Long-term outcomes in heart failure with preserved ejection fraction: Predictors of cardiac and non-cardiac mortality
Angiza Shahim, Marion Hourqueig, Erwan Donal, and Camilla Hage contributed equally.
Abstract
Aims
Heart failure (HF) with preserved ejection fraction (HFpEF) is associated with cardiovascular (CV) and non-CV events, but long-term risk is poorly studied. We assessed incidence and predictors of the long-term CV and non-CV events.
Methods and results
Patients presenting with acute HF, EF ≥ 45%, and N-terminal pro-brain natriuretic peptide > 300 ng/L were enrolled in the Karolinska-Rennes study in 2007–11 and were reassessed after 4–8 weeks in a stable state. Long-term follow-up was conducted in 2018. The Fine–Gray sub-distribution hazard regression was used to detect predictors of CV and non-CV deaths, investigated separately from baseline acute presentation (demographic data only) and from the 4–8 week outpatient visit (including echocardiographic data). Of 539 patients enrolled [median age 78 (interquartile range: 72–84) years; 52% female], 397 patients were available for the long-term follow-up. Over a median follow-up time from acute presentation of 5.4 (2.1–7.9) years, 269 (68%) patients died, 128 (47%) from CV and 120 (45%) from non-CV causes. Incidence rates per 1000 patient-years were 62 [95% confidence interval (CI) 52–74] for CV and 58 (95% CI 48–69) for non-CV death. Higher age and coronary artery disease (CAD) were independent predictors of CV death, and anaemia, stroke, kidney disease, and lower body mass index (BMI) and sodium concentrations of non-CV death. From the stable 4–8 week visit, anaemia, CAD, and tricuspid regurgitation (>3.1 m/s) were independent predictors of CV death, and higher age of non-CV death.
Conclusions
In patients with acute decompensated HFpEF, over 5 years of follow-up, nearly two-thirds of patients died, half from CV and the other half from non-CV causes. CAD and tricuspid regurgitation were associated with CV death. Stroke, kidney disease, lower BMI, and lower sodium were associated with non-CV death. Anaemia and higher age were associated with both outcomes. [Correction added on 24 March 2023, after first online publication: In the first sentence of the Conclusions, ‘two-thirds’ has been inserted before ‘of patients died...’ in this version.]
Introduction
Heart failure (HF) affects 1–2% of adults worldwide, causing a major global public health burden due to poor functional capacity and quality of life, repeated hospitalizations, high risk of cardiovascular (CV) and non-cardiovascular (non-CV) mortality, and high associated costs.1, 2 HF with preserved ejection fraction (EF ≥ 50%; HFpEF) is heterogeneous and complex,1 and many comorbidities are both drivers of the phenotype3 and predictors of outcomes.4
After an acute hospitalization for HFpEF, short-term mortality rates are comparable with HF with reduced ejection fraction (EF < 40%; HFrEF).5 In clinical trials, CV causes are the primary cause of death in HFpEF, ranging from 60% to 70%.6 However, in observational studies that include older age groups and with longer follow-up, the proportion of CV death appears somewhat lower, that is, approximately 50–60%.6 The proportion of non-CV deaths is significantly higher in HFpEF compared with HFrEF.7 Although HFpEF characteristics and outcomes have been characterized in the short and, to some extent, medium terms, long-term outcomes have not been described.
The observational Karolinska-Rennes (KaRen) study was designed to characterize HFpEF in detail.8 In the present analysis, we aimed to investigate rates of and long-term predictors of CV and non-CV mortality in HFpEF, up to 10 years after enrolment in the KaRen study.
Material and methods
Study population
The bi-national, multicentre, observational, prospective KaRen study was designed to describe and define clinical, biomarker, and imaging characteristics and cause-specific outcomes in patients with HFpEF. Between May 2007 and December 2011, a total of 539 patients with HFpEF were enrolled in 11 centres in France [n = 334 (62%)] and 3 centres in Sweden [n = 205 (38%)], whereas the majority of patients (74%) was recruited in the 3 Swedish centres and in 1 French centre in Rennes. Supporting Information, Table S1 shows an overview of patients enrolled in respective country and centre. The study design has been previously reported.8 Patients presenting with symptoms and signs of acute HF according to the Framingham criteria, N-terminal pro-brain natriuretic peptide (NT-proBNP) > 300 ng/L and EF ≥ 45% by echocardiography within the first 72 h of the acute presentation, were enrolled. Exclusion criteria included primary hypertrophic or restrictive cardiomyopathy or pericardial constriction, known cause of right HF not related to left ventricular dysfunction, any CV disorder with an indication for surgical or percutaneous intervention (e.g. valve disease requiring intervention, even if such intervention was not offered due to, for example, frailty or age), existing cardiac resynchronization therapy, renal disease requiring dialysis, or pulmonary disease requiring chronic supplemental oxygen. After discharge from acute admission, patients who were alive and agreed to follow-up returned after 4–8 weeks for a clinical visit and underwent clinical evaluation, blood sampling, electrocardiogram, and detailed echocardiography. In the current long-term follow-up study, patients with data from both baseline acute presentation returning for the 4–8 week stable state visit were considered (n = 397).
Clinical data and echocardiography
Coronary artery disease (CAD) was defined as a history of coronary artery bypass or angioplasty, >50% coronary artery stenosis, or acute myocardial infarction. Estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI). Anaemia was defined according to WHO as haemoglobin < 13 g/dL in males and <12 g/dL in females. Echocardiographic data were acquired using ViVid 7 and a standard 2.5 MHz adult transthoracic probe (GE Healthcare, Horten, Norway), according to the American Society of Echocardiography (ASE) and the European Association for Cardiovascular Imaging (EACVI) recommendations,9 with cut-off values published in a consensus paper for diagnosis of HFpEF,10 and in accordance with previous KaRen studies.11 The measurements were performed three times and averaged by an echocardiographer blinded to the specific clinical history of the patient, and all examinations were analysed in the Core Lab (Rennes University Hospital, CIC 1414, France).8
Outcomes and follow-up
All deaths and hospitalizations were predefined according to prespecified criteria and subclassified as due to a CV cause, a non-CV cause, or an unknown cause. CV death was defined as death from HF, myocardial infarction, stroke, or sudden cardiac death, and non-CV death representing all other known causes of death, including cancer, pulmonary diseases, kidney disease, liver disease, infection, diabetes, trauma, and peripheral (non-cerebral) vascular diseases, and the remaining as unknown causes of death. All patients were followed prospectively by telephone call every 6 up to 18 months until November 2012. Subsequently, long-term follow-up was conducted by telephone contact with patients, personal doctors, and/or medical institutions including patient charts at the end of September 2018. Additionally, the Swedish Cause of Death Registry was used in Sweden. If the cause of death was not found, it was considered unknown. There was no formal adjudicating committee; however, in all cases where there was an uncertainty of the subclassification, it was discussed with the KaRen investigators and a final classification was decided by consensus.
Patients were followed until the occurrence of CV, non-CV, and unknown cause of death or censored alive at the last contact with medical institution. Ten out of 11 French centres (n = 142 patients) did not participate in the present long-term follow-up study due to lack of resources as this was an amendment to the initial study protocol; therefore, only Rennes where the bulk of the French patients originated from was able to participate.
Statistical analysis
Patient characteristics and echocardiographic variables are reported as median and interquartile range (IQR) for continuous variables and as frequencies and percentages for categorical variables. Patients with missing data for the variables included in the models were excluded (but Table 1 shows descriptive data for the entire cohort). We used a competing risk regression approach with the Fine and Gray method to estimate the sub-distribution hazard ratio (SHR) and 95% confidence intervals (CIs) for each outcome, that is, CV, non-CV, and unknown cause of death. Cumulative incidence curves are assessed for the competing outcomes CV, non-CV, and unknown causes of death. As a sensitivity analysis, Cox proportional hazard models (which do not account for competing risk) were also performed, and results are provided in Supporting Information, Tables S4 and S6.
| Variable | Missing (%) | Overall (n = 397) |
|---|---|---|
| Clinical | ||
| Age (years) | 78 (72, 84) | |
| Female sex | 206 (52) | |
| LVEF, at presentation (%) | 2 (0.5%) | 55 (50, 60) |
| NYHA class in stable state before admission, n (%) | 9 (2.3%) | |
| I | 78 (20%) | |
| II | 241 (62%) | |
| III | 67 (17%) | |
| IV | 2 (1%) | |
| NYHA class at presentation, n (%) | 1 (0.3%) | |
| I | 1 (0.3%) | |
| II | 27 (7%) | |
| III | 161 (41%) | |
| IV | 206 (52%) | |
| Peripheral oedema | 276 (70%) | |
| Pulmonary oedema on X-ray | 68 (17%) | 188 (57%) |
| Systolic blood pressure (mmHg) | 2 (0.5%) | 150 (130, 170) |
| Diastolic blood pressure (mmHg) | 2 (0.5%) | 76 (65, 90) |
| Mean arterial blood pressure (mmHg) | 2 (0.5%) | 100 (88, 114) |
| Supine heart rate (b.p.m.) | 3 (0.8%) | 80 (68, 100) |
| Tachycardia (>100 b.p.m.) | 1 (0.3%) | 127 (32%) |
| BMI (kg/m2) | 11 (2.8%) | 28 (24, 32) |
| Obese (BMI ≥ 30 kg/m2) | 11 (2.8%) | 144 (37%) |
| Cardiovascular comorbidities, n (%) | ||
| Known heart failure prior to presentation | 158 (40%) | |
| Coronary artery disease | 3 (0.8%) | 130 (33%) |
| History of myocardial infarction | 65 (16%) | 66 (20%) |
| History of syncope due to bradyarrhythmia | 8 (2.0%) | 10 (3%) |
| History of syncope due to tachyarrhythmia | 39 (10%) | |
| Diabetes mellitus | 2 (0.5%) | 105 (27%) |
| Stroke | 1 (0.3%) | 43 (11%) |
| Peripheral vascular disease | 3 (0.8%) | 60 (15%) |
| Non-cardiovascular comorbidities, n (%) | ||
| Any pulmonary disease | 2 (0.5%) | 100 (25%) |
| COPD | 2 (0.5%) | 62 (16%) |
| Asthma | 2 (0.5%) | 22 (5.8%) |
| Cancer | 1 (0.3%) | 67 (17%) |
| History of non-cardiac syncope | 38 (10%) | |
| Liver disease | 8 (2%) | |
| Age-related/mixed comorbidities, n (%) | ||
| Valve diseasea | 1 (0.3%) | 93 (23%) |
| Hypertension | 1 (0.3%) | 310 (78%) |
| Atrial fibrillation or flutterb | 251 (63%) | |
| Paroxysmal | 87 (35%) | |
| Smoking | 14 (3.5%) | 175 (46%) |
| History of renal disease | 123 (31%) | |
| Anaemia (<12 g/dL in females and <13 g/dL in males) | 4 (1.0%) | 177 (45%) |
| History of interventions, n (%) | ||
| Conventional pacemaker | 53 (13%) | |
| Implantable cardioverter defibrillator | 2 (0.5%) | 1 (0.3%) |
| Percutaneous coronary intervention | 2 (0.5%) | 50 (13%) |
| Coronary artery bypass grafting | 31 (8%) | |
| Any valve intervention | 2 (0.5%) | 3 (1%) |
| Medications at discharge, n (%) | ||
| ACEi or ARB | 88 (22%) | 237 (78%) |
| Beta-blocker | 88 (22%) | 248 (80%) |
| MRA | 88 (22%) | 86 (28%) |
| Loop diuretic | 88 (22%) | 264 (85%) |
| Thiazide diuretic | 88 (22%) | 29 (9%) |
| Calcium channel blocker | 88 (22%) | 81 (26%) |
| Digoxin | 88 (22%) | 29 (9%) |
| Nitrate | 88 (22%) | 41 (13%) |
| Anti-arrhythmic | 88 (22%) | 40 (13%) |
| Anticoagulant | 88 (22%) | 178 (58%) |
| Oral anticoagulants among patients with atrial fibrillation/flutter | 163 (53%) | |
| Antiplatelets | 88 (22%) | 95 (31%) |
| Statins | 88 (22%) | 141 (46%) |
| Glucose-lowering medication | 88 (22%) | 76 (25%) |
| Insulin among patients with glucose-lowering medication | 46 (60%) | |
| Laboratory data | ||
| eGFR (mL/min/1.73 m2) | 62 (46, 79) | |
| ≥30 | 367 (92%) | |
| <30 | 30 (8%) | |
| NT-proBNP (ng/L) | 1 (0.3%) | 2469 (1319, 4860) |
| NT-proBNP among patients with atrial fibrillation/flutter | 2605 (1469, 4941) | |
| NT-proBNP among patients without atrial fibrillation/flutter | 2292 (1120, 4630) | |
| Haemoglobin (g/dL) | 4 (1.0%) | 12 (11, 14) |
| Sodium (mmol/L) | ||
| Low (<135) | 59 (15%) | |
| Normal (135–145) | 335 (84%) | |
| High (>145) | 3 (1%) | |
| Potassium (mmol/L) | 3 (0.8%) | |
| Low (<3.5) | 34 (9%) | |
| Normal (3.5–5.0) | 324 (82%) | |
| High (>5.0) | 36 (9%) | |
| Echocardiographic parameters from 4–8 week clinical visit | ||
| LVEF (%) | 155 (39%) | 63 (56, 67) |
| Tricuspid regurgitation Vmax (m/s) | 182 (46%) | 2.9 (2.5, 3.3) |
| Low (<2.8) | 86 (40%) | |
| Medium (2.8–3.1) | 66 (31%) | |
| High (>3.1) | 63 (29%) | |
| E/e′ ratio | 163 (41%) | 11 (8.5, 15.1) |
| >13 | 81 (35%) | |
| ≤13 | 153 (65%) | |
| LV GLS (%) | 318 (80%) | 16 (13, 18) |
| LAVI (mL/m2) | 318 (80%) | 31 (21, 40) |
| LAVI > 34 | 31 (39%) | |
| TAPSE (mm) | 148 (37%) | 17 (13, 20) |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; TAPSE, tricuspid annular plane systolic excursion; Vmax, maximal velocity.
- Continuous variables are presented as median (interquartile range), and categorical variables as numbers (n) and percentages.
- a Confirmed mild to moderate valve disease; surgical indication (even if such intervention was not offered due to, for example, frailty or age) was an exclusion criterion.
- b Atrial fibrillation/flutter included all patients with (1) permanent (during 1 year and cardioversion has not been attempted or has failed); (2) persistent (more than 7 days and failing to self-terminate); (3) permanent or persistent (but unsure which); and (4) paroxysmal (2 or more episodes lasting 7 or fewer days and usually <24 h).
We chose clinically relevant and previously shown clinically important variables in the multivariable models as potential predictors of outcomes. Two separate multivariable models were used: (1) The first model used acute presentation as index date and consisted of predictors assessed at baseline acute presentation and included the following 18 variables: age (per 5 year increase), sex, New York Heart Association (NYHA) functional classification, NT-proBNP, EF, body mass index (BMI) (per 5 kg/m2 increase), systolic blood pressure (per 5 mmHg increase), sodium concentrations (per 5 mmol/L increase), known HF diagnosis prior to presentation, CAD, pulmonary disease, atrial fibrillation (AF) or flutter, anaemia, kidney disease, diabetes mellitus (DM), stroke, non-cardiac syncope, and cancer; (2) the second model used the stable 4–8 week visit as index date and consisted of predictors assessed at the 4–8 week follow-up visit when detailed echocardiography was performed and included the following 12 variables: age, male sex, anaemia, AF, DM, pulmonary disease, hypertension, CAD, kidney disease, stroke, and the echocardiographic variables tricuspid regurgitation maximal velocity (<2.8, 2.8–3.1, and >3.1 m/s) and E/e′ ratio (≤13 and >13). Tricuspid regurgitation and E/e′ were included as previously shown to be predictors of mortality in the KaRen cohort,11 as well as other studies.10 All P-values were two-sided and statistical significance was set at 0.05. Statistical analyses were performed in R Version 4.0.4.12
Ethical considerations
The KaRen study, with a separate amendment for the long-term follow-up, had ethics approval by the local French and Swedish ethics committees and was conducted according to the International Conference on Harmonization and Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
Results
Baseline characteristics and outcomes
In total, 539 patients (334 from 11 French centres and 205 from 3 Swedish centres) were enrolled in the KaRen study. Due to lack of resources, 142 French patients did not participate (26% of the total study population) in the present long-term follow-up study, leaving 397 available (192 from France and 205 from Sweden) for the present analysis. Of these 397 patients, 7 French patients were lost to long-term follow-up and therefore censored alive at last known follow-up (Figure 1).
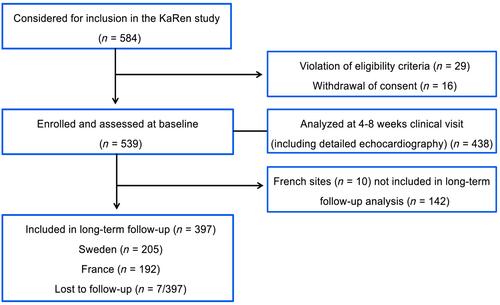
Prior to baseline acute presentation, 40% had an HF diagnosis. At baseline acute presentation, the overall study population was median (IQR) age 78 (72–84) years, and 206 (52%) were female. Most patients were in NYHA class II in stable state prior to admission and in III–IV on admission with median (IQR) EF of 55% (50, 60). The majority had a high burden of CV risk factors and comorbidities, 78% had hypertension, 63% had AF/flutter (35% of which was paroxysmal), 27% had any type of DM, 33% (39% male and 27% female) had CAD, 31% had a history of kidney disease (creatinine ≥ 100 μmol/L or ≥1.1 mg/dL), 45% had anaemia, and 37% were obese (BMI ≥ 30 kg/m2). Three per cent of all patients had a history of syncope due to bradyarrhythmia and 10% due to tachyarrhythmia, and 10% had non-cardiac syncope. NT-proBNP levels were higher in patients with vs. without AF/flutter: median (IQR) 2605 (1469, 4941) ng/L vs. 2292 (1120, 4630) ng/L, respectively. Furthermore, at discharge, the majority was treated with angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) (78%) and/or loop diuretics (85%) (Table 1).
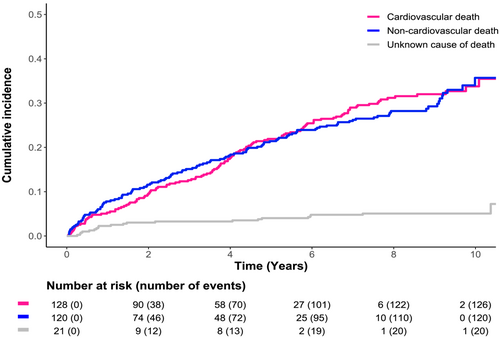
Over a median follow-up of 5.4 (IQR 2.1, 7.9) years, there were 269 (68%) deaths, 128 (47%) CV deaths, 120 (45%) non-CV deaths, and 21 (8%) unknown causes of death, for a total of 130 (95% CI 115–147), 62 (95% CI 52–74) CV, and 58 (95% CI 48–69) non-CV deaths per 1000 patient-years. Similar proportions of CV and non-CV deaths were observed when stratified by country (Sweden vs. France); however, France had higher proportion and rate of unknown cause of death (Supporting Information, Table S2). In the overall population, the incidence of CV, non-CV, and unknown causes of death was proportional over time, with a slightly steeper increase in the first year and slightly lower CV risk in early years and slightly higher CV risk in later years (Figure 2).
Predictors of cardiovascular and non-cardiovascular deaths
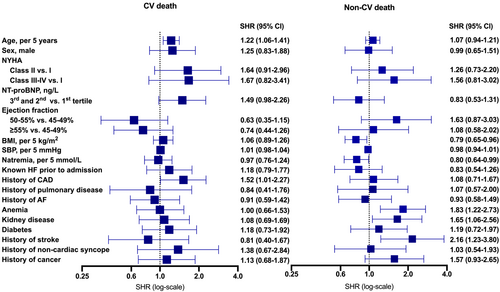
The first multivariable model considering patient characteristics from time of baseline acute presentation included 368 observations with complete data. Higher age (per 5 years) and CAD were independently associated with higher risk of CV death. Of note, higher NT-proBNP appeared to be associated with greater risk of CV death but not non-CV death. Furthermore, higher EF appeared to be associated with lower risk of CV death but if anything, possibly higher risk of non-CV death. Correspondingly, the non-CV conditions anaemia, kidney disease, stroke, and lower sodium concentrations (per 5 mmol/L) and BMI (per 5 kg/m2) were independently associated with increased risk of non-CV death (Figure 3).
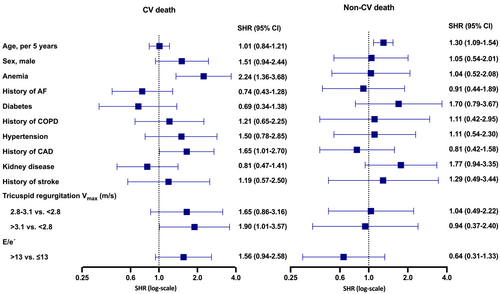
The second multivariable model with data from the 4–8 week stable state visit (including patient characteristics and echocardiographic data) consisted of fewer observations (n = 194) due to missing echocardiographic data, which has previously been described in detail.13 Anaemia and CAD were independently associated with higher risk of CV death. History of kidney disease appeared to be, when assessed from both index dates, associated with higher risk of both causes of death, although in the 4–8 week stable state visit, it was associated with lower risk of CV death. Among the echocardiographic variables, higher tricuspid regurgitation (2.8–3.1 and >3.1 vs. <2.8 m/s) was associated with greater risk of CV death but not non-CV death. Higher E/e′ (>13 vs. ≤13) seemed to be associated with higher risk of CV death but, conversely, lower risk of non-CV death (Figure 4).
In addition to the predictors from the competing risk models, the Cox model from time of baseline acute presentation showed that higher NYHA class (II and III–IV vs. I) was associated with higher risk of CV death, whereas higher age (per 5 years) and NYHA class (III–IV vs. I) were associated with greater risk of non-CV death (Supporting Information, Table S4). In the Cox model from time of 4–8 week stable state, CAD was no longer significantly associated with CV death; however, male sex was found to be independently associated with higher risk of CV death (Supporting Information, Table S6).
Discussion
In this well-characterized HFpEF cohort followed for a median of 5.4 (2.1–7.9) years after an acute admission, we found that nearly two-thirds of the patients died with a similar proportion of CV and non-CV deaths. In the competing risk model assessed at baseline acute presentation, higher age and a history of CAD were independently associated with CV death, whereas stroke, kidney disease, lower BMI, and lower sodium (perhaps due to volume overload) with non-CV death. Moreover, higher natriuretic peptides appeared to be associated with greater risk of CV death but not non-CV death. There were no significant associations observed between EF and outcomes. At the 4–8 week stable state visit, anaemia, CAD, and higher tricuspid regurgitation (>3.1 m/s) were independently associated with CV death, and higher age with non-CV death. Underlying cardiac dysfunction (tricuspid regurgitation), CAD, and anaemia, the latter a marker of the numerous underlying comorbidities, primarily contributed to CV deaths in this elderly population.
Cause of death in heart failure with preserved ejection fraction
Prior HFpEF studies have reported mortality and studies show greater non-CV comorbidity burden in HFpEF compared with HFrEF, suggesting also a higher proportion of non-CV causes of death in HFpEF. However, most studies assessing cause-specific mortality lack long-term follow-up data and have not consistently taken into account the competing risk of CV and non-CV deaths, which could lead to incorrect estimation of event rates.14 Also, the risk of death after acute HF presentation may change over time and differ based on patient characteristics. We demonstrate an overall mortality rate of 68% over a median of ~5 years, which is considerably higher than the 5 year mortality generally reported for HFrEF.15 The cumulative incidence of CV and non-CV deaths was similar, which is inconsistent with randomized controlled trials where >60% of deaths have been due to CV causes and the majority to sudden death and HF death.6, 16 Randomized clinical trials often select HFpEF patients with stringent eligibility criteria and exclude older patients with advanced non-CV comorbidity to ensure high modifiable event rates.17 Our study was an observational study enrolling a relatively unselected HFpEF cohort more similar to other epidemiological studies that consistently reported lower rates of CV mortality compared with trials.6 HFpEF patients die almost twice as often of non-CV causes compared with HFrEF, reflecting the high burden of non-CV comorbidities in HFpEF. Prior literature with short-term follow-up, including the KaRen study,18 has previously identified non-CV conditions such as DM, kidney failure, anaemia, and obesity as drivers of disease and poor prognosis. Since the performance of this study, newer therapies such as the sodium-glucose cotransporter 2 (SGLT2) inhibitors for HF have been introduced, which have reduced CV events.19 Had the present study population been treated with SGLT2 inhibitors, associations with CV and non-CV outcomes may have been different.
Independent predictors of cardiovascular death
In a previous long-term follow-up from the KaRen cohort, the association between non-CV conditions and comorbidities and all-cause mortality and first HF hospitalization were highlighted. This displayed the need for a more in-depth investigation of the role of non-CV comorbidities on cause-specific outcomes.20 Given the high comorbidity burden and high risk of non-CV death, it is important for CV and HF trialists to identify predictors of CV events, which may be used as enrichment factors in future HFpEF trials. The prognostic implication of CAD in HFpEF has been inconsistently reported. Hwang et al. reported a greater deterioration in systolic function and worse survival in patients with HFpEF when CAD was present and angiographically diagnosed.21 We found history of CAD as a predictor of CV death and patients with CAD were more likely to die from a CV cause compared with non-CV over the long term, suggesting, not surprisingly, that CAD contributes to the high rates of CV death in HFpEF. In HFpEF, macrovascular atherosclerosis may also reflect microvascular damage causing myocardial stiffness and diastolic dysfunction.3 This is reflected by increased concentrations of inflammatory biomarkers, and biomarkers indicating cell apoptosis and fibrosis.22 Moreover, in HFpEF, proteins linked with systemic inflammation and endothelial dysfunction are associated with systemic ischaemia even in the absence of apparent CAD.23, 24 Microvascular disease has also been associated with HFpEF in DM, a common comorbidity in HFpEF (26% in our study).25
Natriuretic peptides are an essential diagnostic1 and prognostic tool in HFpEF.26 We observed that increased NT-proBNP levels, assessed during acute admission, appeared to be associated with the risk of death from a CV cause over the long term, as has been previously shown over the short term.26 Natriuretic peptides reflect HF severity and also comorbidities such as kidney disease and cachexia have been reported to be associated also with non-CV risk,27 but in the present study, NT-proBNP was not associated with non-CV death. There was no apparent association between AF/flutter and outcomes. Patients with AF/flutter had higher natriuretic peptide levels compared with those without and 35% of AF/flutter were paroxysmal, which could imply that AF/flutter triggered an HF event in some otherwise asymptomatic patients. According to the definition of HFpEF at the time, the KaRen study did not have different inclusion or exclusion criteria for patients with AF/flutter.
Stroke is a common cause of death in HFpEF,6 irrespective of the presence of AF.28 We identified that patients with a history of stroke had a two-fold increased risk of non-CV death. Furthermore, at enrolment, 11% of our patients had a history of stroke, 63% had a history of AF, and only 41% of these were on oral anticoagulants. Our analysis highlights that suboptimal use of anticoagulants may be a considerable risk factor to consider in patients with AF and HFpEF.
The greater burden of non-CV comorbidities in HFpEF might contribute to the higher proportions of non-CV deaths seen in HFpEF compared with HFrEF. Anaemia is more frequent in HFpEF than HFrEF but similarly associated with worse outcomes regardless of EF.29 Patients with HF and anaemia are older, with worse kidney function and more comorbidities.29 Notably, after extensive adjustment for comorbidities and risk factors, our analysis showed that anaemia in the acute setting was associated with non-CV death, whereas anaemia in the stable setting was associated with CV death. Hyponatraemia is common in all HF phenotypes and reflects neurohormonal activation and volume retention.30 In the previous short-term follow-up study from the KaRen population, lower sodium was associated with both the combined endpoint all-cause mortality/HF hospitalization and mortality alone.18 Our study replicated these findings for the risk of non-CV death also over the long term. Kidney disease is highly prevalent in HF and may be more common in HFpEF.31 It is associated with increased risk of adverse outcomes but more so in HFrEF and HF with mildly reduced EF (HFmrEF), where it may reflect HF severity, and less so in HFpEF, where it may be a comorbidity developing over the long term from the same risk factors as HFpEF itself.32 Indeed, we observed an independent association between kidney disease and non-CV death in HFpEF. Obesity is a major risk factor for incident HF, and especially important in the development of HFpEF by adversely affecting cardiac structure and function.3 Similar to other cohorts, most patients in KaRen were overweight and more than a third were obese. More interestingly, in chronic HFrEF, higher BMI appears associated with better outcomes.32 We confirm this for HFpEF and in particular for long-term follow-up, where higher BMI seems to have a protective effect on survival.
Echocardiographic predictors of cardiovascular and non-cardiovascular deaths
In patients with HFpEF, low serum sodium is also associated with an increased risk of adverse events, although there is uncertainty regarding its mechanisms and treatment. Reflecting elevated left ventricular filling pressure and proportionally related to systolic pulmonary artery pressure and right heart modelling, pulmonary pressures have shown important diagnostic and prognostic significance in HFpEF.10 In previous publications from the KaRen cohort, higher tricuspid regurgitation velocity during exercise was independently associated with all-cause mortality and HF hospitalization during both short-term33 and long-term follow-ups.20 In the current analysis, we extend these findings by demonstrating that high tricuspid regurgitation (>3.1 m/s) was associated with increased risk of CV death. The mechanism of tricuspid regurgitation in HFpEF is likely multifactorial. However, a relationship between cardiac abnormalities such as higher tricuspid regurgitation and the comorbidity-inflammation burden in HFpEF has been reported by Sanders-van Wijk et al.22 Hence, the role of tricuspid regurgitation in addition to cardiac dysfunction may be key to understand the pathophysiology of HFpEF.
Limitations
The KaRen study includes a large number of well-characterized HFpEF patients; however, several limitations can be identified in our study. We were unable to identify the cause of death in 15% and 1.4% of the patients in France and Sweden, respectively. Some French patients were not available for follow-up analysis and models were restricted to patients with complete baseline and follow-up data, reducing the sample size and potentially introducing selection bias. Furthermore, the modest sample size may have been inadequate for adjusted analysis to detect important predictors of outcome, and our analysis did not include HFrEF or a healthy control population, which limits our comparisons to data from the literature. Patients with EF ≥ 45% were recruited in the KaRen study,8 and for consistency and comparison through previous KaRen studies, the current analysis included patients (n = 6) with HFmrEF according to recent diagnostic criteria for HF. The causes of syncope due to tachyarrhythmia were not available and only one patient (0.3% of all patients) had implantable cardioverter defibrillator (ICD) prior to admission. However, there was no certain indication for more use of HF devices as 60% of all patients had de novo HF, with preserved EF and mild HF symptoms in stable state prior to admission.
Conclusions
In patients with acute decompensated HFpEF and with over 5 years of follow-up, nearly two-thirds of patients died, half from CV and the other half from non-CV causes. CAD and tricuspid regurgitation were associated with CV death. Stroke, kidney disease, lower BMI, and lower sodium were associated with non-CV death. Anaemia and higher age were associated with both outcomes.
Acknowledgements
The authors are grateful to Marie Guinoiseau and Valerie Le Moal for patient care and management of the study in Rennes, and Amelie Reynaud for work at the core lab.
Conflict of interest
L.H.L. receives research grants from the Swedish Research Council, the Swedish Heart Lung Foundation, and the Stockholm County Council; research grants from AstraZeneca, Novartis, Boerhinger Ingelheim, ViforPharma, and Boston Scientific; and consulting or speaker's honoraria from AstraZeneca, Novartis, Boehringer Ingelheim, ViforPharma, Bayer, Sanofi, Fresenius, Merck, Myokardia, MedScape, Radcliffe Cardiology, and Lexicon. G.S. reports grants and personal fees from Vifor and AstraZeneca; grants and non-financial support from Boehringer Ingelheim; personal fees from Società Prodotti Antibiotici, Roche, Servier, GENESIS, Cytokinetics, and Medtronic; and grants from MSD and Novartis, outside the submitted work. C.L. receives research grants from the Swedish Heart Lung Foundation and the Stockholm County Council and speaker honoraria from Medtronic, Abbot, Microport, Boston Scientific, Novartis, Vifor, Impulse Dynamics, and Bayer. C.H. is supported by the Stockholm Country Council (grant 20180899) and has received consulting fees from Novartis and Roche Diagnostics, and speaker and honoraria from MSD and Novartis. E.D. receives research facilities from General Electric Healthcare, grant from Novartis, and teaching facilities from Bristol Myers Squibb. Other authors have no conflict of interest to declare.
Funding
This work was supported by grants from the Swedish Research Council (grants 2013-23897-104604-23, 523-2014-2336), the Swedish Heart Lung Foundation, and the Stockholm Country Council (grant 20110120) to L.H.L.'s institution. The prospective KaRen study was supported in part by grants from the Fédération Française de Cardiologie/Société Française de Cardiologie, France, and the Medtronic Bakken Research Center, Maastricht, the Netherlands. No funding agency had any role in the design and conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.




