolism has not been fully elucidated. We retrospectively investigated the effect of ARNI on abnormal glucose metabolism in patients with stable chronic heart failure using an additional structural equation model (SEM) analysis.
Methods
We analysed 34 patients who regularly visited to the outpatient department of our institute with heart failure from October 2021 and July 2022 and who were taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). Seventeen patients switched from ACE inhibitors or ARBs to an ARNI (ARNI group), and the other 17 patients continued treatment with ACE inhibitors or ARBs (control group).
Results
At baseline, although the ARNI group included fewer patients with heart failure with preserved ejection fraction in comparison with the control group (P = 0.004), patients with heart failure with mildly reduced ejection fraction, and heart failure with reduced ejection fraction were mostly biased towards the ARNI group (although not statistically significant). The baseline insulin resistance in the ARNI group was already significantly higher in comparison with the control group [fasting blood insulin, 9.7 (7.4, 11.6) vs. 7.8 (5.2, 9.2) μU/mL, P = 0.033; homoeostasis model assessment of insulin resistance (HOMA-IR), 3.10 (1.95, 4.19) vs. 2.02 (1.56, 2.42), P = 0.014].
Three months later, the fasting blood insulin and the HOMA-IR levels were both found to have decreased in comparison with the baseline values [baseline to 3 months: insulin, 9.7 (7.4, 11.6) to 7.3 (4.6, 9.4) μU/mL, P < 0.001; HOMA-IR, 3.10 (1.95, 4.19) to 1.96 (1.23, 3.09), P < 0.001]. An additional SEM analysis demonstrated that the initiation of ARNI had caused a reduction in the fasting blood insulin and the HOMA-IR levels at 3 months independently of the baseline fasting blood insulin and HOMA-IR levels, respectively. Similarly, the initiation of ARNI resulted in a significant reduction in serum uric acid levels (6.28 ± 0.35 to 5.80 ± 0.30 mg/dL, P = 0.008).
Conclusions
In conclusion, even in a short period of only 3 months, the administration of ARNI improved insulin resistance and consequently reduced the serum uric acid levels in patients with stable chronic heart failure. Although the ARNI group already had high insulin resistance at baseline, an additional SEM analysis revealed that the decreased insulin resistance was truly due to the effect of ARNI.
Introduction
In several previous clinical trials, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have been shown to reduce cardiovascular events in patients with congestive heart failure and a low ejection fraction, and therefore, they have long been the cornerstone of treatment for severe heart failure.1, 2 After that, the PARADIGM-HF [Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor with ACE Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure] trial showed that angiotensin receptor-neprilysin inhibitor (ARNI) reduced cardiovascular deaths and all-cause mortality in comparison with ACE inhibitors in patients with chronic heart failure and a reduced ejection fraction.3 ARNIs are now replacing ACE inhibitors and ARBs as the central drugs of severe heart failure treatment.4
Previous reports suggested that ARNI can improve the functional capacity and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction (HFrEF)5 and that this anti-remodelling effect might inhibit ventricular arrhythmia and even sudden death.6 In addition to improving the haemodynamic status and prognosis, ARNI has been reported to have some pleiotropic effects.7 In a post hoc analysis of the PARADIGM-HF trial, ARNI was associated with a greater reduction in haemoglobin A1c (HbA1c) and new use of insulin treatment in patients with diabetes and HFrEF in comparison with ACE inhibitor.8 The PARAGON-HF trial [Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction] similarly showed that ARNI reduced HbA1c values and the need for new insulin therapy in patients with heart failure with preserved ejection fraction (HFpEF) and diabetes,9 but the detailed efficacy of ARNI in the amelioration of glucose metabolism has not been fully clarified.
We previously reported a case of severe acute heart failure in which the initiation of ARNI markedly ameliorated the patient's deranged glucose metabolism, in addition to improving their haemodynamics.10 However, advanced heart failure by itself leads to increase insulin resistance, and the severity of heart failure was associated with the subsequent risk of developing diabetes.11, 12 Therefore, in our case reports, it was possible that the amelioration of glucose metabolism may have been influenced by the improvement in heart failure severity.
To clarify the effect of ARNI itself on glucose metabolism in heart failure patients, it is necessary to limit the study population to patients whose haemodynamics remain stable over a long period of time. In the present study, using an additional structural equation model (SEM) analysis, we retrospectively investigated the effect of ARNI-induced improvement of deranged glucose metabolism in ambulatory patients with chronic heart failure in stable condition.
Methods
Patient population
The study population consisted of 71 patients who regularly visited the outpatient department of Jikei University School of Medicine with heart failure from October 2021 to July 2022 and who were taking ACE inhibitors or ARBs at any dose (Figure 1). ACE inhibitors or ARBs were initiated in all patients of the present study at least 4 weeks before screening. We excluded 37 patients (as described later). Finally, we analysed 34 patients in the present study. Patients who switched from ACE inhibitors or ARBs to ARNI were classified into the ARNI group (n = 17), whereas those who continued treatment with ACE inhibitors or ARBs were classified into the control group (n = 17). ARNI treatment was initially started at 100 or 200 mg per day by carefully monitoring blood pressure and other clinical parameters. For all patients in the present study, no other medications were added or changed from 4 weeks prior to screening to the end of the observation period.
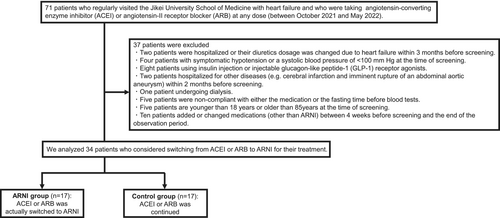
Patient flow chart for the present study. We excluded 37 patients and finally analysed 34 patients who considered switching from ACE inhibitor or ARB to ARNI.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor.
- Patients who were hospitalized or whose dose of diuretics was changed due to heart failure within 3 months before screening (n = 2).
- Symptomatic hypotension, a systolic blood pressure of <100 mmHg at the time of screening (n = 4).
- Patients using insulin injection or injectable glucagon-like peptide-1 (GLP-1) receptor agonists (n = 8).
- Patients hospitalized for other diseases (e.g. cerebral infarction and imminent rupture of an abdominal aortic aneurysm) within 2 months before screening (n = 2).
- Patient undergoing dialysis (n = 1).
- Patients who were non-compliant with either the medication or the fasting time before blood tests (n = 5).
- Patients of <18 years of age or >85 years of age at the time of screening (n = 5).
- Patients who added or changed medications (other than ARNI) between 4 weeks prior to screening and the end of the observation period (n = 10).
Data collection
The clinical characteristics of patients were measured at baseline and 3 months later and were retrospectively collected from their medical records. The left ventricle ejection fraction (LVEF) was measured by transthoracic echocardiography (TTE). Blood pressure was measured at rest in an outpatient department. Blood samples were collected after at least 11 h of fasting. Whole blood sugar levels were measured by the amperometric method with glucose oxidase (GOD)-immobilized enzyme membrane and hydrogen peroxide electrodes (ADAMS Glucose GA-1172, ARKRAY, Inc., Kyoto, Japan). Serum insulin levels were measured using a chemiluminescent microparticle immunoassay on the Alinity system (Abbott Laboratories, Abbott Park, IL, USA). The homoeostasis model assessment of insulin resistance (HOMA-IR) index [fasting blood sugar (mg/dL) × fasting blood insulin (μU/mL)/405] and homoeostasis model assessment of β-cell function (HOMA-β) [(fasting blood insulin (μU/mL) × 360)/(fasting blood sugar (mg/dL) −63)] were used to evaluate insulin resistance and the β-cell function.13 Serum uric acid (UA) levels were measured using the uricase-N-(3-sulfopropyl)-3-methoxy-5-methylaniline (HMMPS) method on an L-type UA/M (FUJIFILM Wako Pure Chemical, Osaka, Japan). We defined the percentage change from baseline to 3 months later in each parameter as %Δ [e.g. %ΔFBS (%) = 100 × (FBS at 3 months later − FBS at baseline)/FBS at baseline] and compared the %Δ values between the ARNI group and the control group.
Statistical analyses
Data are expressed as the mean ± standard error (SE) or as the median (25th or 75th percentile) for significantly skewed variables. For continuous variables, differences between two groups were evaluated either by an unpaired Student's t-test or the Mann–Whitney rank-sum test, and differences within groups were evaluated using a paired t-test or Wilcoxon signed-rank test. For discrete variables, which were expressed as counts and percentages, differences between the two groups were analysed by the chi-squared test, unless the expected value in any cell was less than 5, in which case Fisher's exact test was used. All statistical analyses were performed using the STATA software program (version 16.0, STATA Corp., College Station, TX, USA). P values of < 0.05 were considered to indicate statistical significance.
This study was designed for heart failure patients who were on ACE inhibitors or ARBs and were considering switching to ARNI as usual care. In this study, all patients were considered for switching from ACE inhibitors or ARBs to ARNI. However, the final decision to switch to ARNI was dependent on the physician's and patient's will (e.g. some patients refused for economic reasons). Therefore, some patients were actually switched from ACE inhibitors or ARBs to ARNI, whereas others continued on ACE inhibitors or ARBs. In some patients, the decision of whether or not to switch ACE inhibitors or ARBs to ARNI may have been made according to the baseline levels of each parameter. Therefore, we cannot deny the possibility that ARNI was inevitably administered for patients at high risk of an exacerbation of heart failure (e.g. the patients with high insulin resistance, an impaired cardiac function, etc.). To eliminate the bias in the background between the two patient groups, we devised this path model. Using an SEM analysis with an appropriate path model enables assessment of the effect of ARNI administration itself on the values of each parameter at 3 months, independently of the values of each parameter at baseline. An appropriate path model was created and analysed by SEM, which could represent the following three basic concepts: (i) the relationship between baseline values and the presence or absence of ARNI treatment; (ii) the relationship between baseline values and the values of 3 months after ARNI treatment; and (iii) the relationship between the presence or absence of ARNI treatment and the values 3 months after ARNI treatment can be calculated independently of (i) and (ii). A path analysis was performed using the IBM SPSS AMOS software program (version 27; Amos Development Corporation, Meadville, PA, USA). The SEM that were obtained were tested and confirmed at a significance level of P < 0.05.
Results
Comparison of clinical characteristics of the two groups at baseline and 3 months later
Tables 1, 2, and S1 –S3 show the baseline clinical characteristics of the patients in this study. The LVEF of the ARNI group was lower in comparison with that of the control group (50.3 ± 3.27 vs. 59.6 ± 2.02%, P = 0.021). Although the ARNI group included fewer patients with HFpEF in comparison with the control group (P = 0.004), patients with heart failure with mildly reduced ejection fraction (HFmrEF) and HFrEF were mostly biased toward the ARNI group (although the difference was not statistically significant). There were no significant differences between the two groups in the administration rates of antidiabetic agents or medications for heart failure. The fasting blood insulin level and HOMA-IR in the ARNI group were significantly higher in comparison with the control group [fasting blood insulin, 9.7 (7.4, 11.6) vs. 7.8 (5.2, 9.2) μU/mL, P = 0.033; HOMA-IR, 3.10 (1.95, 4.19) vs. 2.02 (1.56, 2.42), P = 0.014]. In fact, the baseline insulin resistance in the ARNI group was significantly higher in comparison with the control group. Furthermore, the baseline triglyceride (TG) and γ-glutamyltranspeptidase (γ-GTP) levels in the ARNI group were significantly higher in comparison to the control group [TG, 148 (114, 186) vs. 96 (80, 134) mg/dL, P = 0.010; γ-GTP, 50 (44, 122) vs. 39 (18, 51) U/L, P = 0.019]. At 3 months, no significant differences were found except for LVEF (Table S4).
| Characteristics | Number (%), mean ± SE or median (25th or 75th percentile) | P | |
|---|---|---|---|
| ARNI group (n = 17) | Control group (n = 17) | ||
| Male (n, %) | 14 (82.4) | 14 (82.4) | NS |
| Age (years) | 62.3 ± 2.90 | 66.4 ± 2.61 | NS |
| BMI (kg/m2) | 25.8 ± 1.05 | 23.8 ± 0.93 | NS |
| SBP (mmHg) | 132 ± 4.98 | 132 ± 2.86 | NS |
| DBP (mmHg) | 78.0 ± 2.74 | 81.0 ± 2.47 | NS |
| Heart rate (beats per minutes) | 79.6 ± 3.07 | 75.1 ± 3.91 | NS |
| LVEF (%) | 50.3 ± 3.27 | 59.6 ± 2.02 | 0.021 |
| FBS (mg/dL) | 110 (100, 141) | 106 (97, 122) | NS |
| Fasting blood insulin (μU/mL) | 9.7 (7.4, 11.6) | 7.8 (5.2, 9.2) | 0.033 |
| HOMA-IR | 3.10 (1.95, 4.19) | 2.02 (1.56, 2.42) | 0.014 |
| HOMA-β (%) | 65.4 (50.4, 87.2) | 58.0 (36.0, 93.3) | NS |
| HbA1c (%) | 6.4 (5.7, 7.0) | 6.1 (5.7, 6.5) | NS |
| TG (mg/dL) | 148 (114, 186) | 96 (80, 134) | 0.010 |
| HDL (mg/dL) | 61.9 ± 4.55 | 60.3 ± 3.18 | NS |
| LDL (mg/dL) | 101 ± 8.35 | 100 ± 7.90 | NS |
| AST (U/L) | 25 (25, 31) | 24 (21, 27) | NS |
| ALT (U/L) | 30 (20, 34) | 22 (16, 27) | NS |
| LDH (U/L) | 179 ± 6.88 | 176 ± 6.34 | NS |
| ALP (U/L) | 82.0 ± 7.29 | 70.8 ± 5.12 | NS |
| γ-GTP (U/L) | 50 (44, 122) | 39 (18, 51) | 0.019 |
| TB (mg/dL) | 0.69 ± 0.06 | 0.65 ± 0.05 | NS |
| BNP (pg/mL) | 21.7 (4.8, 91.4) | 23.8 (10.7, 55.6) | NS |
| eGFR (mL/min/1.73 m2) | 67.5 ± 5.34 | 63.8 ± 5.21 | NS |
| UA (mg/dL) | 6.28 ± 0.35 | 6.29 ± 0.26 | NS |
| Underlying main cardiovascular diseases | |||
| Ischaemic heart disease (n, %) | 9 (52.9) | 7 (41.2) | NS |
| Cardiomyopathy (n, %) | 6 (35.3) | 2 (11.8) | NS |
| Arrhythmia (n, %) | 1 (5.9) | 7 (41.2) | 0.020 |
| Valvular disease (n, %) | 0 (0) | 1 (5.9) | NS |
| Congenital heart disease (n, %) | 1 (5.9) | 0 (0) | NS |
| Co-morbidities | |||
| Diabetes mellitus (n, %) | 6 (35.3) | 4 (23.5) | NS |
| Hypertension (n, %) | 13 (76.5) | 14 (82.4) | NS |
| Dyslipidaemia (n, %) | 15 (88.2) | 15 (88.2) | NS |
| Renal dysfunction (n, %) | 5 (29.4) | 8 (47.1) | NS |
| Medications | |||
| Beta-blockers (n, %) | 15 (88.2) | 13 (76.5) | NS |
| MRAs (n, %) | 9 (52.9) | 5 (29.4) | NS |
| Diuretics (n, %) | 8 (47.1) | 9 (52.9) | NS |
| Lipid-lowering drugs (n, %) | 15 (88.2) | 13 (76.5) | NS |
| Antidiabetic agentsa (n, %) | 3 (17.6) | 4 (23.5) | NS |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARNI, angiotensin receptor-neprilysin inhibitor; AST, aspartate aminotransferase; BMI, body mass index; BNP, B-type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBS, fasting blood sugar; Hb, haemoglobin; HDL, high-density lipoprotein cholesterol; HOMA-IR, homoeostasis model assessment of insulin resistance; HOMA-β, homoeostasis model assessment of β-cell function; LDH, lactate dehydrogenase; LDL, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; SD, standard error; TB, total bilirubin; TG, triglyceride; UA, uric acid; γ-GTP, γ-glutamyltranspeptidase.
- a Antidiabetic agents include sodium-glucose cotransporter 2 inhibitors.
| HF subtypes | ARNI group (n = 17) | Control group (n = 17) | P |
|---|---|---|---|
| HFpEF (≥50%) (n) | 7 | 15 | 0.004 |
| HFmrEF (41–49%) (n) | 5 | 1 | NS |
| HFrEF (≤40%) (n) | 5 | 1 | NS |
- Abbreviations: ARNI, angiotensin receptor-neprilysin inhibitor; HF, heart failure; HFmrEF, HF with mildly reduced ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; LVEF, left ventricular ejection fraction.
Change in clinical factors from baseline to 3 months
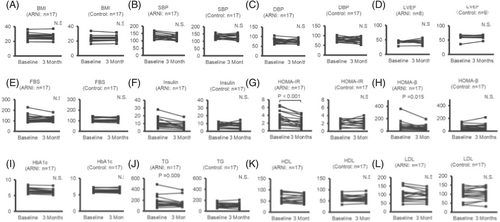
Figures 2 and 3 demonstrated that, at 3 months, the fasting blood insulin, HOMA-IR, HOMA-β, TG, alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-GTP, and UA levels had decreased in comparison to the baseline levels [baseline to 3 months: insulin, 9.7 (7.4, 11.6) to 7.3 (4.6, 9.4) μU/mL, P < 0.001; HOMA-IR, 3.10 (1.95, 4.19) to 1.96 (1.23, 3.09), P < 0.001; HOMA-β, 65.4 (50.4, 87.2) to 47.8 (35.2, 80.6)%, P = 0.015; TG, 148 (114, 186) to 117 (106, 145) mg/dL, P = 0.009; ALT, 30 (20, 34) to 24 (17, 33) U/L, P = 0.029; ALP, 82.0 ± 7.29 to 75.4 ± 6.48 U/L, P = 0.034; γ-GTP, 50 (44, 122) to 52 (32, 64) U/L, P = 0.021; and UA, 6.28 ± 0.35 to 5.80 ± 0.30 mg/dL, P = 0.008]. In the ARNI group, no significant change were observed between baseline and 3 months in body mass index (BMI), LVEF, or B-type natriuretic peptide; that is to say, during the observational period, there was no obvious hemodynamic changes in the ARNI group. In the control group, there were no significant changes between baseline and 3 months in any clinical factors.
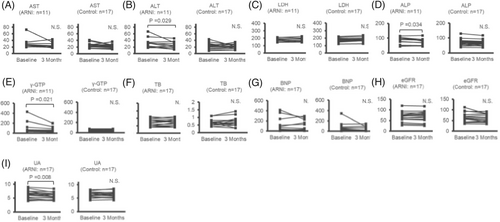
Changes in clinical factors from baseline to 3 months after the initiation of ARNI in patients with heart failure. (A) AST, (B) ALT, (C) LDH, (D) ALP, (E) γ-GTP, (F) TB, (G) BNP, (H) eGFR, (I) UA. ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARNI, angiotensin receptor-neprilysin inhibitor;
AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; TB, total bilirubin; UA, uric acid; γ-GTP, γ-glutamyltranspeptidase.
Table S5 shows the percentage change (%⊿) from baseline to 3 months, demonstrating significant decreased values for the fasting blood insulin, HOMA-IR, HOMA-β, γ-GTP, and UA levels (insulin, −28.0 ± 4.95 vs. −6.60 ± 7.63%, P < 0.001; HOMA-IR, −31.3 ± 5.63 vs. 6.32 ± 7.92%, P < 0.001; HOMA-β, −18.1 ± 6.96 vs. −9.08 ± 8.43%, P = 0.018; γ-GTP, −19.9 ± 6.28 vs. −0.15 ± 4.75%, P = 0.016; and UA, −6.66 ± 2.27 vs. 1.37 ± 1.91%, P = 0.011) and significantly increased values for B-type natriuretic peptide in the ARNI group in comparison to the control group [24.9 (−26.8, 79.6) vs. -8.21 (−40.2, 4.99) %, P = 0.046].
The results of the proposed path model indicating the effect of ARNI on insulin resistance
As Table 1 showed, the insulin resistance in the ARNI group was already higher than that at baseline. Then, to clarify that the effect of ARNI on insulin resistance, we created an appropriate path model and performed an additional analysis by SEM. The theoretical path model is illustrated in Figure 4, and the results of this statistical analysis are presented in Table 3. Path model (F) was drawn in a parallel manner with the baseline insulin level (‘Insulin Baseline’) and the presence or absence of ARNI (‘ARNI’). The paths were drawn from the independent to the dependent variables with directional arrows for each regression model (i.e. from ‘Insulin Baseline’ and ‘ARNI’ to ‘Insulin 3 Months’). The associations between the factors (‘Insulin Baseline’ and ‘ARNI’) are illustrated with two-way arrows. The results of this statistical analysis are presented in Table 3. A theoretical path model based on the covariance structure analysis using two dependent variables in the same equation model was proposed. Path model (F) demonstrated that, at baseline, patients with higher insulin levels also had higher insulin levels at 3 months [‘Insulin 3 Months’ was significantly and positively associated with ‘Insulin Baseline’ (standardized regression coefficient [St. β] = 0.833, P < 0.001)]. Even though insulin levels at baseline were higher in the ARNI group in comparison with the control group (‘Insulin Baseline’ showed a significant positive association with ‘ARNI’), the insulin levels at 3 months were lower in the ARNI group [‘Insulin 3 Months’ showed a significant negative association with ‘ARNI’ (St. β = −0.299, P = 0.013)]. This result indicates that the administration of ARNI decreased the insulin level at 3 months independently of the baseline insulin level. Similarly, Path model (G) demonstrated that patients with higher HOMA-IR levels at baseline also had higher HOMA-IR levels at 3 months [‘HOMA-IR 3 Months’ showed a significant positive association with ‘HOMA-IR Baseline’ (St. β = 0.881, P < 0.001)]. Even though the HOMA-IR levels at baseline were higher in the ARNI group in comparison with the control group (‘HOMA-IR Baseline’ showed a significant positive association with ‘ARNI’), the HOMA-IR levels at 3 months were lower in the ARNI group [‘HOMA-IR 3 Months’ showed a significant negative association with ‘ARNI’ (St. β = −0.336, P = 0.006)]. The results of Path model (G) indicate that the administration of ARNI decreased HOMA-IR at 3 months independently of the baseline HOMA-IR value. These results showed that ARNI itself is effective on improving insulin resistance in patients with heart failure.

| Path model | Clinical factors | Estimate | Standard error | Test statistic | P value | ||
|---|---|---|---|---|---|---|---|
| (A) | BMI 3 Months | ← | BMI Baseline | 0.919 | 0.027 | 34.371 | <0.001 |
| ← | ARNI | −0.024 | 0.219 | −0.109 | NS | ||
| BMI Baseline | ↔ | ARNI | 0.509 | 0.367 | 1.384 | NS | |
| (B) | SBP 3 Months | ← | SBP Baseline | 0.499 | 0.149 | 3.341 | <0.001 |
| ← | ARNI | −5.088 | 4.858 | −1.047 | NS | ||
| SBP Baseline | ↔ | ARNI | 0.132 | 1.414 | 0.094 | NS | |
| (C) | DBP 3 Months | ← | DBP Baseline | 0.592 | 0.163 | 3.639 | <0.001 |
| ← | ARNI | 1.423 | 3.429 | 0.415 | NS | ||
| DBP Baseline | ↔ | ARNI | −0.750 | 0.927 | −0.809 | NS | |
| (D) | LVEF 3 Months | ← | LVEF Baseline | 0.692 | 0.113 | 6.142 | <0.001 |
| ← | ARNI | −4.512 | 2.665 | −1.693 | NS | ||
| LVEF Baseline | ↔ | ARNI | −2.334 | 1.106 | −2.110 | 0.035 | |
| (E) | FBS 3 Months | ← | FBS Baseline | 0.638 | 0.054 | 11.830 | <0.001 |
| ← | ARNI | −3.591 | 3.009 | −1.193 | NS | ||
| FBS Baseline | ↔ | ARNI | 3.088 | 2.486 | 1.242 | NS | |
| (F) | Insulin 3 Months | ← | Insulin Baseline | 0.620 | 0.090 | 6.910 | <0.001 |
| ← | ARNI | −2.176 | 0.878 | −2.478 | 0.013 | ||
| Insulin Baseline | ↔ | ARNI | 0.944 | 0.457 | 2.068 | 0.039 | |
| (G) | HOMA-IR 3 Months | ← | HOMA-IR Baseline | 0.669 | 0.093 | 7.190 | <0.001 |
| ← | ARNI | −0.740 | 0.270 | −2.746 | 0.006 | ||
| HOMA-IR Baseline | ↔ | ARNI | 0.338 | 0.139 | 2.428 | 0.015 | |
| (H) | HOMA-β 3 Months | ← | HOMA-β Baseline | 0.566 | 0.079 | 7.149 | <0.001 |
| ← | ARNI | −19.331 | 9.578 | −2.018 | 0.044 | ||
| HOMA-β Baseline | ↔ | ARNI | 5.519 | 5.353 | 1.031 | NS | |
| (I) | HbA1c 3 Months | ← | HbA1c Baseline | 0.872 | 0.047 | 18.571 | <0.001 |
| ← | ARNI | −0.068 | 0.064 | −1.071 | NS | ||
| HbA1c Baseline | ↔ | ARNI | 0.062 | 0.060 | 1.027 | NS | |
| (J) | TG 3 Months | ← | TG Baseline | 0.792 | 0.063 | 12.573 | <0.001 |
| ← | ARNI | −16.750 | 10.269 | −1.631 | NS | ||
| TG Baseline | ↔ | ARNI | 16.897 | 7.682 | 2.200 | 0.028 | |
| (K) | HDL 3 Months | ← | HDL Baseline | 0.893 | 0.078 | 11.409 | <0.001 |
| ← | ARNI | −2.125 | 2.461 | −0.863 | NS | ||
| HDL Baseline | ↔ | ARNI | 0.397 | 1.369 | 0.290 | NS | |
| (L) | LDL 3 Months | ← | LDL Baseline | 0.798 | 0.071 | 11.278 | <0.001 |
| ← | ARNI | −6.140 | 4.603 | −1.334 | NS | ||
| LDL Baseline | ↔ | ARNI | 0.191 | 2.831 | 0.068 | NS | |
| (M) | AST 3 Months | ← | AST Baseline | 0.389 | 0.099 | 3.948 | <0.001 |
| ← | ARNI | −0.245 | 2.067 | −0.119 | NS | ||
| AST Baseline | ↔ | ARNI | 1.466 | 1.026 | 1.429 | NS | |
| (N) | ALT 3 Months | ← | ALT Baseline | 0.599 | 0.104 | 5.752 | <0.001 |
| ← | ARNI | −0.188 | 2.856 | −0.066 | NS | ||
| ALT Baseline | ↔ | ARNI | 2.707 | 1.360 | 1.991 | 0.047 | |
| (O) | LDH 3 Months | ← | LDH Baseline | 0.907 | 0.085 | 10.713 | <0.001 |
| ← | ARNI | −6.860 | 3.978 | −1.725 | NS | ||
| LDH Baseline | ↔ | ARNI | 0.592 | 2.184 | 0.271 | NS | |
| (P) | ALP 3 Months | ← | ALP Baseline | 0.831 | 0.058 | 14.438 | <0.001 |
| ← | ARNI | −1.521 | 2.597 | −0.585 | NS | ||
| ALP Baseline | ↔ | ARNI | 2.487 | 2.133 | 1.166 | NS | |
| (Q) | Γ-GTP 3 Months | ← | Γ-GTP Baseline | 0.433 | 0.027 | 16.081 | <0.001 |
| ← | ARNI | 2.943 | 4.337 | 0.679 | NS | ||
| Γ-GTP Baseline | ↔ | ARNI | 14.772 | 7.844 | 1.883 | NS | |
| (R) | TB 3 Months | ← | TB Baseline | 0.909 | 0.121 | 7.515 | <0.001 |
| ← | ARNI | −0.019 | 0.051 | −0.378 | NS | ||
| TB Baseline | ↔ | ARNI | 0.012 | 0.018 | 0.637 | NS | |
| (S) | BNP 3 Months | ← | BNP Baseline | 0.593 | 0.066 | 8.947 | <0.001 |
| ← | ARNI | 21.872 | 14.170 | 1.544 | NS | ||
| BNP Baseline | ↔ | ARNI | 8.109 | 9.414 | 0.861 | NS | |
| (T) | eGFR 3 Months | ← | eGFR Baseline | 0.915 | 0.059 | 15.635 | <0.001 |
| ← | ARNI | 4.962 | 2.481 | 2.000 | 0.045 | ||
| eGFR Baseline | ↔ | ARNI | 0.926 | 1.852 | 0.500 | NS | |
| (U) | UA 3 Months | ← | UA Baseline | 0.831 | 0.070 | 11.835 | <0.001 |
| ← | ARNI | −0.530 | 0.179 | −2.969 | 0.003 | ||
| UA Baseline | ↔ | ARNI | 0.027 | 0.111 | 0.242 | NS | |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARNI, angiotensin receptor-neprilysin inhibitor; AST, aspartate aminotransferase; BMI, body mass index; BNP, B-type natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FBS, fasting blood sugar; Hb, haemoglobin; HDL, high-density lipoprotein cholesterol; HOMA-IR, homoeostasis model assessment of insulin resistance; HOMA-β, homoeostasis model assessment of β-cell function; LDH, lactate dehydrogenase; LDL, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TB, total bilirubin; TG, triglyceride; UA, uric acid; γ-GTP, γ-glutamyltranspeptidase.
Although the TG, ALT, ALP, and γ-GTP levels at 3 months decreased in comparison with the baseline levels (Figures 2 and 3), the SEM analyses did not show that the observed reduction in the TG, ALT, ALP, and γ-GTP levels at the 3 months were due to ARNI treatment (Figure 4).
The results of the proposed path model indicating the effect of ARNI on UA
In addition, Path model (U) revealed that ‘UA 3 Months’ was also positively associated with ‘UA Baseline’ (St. β = 0.887, P < 0.001). Although there was no significant relationship between “UA Baseline” and “ARNI”, “UA 3 Months” was negatively associated with ‘ARNI’ (St. β = −0.223, P = 0.003). These results indicated that ARNI was effective for decreasing UA in patients with heart failure.
Discussion
In this study, the initiation of ARNI improved insulin resistance indices, such as HOMA-IR, in patients with stable chronic heart failure, even in the short period of only 3 months. Although the ARNI group was already highly insulin resistant at baseline, an additional SEM analysis with an appropriate path model revealed that the improvement in insulin resistance was truly due to the effect of ARNI. Additionally, the present study indicated that the initiation of ARNI also decreased serum UA levels in patients with heart failure.
Renin–angiotensin system inhibition with ACE inhibitors or ARBs significantly reduced the incidence of the new-onset diabetes.14 A previous study reported that increased levels of tumour necrosis factor-α (TNF-α), a pre-inflammatory cytokine, in skeletal muscle contribute to insulin resistance and that ACE inhibitors and ARBs improve insulin resistance by suppressing TNF-α.15 Another previous report indicated that adiponectin, an adipocyte-derived protein, is closely related to insulin sensitivity, and that renin–angiotensin system inhibition increases the level of adiponectin in the blood and improves insulin resistance.16 Moreover, we previously reported that natriuretic peptides, which are increased by neprilysin inhibition, could halt the progression to diabetes by improving insulin resistance under the metabolically harmful condition of heart failure.17 In addition, we reported that in mice fed a high-fat diet, the administration of exogenous A-type natriuretic peptide ameliorated systemic insulin resistance by attenuating hepatic steatosis and inducing adipose tissue browning and furthermore ameliorated myocardial insulin resistance and protected against ischaemia–reperfusion injury.18, 19 In the present study, the levels of liver enzymes, such as ALT, ALP, and γ-GTP, tended to decrease after ARNI treatment. However, the SEM analyses failed to show a significant relationship between the initiation of ARNI treatment and improvement in the liver function due to missing data. Therefore, ARNI, a dual inhibitor of the angiotensin-receptor and neprilysin inhibitor, might have a substantial insulin resistance ameliorating effect in patients with heart failure.
This study showed that the initiation of ARNI reduced serum UA levels in patients with heart failure. In the PARADIGM-HF trial, ARNI reduced serum UA levels in comparison with ACE inhibitor.20 Similarly, in the PARAGON-HF trial, ARNI reduced serum UA levels and the initiation of serum UA-related therapy in comparison with ARB.21 A previous study suggested that, in a diabetic state, insulin significantly increased urate transporter 1 (URAT1) and decreased ATP-binding cassette subfamily G member 2 (ABCG2) levels and consequently increased UA reabsorption in the kidney.22 Another study reported that insulin resistance and hyperinsulinaemia increase the reabsorption of sodium and monocarboxylic acids, such as lactate and ketone, via sodium and monocarboxylic acid cotransporter with UA in the exchange of monocarboxylic acid excretion via URAT1, resulting in an increase in serum UA levels.23 In brief, the ameliorating effect of ARNI on insulin resistance may reduce UA levels by inhibiting URAT1 in the renal proximal tubules and consequently reducing UA reabsorption. In addition to the lower reabsorption of UA in the kidney via URAT1 induced by ARNI, the reduction in the serum UA level itself reduces the entry of UA into cells via URAT1 in the liver24 and adipose tissues,25 which may result in the improvement of systemic insulin resistance through the inhibition of reactive oxygen species and inflammation in these cells.26
The present study included two novel features. First, the previous PARADIGM-HF and PARAGON-HF studies both showed that ARNI reduces HbA1c and even reduces new insulin use in heart failure patients with diabetes mellitus. However, both studies were based on the results of long-term follow-up (>1 year). Furthermore, the mechanism through which ARNI improves glucose metabolism has not been clarified. In this study, it was demonstrated that the mechanism of ARNI's effect on glucose metabolism was significantly related to the improvement of insulin resistance and that this effect is already demonstrated in a short follow-up period, such as within 3 months after the initiation of ARNI. Second, this was a retrospective study, and at baseline, there was already a bias towards patients with higher insulin resistance in the ARNI group in comparison with the control group. These differences disappeared after 3 months of ARNI treatment. It was unclear whether the administration of ARNI itself improved insulin resistance or whether patients with high insulin resistance were more likely to improve spontaneously. In this study, using an SEM analysis with an appropriate path model, we were able to clarify that these improvements in insulin resistance were due to the administration of ARNI itself.
The present study was associated with several limitations. First, this was a retrospective, single-centre study with a very small population, and patients in this study had a better LVEF than those who generally take ARNIs. However, due to this patient selection, we believe that we were able to demonstrate the effect of ARNI itself on glucose metabolism, independent of the cardiac function. Second, the final decision to switch from ACE inhibitors or ARBs to ARNI was dependent on the physician's and patient's will (e.g. some patients refused for economic reasons), which could have per se influenced the metabolic status. Actually, at baseline, patients with high insulin resistance were more likely to be classified into the ARNI group than the control group. Hence, to solve the problem of bias and to accurately assess the effect of ARNI on each clinical factor, we created an appropriate path model and performed an SEM analysis. Third, at baseline, the type and internal dose of ACE inhibitors or ARBs varied from case to case, as shown in 3, and the variation in these medications may also influence some clinical factors. Finally, in the present study, we use HOMA indices to evaluate insulin resistance and the β-cell function, instead of using the gold standard of either the hyperglycaemic–euglycaemic glucose clamp method or the oral glucose tolerance test. In addition, C-peptide should have been measured to assess the insulin secretory reserve; however, but there were no data on the C-peptide levels in the patients of this study.
In conclusion, even in a short period of only 3 months, the administration of ARNI improved insulin resistance and consequently reduced the serum UA level in patients with stable chronic heart failure. Although the ARNI group of the present study already showed high insulin resistance at baseline, an additional SEM analysis with an appropriate path model proved that the improvement in insulin resistance was due to the effect of ARNI treatment.
Acknowledgements
We would like to thank Brian Quinn, Japan Medical Communication, for reviewing the manuscript.
Conflict of interest
There are no conflicts of interest to disclose directly related to this study. Outside this study, Michihiro Yoshimura received research funds from Teijin Pharma Ltd., Shionogi & Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Mochida Pharmaceutical Co. Ltd., and speaker's honorarium from Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd, Astellas Pharma Inc., Bayer Yakuhin Ltd., Novartis Pharma K.K., and Mochida Pharmaceutical Co., Ltd.
Funding
None.




