Prognosis evaluation of chronic inflammatory cardiomyopathy with ring-like late gadolinium enhancement
Abstract
Aims
Ring-like late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging is a special LGE pattern in patients with chronic inflammatory cardiomyopathy (infl-CMP), which is associated with cardiac dysfunction and ventricular remodelling and attributed to viral infection followed by bacterial and parasitic infections. Data on the combination of CMR imaging and clinical parameters that can add long-term prognostic value in patients with infl-CMP are still rare. We aimed to evaluate the prognostic value of CMR in risk stratification in this kind of patients.
Methods and results
A total of 319 consecutive patients with clinically suspected myocarditis were retrospectively identified. Forty-seven patients with ring-like LGE on CMR who diagnosed as infl-CMP, and 72 patients with other LGE pattern were eligible for standardized follow-up. The left ventricle (LV) and right ventricle function and mass were analyses by CMR. Myocardial strain of the ventricles was evaluated by feature tracking. Major (cardiac death, resuscitated cardiac arrest, ventricular assist device, transplantation, and appropriate implantable cardioverter-defibrillator shock) and minor (rehospitalization due to heart failure and sustained atrial fibrillation) adverse cardiovascular events were assessed during follow-up since the date of nCMR examination. Cox proportional hazards model was used to investigate which of the prognostic factors identified by univariable analysis were significantly associated with cardiac events. In the ring-like LGE group, adverse cardiac events occurred in 14 (31.11%) patients, including 7 deaths (15.56%), 6 (13.33%) heart-failure hospitalizations, and 1 (2.22%) case of sustained atrial fibrillation during the mean follow-up period of 70.15 ± 45.68 months (interquartile range: 32.83–103.71 months). No major or minor adverse cardiac event occurred in the other LGE pattern group, except rehospitalization in one patient due to arrhythmia. Further analyses of ring-like LGE group by univariable and Multivariable Cox proportional hazard regression analysis showed that body mass index (BMI) (hazard ratio [HR]: 0.54, 95% confidence interval [CI]: 0.40–0.73, P = 0.000), right ventricle cardiac index (RVCI) (HR: 0.31, 95% CI: 0.14–0.71, P = 0.002), LV basal peak circumferential strain (LV-PCSbasal) (HR: 1.26, 95% CI: 1.11–1.43, P = 0.000) were independently associated with the long-term outcome. Receiver operator characteristic curve indicated that the cut-off of LV-PCSBasal was −7.95%, and it has added prognostic value to BMI and RVCI.
Conclusions
For infl-CMP patients with ring-like LGE on CMR, low BMI and RVCI were associated with a poor prognosis. LV-PCSBasal with a cut-off of −7.95% can add prognostic value for patients with infl-CMP who have ring-like LGE.
Introduction
Chronic inflammatory cardiomyopathy (infl-CMP), which is associated with cardiac dysfunction and ventricular remodelling, refers to a broad group of disorders characterized by myocardial inflammation.1-3 Infl-CMP typically indicates myocardial inflammation-induced dilated cardiomyopathy (DCM) with prolonged symptoms (typically >1 month) with or without persistent inflammation and mostly attributed to viral infection followed by bacterial and parasitic infections.4 With the advancements in cardiac magnetic resonance (CMR), it has become feasible to not only diagnose myocarditis but also obtain information on prognosis in patients with myocardial inflammation, especially in the acute inflammation stage.5
Two patterns (epicardium in the lateral wall and mid wall of the interventricular septum) of myocardial damage that show late gadolinium enhancement (LGE) have been reported to be associated with the clinical course of myocarditis.6 However, another LGE pattern—more than three contiguous segments with subepicardial and/or mid-wall LGE in the same slice (ring-like LGE)7—was not fully discussed in the previous studies. In patients with non-ischaemic DCM, ring-like LGE may associate with an increased risk of ventricular tachyarrhythmias.7 Apart from LGE, CMR can also provide strain parameters, which have prognostic value in patients with heart failure (HF).8 Yet, to the best of our knowledge, no study has explored the prognostic value of CMR in risk stratification in patients with infl-CMP who present with ring-like LGE. Hence, the present study aimed to explore the combination of different CMR and clinical parameters that can add long-term prognostic value in this kind of patients.
Methods
Study population
Study population and study design
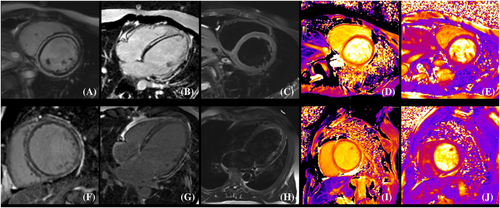
From April 2016 to December 2021, a total of 319 consecutive patients with clinically suspected myocarditis who were undergone CMR were retrospectively identified. Clinically suspected myocarditis refers to symptomatic patients of (i) ≥1 clinical presentation (acute chest pain or new-onset dyspnoea [days up to 3 months] or in subacute/chronic dyspnoea [>3 months] or palpitations/unexplained arrhythmia symptoms or unexplained cardiogenic shock) and (ii) ≥1 diagnostic criteria from different categories (electrocardiographic features of cardiac injury, elevated markers of myocardial necrosis, functional/structural abnormalities on echocardiogram/angiogram or cardiac magnetic resonance imaging [CMRI], tissue characterization by CMRI). Infl-CMP was diagnosed based on the following features: (i) previous myocarditis >1 month ago and (ii) in conjunction with consistent ejection fraction-reduced HF measured by transthoracic echocardiography with or without consistent inflammation (myocardium oedema on CMRI and/or elevated troponin T or I).2, 3, 9 Ejection fraction-reduced HF was diagnosed according to the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF.10 None of the patients underwent immunosuppressive therapy. Patients were excluded if they had coronary artery disease (>50% stenosis of coronary artery confirmed by coronary artery computed tomography angiography or coronary artery angiography), congenital heart disease, infiltrative cardiomyopathy (i.e. amyloidosis), significant valvular heart disease, previous myocardial infarction, or a history of cardiac resynchronization therapy and implantable cardioverter defibrillator implantation. Cardiac sarcoidosis was excluded based on clinical guidelines combining proof of extracardiac disease with evidence of cardiac involvement.11 Ring-like LGE was defined as more than three contiguous segments with subepicardial and/or mid-wall LGE in the same slice on short-axis CMR LGE images (Figure 1).
At the time of CMR evaluation, all enrolled patients were treated only with oral medications to resolve myocarditis and HF. This study protocol was approved by the local ethics committee. All included patients provided written informed consent. All the experiments were performed in accordance with the relevant guidelines and regulations.
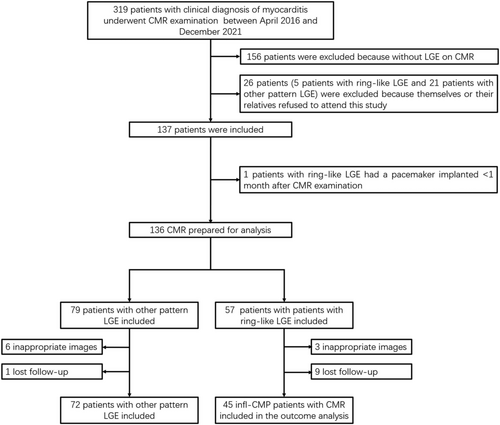
In the current study, we also excluded patients who refused or were lost to follow-up and those with low image quality that cannot be analysed (Figure 2).
Cardiac magnetic resonance imaging protocol
Cardiac magnetic resonance data acquisition
All CMR studies were performed on a 3.0-T scanner (Ingenia CX, Philips Healthcare or Magnetom Verio; Siemens AG Healthcare, Erlangen, Germany, or MR750W, General Electric Healthcare, Waukesha, Wisconsin, USA) with retrospective electrocardiogram (ECG) gating and a 32-channel phased-array coil. Standardized imaging protocols were followed, which comprised balanced steady-state free-precession (bSSFP) breath-hold cine images, black-blood fat-suppressed T2 weighted images (BB-T2WI), and LGE images. The bSSFP was with repetition time/echo time (TR/TE) = 3.0/1.52 ms, flip angle (FA) = 45°, voxel size = 1.8 × 1.8 × 8 mm3, field-of-view (FOV) = 270 × 270 mm2, and acceleration factor = 1.5 or 3.6 depending on the number of slices acquired per breath hold. For short-axis cine imaging, a minimum of nine slices were acquired to cover the entire left ventricle (LV). Long-axis planes (2-, 4-, and 3-chamber views) were of 5-mm slice thickness with no spacing intersection gap. BB-T2WI was performed in short-axis and/or four-chamber views using multi-shot turbo spin echo sequence with TR = 2 heart beat periods, TE = 75 ms, voxel size = 1.7 × 1.7 × 8 mm3, FOV = 380 × 380 mm2, and FA = 90°. LGE images were acquired 10 min after the intravenous infusion of the gadolinium chelate contrast agent (Magnevist, Bayer Schering, Germany, 0.2 mmol/kg) using a prospectively ECG-gated gradient echo sequence with an inversion prepulse. The inversion times were optimized to null normal myocardium. The imaging parameters were as follows: TR/TE = 4.1/1.6 ms; FA = 20°; image matrix = 256 × 130.
Cardiac magnetic resonance analysis
The LV and right ventricle (RV) function and mass and myocardial strain analyses were performed on a commercially available workstation CVI42 software (Version 5.13.7 Circle Cardiovascular Imaging, Calgary, Canada). Short-axis cine images and 4-, 2-, or 3-chamber images were used for semi-automated analysis. Endocardial and epicardial borders as well as the RV insertion sites (marker of the outer borders of the antero-septum and infra-septum) were drawn automatically and amended by a radiologist on the short-axis cine images. Papillary muscles were considered as part of the LV volume. The longitudinal ranges of LV and RV were marked on 4-chamber images. The volume and mass of the LV were normalized to the body surface area (BSA). Myocardial oedema was defined as regional or global signal hyperintensity on T2WI.5 Myocardial oedema ratio was defined as a ratio of >1.9 between the myocardial and skeletal muscle signal intensities. The high signal area of inadequately suppressed slow-flowing cavitary blood was carefully excluded.7 Epicardial and endocardial contours were placed manually on the short-axis LGE images. LGE was defined as any area with a signal intensity 5 standard deviations above the normal myocardium and was detected automatically and subsequently amended by a radiologist (W. H.) with >10 years of experience in CMR. The percentage of myocardial LGE was quantified by obtaining the area of LGE in each slice automatically, which was calculated as (total area of myocardial LGE/total area of myocardium)*100%.
The myocardial strain of LV and RV was obtained using CVI42. Circumferential strain (CS) and radial strain were calculated based on short-axis cine images, and longitudinal strain was obtained from 4- or 2-chamber cine images. The peak strain of the basal, middle, and apical segments as well as the global strain were calculated and recorded. End-diastolic phase was automatically selected as the reference phase and was redefined manually. The anterior and inferior insertion points of the endo- and epicardial short-axis contours were same as those in function analysis slices.
Follow-up and study endpoints
After the CMR examination, all patients were followed up by the investigators. A clinical questionnaire was compiled by contacting the patients or their relatives via telephone or face-to-face communication. The clinical questionnaire included the definitions of several cardiac major and minor events. The major events were cardiac death, resuscitated cardiac arrest, ventricular assist device, transplantation, and appropriate implantable cardioverter-defibrillator shock. The minor event was rehospitalization due to HF and sustained atrial fibrillation, as sustained atrial fibrillation was independently associated with an adverse outcome.12 The patients were followed up until the end of September 2021.
Statistical analysis
The continuous variables were presented as mean ± standard deviation (normal distribution) or medians and interquartile ranges (skewed distribution). The categorical variables were presented as numbers (%). The continuous variables were compared using an unpaired Student's t-test or a Mann–Whitney nonparametric test between two groups. The categorical variables were compared using the Pearson χ2 test and the Fisher exact test between groups. P < 0.05 was considered statistically significant. A multivariable Cox proportional hazards model was used to investigate which of the prognostic factors identified by univariable analysis were significantly associated with cardiac events. The interactions among the subgroups were examined using multivariate adjusted Cox regression models. Furthermore, time-dependent receiver operating characteristic (ROC) curves were used to compare the identified prognostic factors and determine the optimal cut-off of the best prognostic factors in this study. The best cut-off values of the prognostic factors for the analysed events were defined based on the highest sum of sensitivity and specificity. The event-free curves were based on Kaplan–Meier analysis stratified by best cut-offs of the prognostic factors and were compared using log-rank test.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Results
Of the 319 patients with clinical diagnosis of myocarditis who were undergone CMR, 156 patients were excluded with no LGE on CMR images. Twenty-six patients (5 patients with ring-like LGE and 21 patients with other pattern LGE) were excluded because themselves or their relatives refused to attend this study. Nineteen patients were excluded because of inappropriate images or lost follow-up, and one patient with ring-like LGE were excluded because he had a pacemaker implanted <1 month after CMR examination. Finally, 45 patients with ring-like LGE on CMR who diagnosed as infl-CMP were included in this observational cohort study. Seventy-two patients with other LGE pattern were included to compare prognosis with patients with ring-like LGE (Figure 2). In the ring-like LGE group, the average age of the patients was 40 years (39.93 ± 16.6) at the time of enrolment. During the mean follow-up period of 70.15 ± 45.68 months (interquartile range: 32.83–103.71 months), adverse cardiac events occurred in 14 (31.11%) patients, including 7 deaths (15.56%), 6 (13.33%) heart-failure hospitalizations, and 1 (2.22%) case of sustained atrial fibrillation. No major or minor adverse cardiac event occurred in the other LGE pattern group, except one patient's rehospitalization due to arrhythmia and only taken some medicine for it.
In ring-like LGE group, myocardial oedema was not detected. The entire ring-like LGE cohort was divided into two groups based on the presence or absence of cardiac events: event-free and event group. The baseline demographic and admission biochemical characteristics of the two groups are presented in Table 1. No differences were found in the patients' sex, age, LV ejection fraction, and RV ejection fraction on CMRI between the two groups. However, when compared with the event-free group, the event group had lower body mass index (BMI, 20.39 ± 3.87 vs. 24.06 ± 3.33, event group vs. event-free group, respectively; P < 0.05), lower RV cardiac output (2.46 ± 1.28 vs. 3.94 ± 1.53, event group vs. event-free group, respectively; P < 0.05), and lower right ventricle cardiac index (RVCI, 1.62 ± 0.84 vs. 2.26 ± 0.84, event group vs. event-free group, respectively; P < 0.05). And after adjust end-diastolic ventricular volumes for BSA, the patients in the event group with low BMI had lager end-diastolic ventricular volumes index (CMRI-LVEDV/BSA, 146.30 ± 46.00 vs. 117.73 ± 45.30, event group vs. event-free group, respectively; P < 0.05).
| Mean + SD | All patients (n = 45) | Event-free patients (n = 31) | Event patients (n = 14) | P value |
|---|---|---|---|---|
| Age, mean (SD) (years) | 39.93 ± 16.6 | 40.19 ± 13.45 | 39.36 ± 22.68 | 0.878 |
| Male (%) | 26 (57.78%) | 16 (51.62%) | 10 (71.42%) | 0.218 |
| BMI, mean (SD) (kg/m2) | 22.91 ± 3.87 | 24.06 ± 3.33 | 20.39 ± 3.87 | 0.019 |
| CMRI-LVEDV (mL) | 210.74 ± 74.37 | 204.12 ± 79.9 | 225.4 ± 60.41 | 0.380 |
| CMRI-LVEDV/BSA | 125.61 ± 45.03 | 117.73 ± 45.30 | 146.30 ± 46.00 | 0.048 |
| CMRI-LVESV (m;) | 154.67 ± 71.17 | 146.81 ± 77.47 | 172.03 ± 53.15 | 0.276 |
| CMRI-LVEF, (%) | 29.05 ± 12.02 | 31.06 ± 12.45 | 24.58 ± 9.88 | 0.094 |
| CMRI-LVMASS (g) | 100.88 ± 36.11 | 101.28 ± 41.35 | 100.02 ± 21.56 | 0.915 |
| CMRI-LVCO | 4.09 ± 1.31 | 4.20 ± 1.16 | 3.86 ± 1.61 | 0.438 |
| CMRI-LVCI | 2.43 ± 0.79 | 2.41 ± 0.65 | 2.49 ± 1.05 | 0.733 |
| CMRI-RVEDV (mL) | 128.79 ± 43.2 | 135.88 ± 44.45 | 113.07 ± 37.01 | 0.102 |
| CMRI-RVESV (mL) | 81.29 ± 38.47 | 82.39 ± 38.8 | 78.86 ± 39.06 | 0.779 |
| CMRI-RVEF (%) | 38.35 ± 15.68 | 40.79 ± 12.01 | 32.95 ± 21.31 | 0.122 |
| CMRI-RVCO | 3.48 ± 1.61 | 3.94 ± 1.53 | 2.46 ± 1.28 | 0.003 |
| CMRI-RVCI | 2.06 ± 0.88 | 2.26 ± 0.84 | 1.62 ± 0.84 | 0.023 |
| LV-SAX-PRSBasal | 16.98 ± 7.2 | 17.81 ± 7.04 | 15.14 ± 7.47 | 0.254 |
| LV-SAX-PRSMid | 11.19 ± 6.89 | 12.15 ± 7.44 | 9.04 ± 5.08 | 0.164 |
| LV-PRSapical | 18.39 ± 16.98 | 21.63 ± 17.93 | 11.21 ± 12.42 | 0.031 |
| LV-PCSbasal | −10.88 ± 5.41 | −11.99 ± 3.75 | −8.40 ± 7.55 | 0.038 |
| LV-SAX-PCSMid | −8.32 ± 4.06 | −8.91 ± 4.24 | −7.02 ± 3.38 | 0.150 |
| LV-PCSapical | −10.84 ± 6.8 | −12.35 ± 6.77 | −7.5 ± 5.78 | 0.025 |
| LV-SAX-PGRS | 13.59 ± 7.01 | 14.65 ± 7.02 | 11.25 ± 6.63 | 0.134 |
| LV-SAX-PGCS | −9.61 ± 3.95 | −10.26 ± 3.89 | −8.17 ± 3.84 | 0.100 |
| LV-LAX-PLSBasal | −14.27 ± 3.63 | −14.67 ± 3.89 | −13.39 ± 2.95 | 0.281 |
| LV-LAX-PLSMid | −7.11 ± 4.82 | −7.94 ± 4.3 | −5.28 ± 5.56 | 0.086 |
| LV-PLSapical | −8.92 ± 4.75 | −10.14 ± 4.54 | −6.21 ± 4.17 | 0.009 |
| LV-PGLS | −9.84 ± 3.65 | −10.62 ± 3.63 | −8.08 ± 3.24 | 0.03 |
| RV-SAX-PRSBasal | 17.38 ± 11.93 | 18.76 ± 12.25 | 14.35 ± 11 | 0.256 |
| RV-SAX-PRSMid | 23.89 ± 13.39 | 22.76 ± 10.7 | 26.4 ± 18.25 | 0.404 |
| RV-SAX-PRSApical | 33 ± 28.72 | 30.36 ± 17.56 | 38.83 ± 45.02 | 0.507 |
| RV-SAX-PCSBasal | −8.79 ± 6.95 | −9.36 ± 6.74 | −7.52 ± 7.51 | 0.415 |
| RV-SAX-PCSMid | −12.73 ± 6.76 | −12.72 ± 5.85 | −12.77 ± 8.7 | 0.98 |
| RV-SAX-PCSApical | −12.52 ± 14.27 | −13.11 ± 13.99 | −11.22 ± 15.34 | 0.686 |
| RV-SAX-PGRS | 20.18 ± 9.45 | 21 ± 8.46 | 18.37 ± 10.91 | 0.384 |
| RV-SAX-PGCS | −11.56 ± 5.18 | −12.15 ± 4.37 | −10.27 ± 6.65 | 0.264 |
| RV-LAX-PLSBasal | −17.27 ± 18.39 | −17.37 ± 19.57 | −17.03 ± 16.14 | 0.951 |
| RV-LAX-PLSMid | −7.67 ± 16.91 | −7.99 ± 17.24 | −6.96 ± 16.76 | 0.852 |
| RV-LAX-PLSApical | −14.69 ± 11.04 | −14.73 ± 10.97 | −14.60 ± 11.62 | 0.971 |
| RV-LAX-PGLS | −13.82 ± 12.03 | 14.22 ± 12.41 | −12.91 ± 11.53 | 0.738 |
| LGE% | 21.04 ± 9.11 | 20.61 ± 9.62 | 21.99 ± 8.12 | 0.643 |
- BMI, Body mass index; CMRI, cardiac magnetic resonance imaging; LV, left ventricle; LVEDV, Left ventricular end-diastolic volume; LVESV, Left ventricular end-systolic volume; LVEF, Left ventricular ejection fraction; LVMASS, Left ventricular mass; LVCO, left ventricle cardiac output; LVCI, left ventricle cardiac index; RV, right ventricle; RVEDV, right ventricular end-diastolic volume; RVESV, right ventricular end-systolic volume; RVEF, right ventricular ejection fraction; RVCO, right ventricle cardiac output; RVCI, right ventricle cardiac; SAX, short axis view; PRS, peak radial strain; PCS, peak circumferential strain; PGRS, peak global radial strain; PGCS, peak global circumferential strain; LAX, long axis view; PLS, peak longitudinal strain; PGLS, peak global longitudinal strain; LGE, Left ventricular late gadolinium enhancement.
The CMRI LV apical peak radial strain (LV-PRSapical) was significantly lower in the event group (11.21 ± 12.42 vs. 21.63 ± 17.93, event-free group vs. event group, respectively; P < 0.05), whereas LV basal segment peak circumferential strain (LV-PCSbasal) (−8.40 ± 7.55 vs. −11.99 ± 3.75), LV apical segment peak circumferential strain (LV-PCSapcial) (−7.5 ± 5.78 vs. −12.35 ± 6.77), LV apical segment peak longitudinal strain (LV-PLSapcial) (−6.21 ± 4.17 vs. −10.14 ± 4.54), and LV peak global longitudinal strain (LV-PGLS) (−8.08 ± 3.24 vs. −10.62 ± 3.63) were higher in the event group when compared with the event-free group; all P < 0.05.
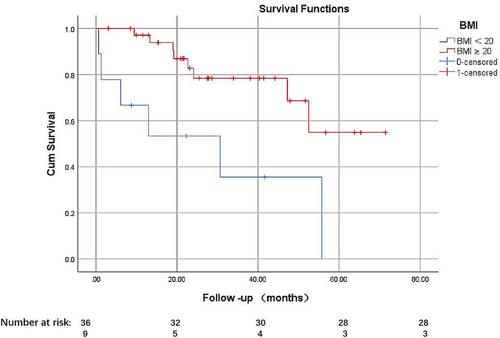
Fourteen patients developed 5 year cardiac events, including 6 deaths and 8 HF rehospitalization. Univariate analyses show that BMI, RVCI, LV-PRSapical, LV-PCSBasal, LV-PCSapical, LV-PLSapical, and LV-PGLS were associated with a significant increase in the incidence of 5 year adverse cardiac events. We performed a forward stepwise regression. BMI, RVCI, and LV-PCSBasal were identified features associated with 5 year adverse cardiac events and then a multivariate Cox regression analysis to further explore the roles of BMI, RVCI, and LV-PCSBasal as prognostic markers. In the multivariable analysis shown in Table 2, BMI (hazard ratio [HR]: 0.54, 95% CI: 0.40–0.73, P < 0.01), RVCI (HR: 0.31, 95% CI: 0.14–0.71, P = 0.002), and LV-PCSBasal HR: 1.26, 95% CI: 1.11–1.43, P < 0.01) were independently associated with the long-term outcome. We further assigned the patients into two categories according to their BMI13: the relatively low-BMI group when the BMI was the lowest and <20 kg/m2, which is close to the mean BMI value of the event group, and relatively high-BMI group when the BMI was ≥20 kg/m2. The low-BMI group had higher cardiovascular events, with an HR of 0.235 (95% confidence interval (CI) 0.081–0.683, P = 0.01) (Figure 3).
| 45 patients | Univariable, HR (95% CI) | Multivariable, HR (95% CI) |
|---|---|---|
| BMI (kg/m2) |
0.70 (0.57–0.86) P < 0.001 |
0.54 (0.40–0.73) P < 0.001 |
| CMRI-RVCO |
0.53(0.35–0.79) P < 0.001 |
|
| CMRI-RVCI |
0.44(0.23–0.85) P < 0.05 |
0.31(0.14–0.71) P < 0.01 |
| LV-PCSbasal |
1.10 (1.02–1.17) P < 0.05 |
1.26(1.11–1.43) P < 0.001 |
| LV-PCSapical |
1.14(1.02–1.27) P < 0.05 |
|
| LV-PLSapical |
1.20(1.05–1.37) P < 0.05 |
|
| LV-PGLS |
1.17 (1.01–1.35) P < 0.05 |
- Note: In bold, significant variables at multivariable analysis for adverse events.
- Abbreviations: BMI, body mass index; CI, confidence interval; CMRI, cardiac magnetic resonance imaging; HR, hazard ratio; LV, left ventricle; PCS, peak circumferential strain; PGLS, peak global longitudinal strain; PLS, peak longitudinal strain; RV, right ventricle; RVCO, right ventricle cardiac output; RVCI, right ventricle cardiac.
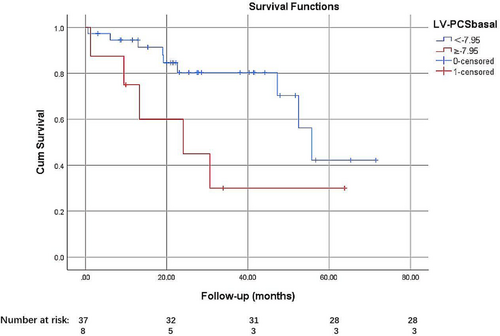
Among all myocardial strains, LV-PCSBasal was the only independent risk factor for 5 year adverse cardiac events (HR: 1.26, 95% CI: 1.10–1.42; P = 0.000). Time-dependent ROC curves were used to compare BMI, RVCI, and LV-PCSBasal and determine the optimal cut-off of LV-PCSBasal for the 5 year primary composite endpoint of −7.95%.
We also paired-up the three of them and combined them to compare the prognostic factors in pairs. LV-PCSBasal can add prognostic value to BMI and RVCI. LGE% was also used to compare the selected parameters. Then, the value of −7.95% was used as dichotomies in Kaplan–Meier survival analysis, which showed a significant difference (Figure 4).
Discussion
The present study investigated the prognostic factors in patients with infl-CMP who had a ring-like LGE pattern. The main findings were as follows: (i) Patients with ring-like LGE infl-CMP who have a low BMI have a poor prognosis. (ii) When compared with LV function, RVCI has a better prognostic value in patients with infl-CMP who exhibit ring-like LGE. (iii) LV-PCSBasal could add additional prognostic value, and −7.95% may be used as the cut-off value. (iv) The BMI and RVCI as well as the LV-PCSBasal have strong prognostic values for patients with infl-CMP who have a ring-like LGE.
The location, pattern, and distribution of LGE have been investigated as prognostic factors in DCM.14, 15 A study examining 10 year outcome data demonstrated that the subepicardial layer of the lateral wall was associated with a favourable outcome, while the septal or mid-wall LGE pattern was linked to the high risk of mortality.15 Mahrholdt et al.6 found that septal LGE was related to ventricular dysfunction and dilatation, and the researchers also found different patterns of LGE associated with different viruses. However, there is another pattern, that is, epicardial and mid-wall LGE (ring-like LGE), which has been detected but rarely analysed as a separate type of LGE.16 Chen et al.7 observed that the ring-like pattern of LGE is independently associated with an increased risk of ventricular tachyarrhythmia in patients with non-ischaemic DCM. Regrettably, the ring-like pattern of LGE induced by myocarditis was excluded in this study.
Obesity is a risk factor for the development of HF,10 but our study signified that a lower BMI is associated with a higher risk of cardiac events (HR = 0.7, P = 0.00) in patients with infl-CMP who exhibit a ring-like LGE. This finding is consistent with the obesity paradox—higher BMI is linked to better survival in patients with HF. Although obesity increases the risk of HF, being overweight or obese exerts a protective effect in patients with a confirmed HF diagnosis.17, 18 Our results indicated that low BMI patients with infl-CMP who exhibit a ring-like LGE on CMR have a poor prognosis. This observation supports the lean paradox,19 with some studies arguing that patients with cardiovascular disease who have a low BMI may have worse clinical outcomes and it could be independent predictor of worse outcomes. The mechanism by which being lean is associated with a poor prognosis is yet to be elucidated.18-21 And our results show that, after adjust end-diastolic ventricular volumes for BSA, the patients with low BMI had lager end-diastolic ventricular volumes index, these may be an explanation for worse clinical outcomes in these patients. Nevertheless, our results do suggest that lean added risk of cardiac events for patients with infl-CMP who exhibit a ring-like LGE on CMR.
In patients with infl-CMP, the prognosis and treatment are generally based on LV function and LGE.1, 5 Nonetheless, RV systolic dysfunction is regarded as an important determinant of symptoms and a powerful marker of poor prognosis in patients with chronic HF.22, 23 Bosch et al. documented that pulmonary artery systolic pressure and RV longitudinal strain (RVLS) were related to the composite endpoint in adjusted analysis in HF.24 Another study also suggested that RVLS is associated with prognostic impact in patients with stage C and D HF.25 However, our results showed the RVCI, rather than RVEF or RVLS, has prognostic value in patients with infl-CMP who exhibit a ring-like LGE on CMR. A likely explanation is that RV dysfunction in infl-CMP is highly afterload-dependent. Severe RV and LV dysfunctions lead to higher RV end-diastolic pressure and lower RV cardiac power output at rest. Subsequently, the RVCI is decreased, which results in an adverse prognosis.26, 27
Previous studies have explored and emphasized the prognostic value of LV strain, mainly global longitudinal strain (GLS), in non-ischaemic DCM.8, 28, 29 However, our results indicated the LV-PCSBasal can add prognostic value in patients with infl-CMP who exhibit ring-like LGE. This result could be attributed to the fact that LGE in our study was mainly located in the middle myocardium of the septal wall and the sub-epicardium of the lateral wall, wherein the torque enhances the shortening in the circumferential direction. The reason is that the circumferential muscle mass in the middle layer consists predominantly of transverse fibres, whereas oblique helical fibres are present in the inner and outer walls.30, 31 Furthermore, the mid-wall fibres, which are oriented transversely, are the most numerous.30 Stokke et al.32 noted that global circumferential strain contributes more than twice as much to ejection fraction than GLS when the geometry is taken into consideration. Carlsson et al.33 explained this phenomenon by observing that circumferential systolic strain is not caused by a separate ‘circumferential fiber function’ but is instead generated by all fibres having varying orientations, including longitudinally oriented fibres. As both the middle myocardium of the septal wall (circumferential fibre) and the sub-epicardium of the lateral wall (longitudinally oriented fibres) were fibrotic in patients with infl-CMP who displayed ring-like LGE, plus basal impairment could be caused by twist differences caused by circumferential fibre damage (resulting in larger basal diameters), a basal contraction impairment34 may add additional prognostic value to BMI and RVCI.
Limitations
First, we did not use myocardial biopsy as the inclusion criteria of infl-CMP. Myocarditis was defined based on symptoms, laboratory results, and CMR findings. Second, the genetic testing was not performed in this study, which would be important and may have interesting results. Third, this is a single-centre study with a relatively limited sample size. The prognostic significance of BMI, RVCI, and basal-peak circumferential strain therefore warrants validation in prospective multicentre investigations. Fourth, some factors associated with the outcomes in myocarditis and HF (e.g. natriuretic peptide and myocardial enzyme) were not analysed in the present study.
Conclusions
Low BMI and low RVCI in patients with infl-CMP who had ring-like LGE on CMR were associated with a poor prognosis. Furthermore, LV-PCSBasal with a cut-off of −7.95% can add prognostic value for patients with infl-CMP who exhibit ring-like LGE.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was supported by grants from the National Natural Science Foundation of China (U1908211 and 82271986) and the Capital's Funds for Health Improvement and Research Foundation of China (2020-1-1052).




