Integrated care for older multimorbid heart failure patients: protocol for the ESCAPE randomized trial and cohort study
Registration:
The study has been registered at the University of Göttingen Medical Centre (UMG Reg. No 02853) and the German Clinical Trials Register (DRKS00025120).
Christine Zelenak and Jonas Nagel share first authorship.
Susanne S. Pedersen and Christoph Herrmannn-Lingen share last authorship.
[Correction added on 30 March 2023, after first online publication: Affiliation 6 has been added to Tim Friede and Christoph Herrmann-Lingen; and the sequence for the rest of the affiliations were reordered accordingly.]
Abstract
ESCAPE
Evaluation of a patient-centred biopsychosocial blended collaborative care pathway for the treatment of multimorbid elderly patients.
Therapeutic Area
Healthcare interventions for the management of older patients with multiple morbidities.
Aims
Multi-morbidity treatment is an increasing challenge for healthcare systems in ageing societies. This comprehensive cohort study with embedded randomized controlled trial tests an integrated biopsychosocial care model for multimorbid elderly patients.
Hypothesis
A holistic, patient-centred pro-active 9-month intervention based on the blended collaborative care (BCC) approach and enhanced by information and communication technologies can improve health-related quality of life (HRQoL) and disease outcomes as compared with usual care at 9 months.
Methods
Across six European countries, ESCAPE is recruiting patients with heart failure, mental distress/disorder plus ≥2 medical co-morbidities into an observational cohort study. Within the cohort study, 300 patients will be included in a randomized controlled assessor-blinded two-arm parallel group interventional clinical trial (RCT). In the intervention, trained care managers (CMs) regularly support patients and informal carers in managing their multiple health problems. Supervised by a clinical specialist team, CMs remotely support patients in implementing the treatment plan—customized to the patients' individual needs and preferences—into their daily lives and liaise with patients' healthcare providers. An eHealth platform with an integrated patient registry guides the intervention and helps to empower patients and informal carers.
HRQoL measured with the EQ-5D-5L as primary endpoint, and secondary outcomes, that is, medical and patient-reported outcomes, healthcare costs, cost-effectiveness, and informal carer burden, will be assessed at 9 and ≥18 months.
Conclusions
If proven effective, the ESCAPE BCC intervention can be implemented in routine care for older patients with multiple morbidities across the participating countries and beyond.


Introduction: Background and scientific rationale
Heart failure (HF) affects over 64 million people worldwide1 and is a major cause of mortality, morbidity, hospitalizations, and disability contributing to substantial healthcare costs across European countries (1–3% of all healthcare expenditure2). Its prevalence is particularly high in older adults, expected to further increase due to an ageing population,1 and medical advances have not reduced its prevalence so far.3 Furthermore, HF contributes significantly to a reduced health-related quality of life (HRQoL).1 For abbreviations refer to Table 1.
| AE | Adverse event |
|---|---|
| (B)CC | (Blended) collaborative care |
| CEA | Cost-effectiveness analysis |
| CONSORT | Consolidated Statement of Reporting Trials |
| CRA | Clinical research associate |
| CTU | Clinical trial unit |
| CVD | Cardiovascular disease |
| DFD | Depression-free days |
| DSMB | Data Safety and Monitoring Board |
| eCRF | Electronic case report form |
| EOS | End of study |
| EOT | End of treatment |
| EQ-5D-5L | RQoL questionnaire w/5 dimensions developed by the EuroQol Group |
| ESCAPE | Evaluation of a patient-centred biopsychosocial blended collaborative care pathway for the treatment of multimorbid elderly patients |
| EU | European Union |
| FAIR principles | Findable Accessible Interoperable Reusable |
| FU | Follow-up |
| GP | General practitioner |
| HADS | Hospital Anxiety and Depression Scale |
| HF | Heart failure |
| HRQoL | Health-related quality of life |
| ICP | Integrated Care Platform |
| ITT | Intention-to-treat |
| iMCQ | Medical Consumption Questionnaire |
| MAM | Meta-algorithm for multi-morbidity |
| CM | Care manager |
| PIC | Patient-informed consent |
| RCT | Randomized controlled trial |
| SAE | Serious adverse event |
| SOP | Standard operating procedure |
| UC | Usual care |
| UMG | Universitätsmedizin Göttingen/University Medical Center Göttingen |
- This table lists the abbreviations used in the publication of the ESCAPE study's protocol.
In addition to problems directly related to HF, most HF patients suffer from somatic and mental multi-morbidity,4 that is, the co-occurrence and possible interaction of multiple, usually chronic, somatic, and/or mental health conditions. Mental distress and disorders are known to interact with heart disease and other physical illnesses in a bidirectional way: They can be triggered or amplified by a physical disease and in turn impair patients' HRQoL and prognosis.5, 6 For example, in HF patients, depression can increase medical encounters, emergency room visits by twofold, hospital readmissions over 6–12 months by ≥50%, and total healthcare costs by 29%.7 Mental co-morbidities also reduce the benefits of cardiac rehabilitation.8 After coronary artery and heart valve surgery, anxiety and lower education are independent predictors of mortality and morbidity.9 Patients with mental co-morbidity or distress are often challenged adhering to the demanding treatment regimens10 for multi-morbidity, and their clinical outcomes are compromised. Yet, mental co-morbidities often go undetected in clinical routine. Finally, multi-morbidity can also pose a psychological and financial strain on patients' informal carers and relatives.11
Continuous and timely care by physicians promotes patients' treatment adherence and can have a protective effect on mortality and rehospitalization.12 However, healthcare providers often lack the time to address the complexity of multi-morbidity and its challenges, leading to a narrowed treatment focus, missed or delayed diagnoses, especially those pertaining to mental disorders, inappropriate prescribing and redundant diagnostics. Treatment of patients with multi-morbidity is typically coordinated by general practitioners (GPs) who are confronted with numerous problems.13 Ineffective communication among providers including various specialists can result in fragmented care, especially if each provider focuses on isolated disease entities,4 resulting among others in high treatment burden on patients6 and polypharmacy that leads to adverse drug reactions14 and drug–drug interactions causing avoidable hospital admissions.15
To address these treatment gaps and fragmented care, it is indispensable that patients with multi-morbidity receive integrated, patient-centred care across all conditions and specialties.6 This is particularly relevant for patients with HF and mental co-morbidities, such as depression, as integrated care treatment of co-morbid mental disorders verifiably increases HRQoL16 and reduces hospital readmissions of HF patients.17
One such integrated care model is collaborative care (CC), a team-based approach across providers. In the USA, CC effectively improved depressive symptoms in cardiac patients.18 However, focusing CC primarily on depression treatment does not seem to render any viable somatic benefit.18, 19 Most relevant, the TeamCare trial, targeting both mental health and cardiovascular risk factors, showed that this ‘blended’ CC strategy (BCC) can improve both conditions20 at no or minimal additional cost.21 The Hopeful Heart trial16 successfully employed the BCC intervention to treat depressive HF patients, but without explicitly addressing physical multi-morbidity or mental conditions besides depression.22
Despite these promising results, BCC interventions have not been tested in multi-morbid patients in Europe. To overcome this structural deficit, the ESCAPE consortium has received funding from the EU (EU Horizon 2020; project no. 945377) to develop and examine an integrated care concept for older patients with somatic-mental multi-morbidity. By introducing our integrated care concept, we extend previous concepts of multi-morbidity care to a vulnerable patient group with a high need for care integration. In particular, we chose to focus on patients with HF and somatic–mental multi-morbidity, given the high prevalence of HF in older adults and its frequent co-occurrence and interaction with other somatic3 and mental5 conditions.
ESCAPE strives to improve quality of care and HRQoL through the delivery of a personalized blended collaborative care (BCC) intervention, integrating a meta-algorithm for multi-morbidity to optimize patient-centred treatment plans23 and supported by advanced information and communication technology. It also attempts to support the informal carers who are expected to benefit (i) emotionally from knowing that their relative has somebody to talk to concerning both emotional and medical questions and (ii) practically by having somebody who coordinates all information relevant for the care of their relative, a task most informal carers have to do without any medical education in their spare time.
Study objectives and design
The current paper describes, in accordance with the SPIRIT reporting guideline24 and objectives and design of the ESCAPE study, a comprehensive cohort study consisting of a longitudinal observational study and an embedded randomized controlled assessor-blinded two-arm parallel group interventional clinical trial (RCT).
The RCT will examine the primary hypothesis that adding a 9-month optimized and targeted BCC intervention to usual care (UC) for older patients with multi-morbidity improves HRQoL more than UC alone. The parallel cohort will allow to assess the external validity of the RCT in terms of both baseline characteristics and outcomes and the extrapolation of the intervention effect to the ‘real world’. It further reflects routine care and provides an opportunity to investigate prognostic factors for the natural disease course.
Approximately 450 patients with HF, ≥2 chronic somatic co-morbidities (i.e. multi-morbidity), and psychological distress or disorder will be enrolled in the study, 300 in the RCT plus 150 in the observational cohort (Figure 1).
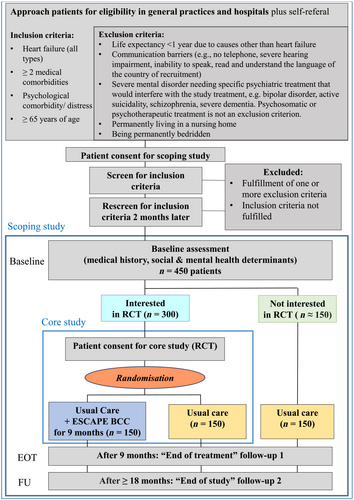
Patients do not receive any direct remuneration or free medication for study participation. However, patients in the RCT have a 50% chance of getting assigned a personal CM from whom they receive emotional and practical support once or twice monthly over 9 months.
Subject population, selection criteria, and recruitment process
Eleven clinical centres in six European countries will participate in the recruitment, intervention delivery, and blinded assessments from either cardiac units or out of community general practices (Germany: University hospitals of Göttingen, Cologne, Leipzig, Hamburg; Denmark: General Practice-University of Southern Denmark; Roskilde and Slagelse hospitals, Region Zealand; Ireland: Beaumont Hospital Dublin; Lithuania: University of Kaunas; Hungary: Semmelweis University Budapest; Italy: Bellaria Hospital Bologna).
The cohort study will include older patients (≥65 years old, any gender) with any type of HF, ≥2 chronic somatic co-morbidities, and elevated psychological distress or diagnosed mental disorder(s) over a recruitment period of 12 months. For inclusion and exclusion criteria, see Figure 1. Regarding the diagnosis of HF, the most important information is the diagnosis confirmed by a cardiologist or internist in a written medical report. In case HF as such has not been mentioned in any written document yet, we rely on the diagnostic criteria as specified in the current ESC guidelines3 with information taken from written medical records and the patient. In order to assure a correct diagnostic procedure, a standard operating procedure for the diagnosis of HF has been developed. Throughout the study, patient contacts take place via telephone, video, or in person. The questionnaires and patient consent forms are sent out by post or handed out in person. For the questionnaires, web-based completion is offered as additional option after providing any patient who wishes so with a specific password for the database that enables them to see only those questionnaires related to themselves. There are three ways of recruitment in ESCAPE: (i) hospital-based, (ii) identification by GP, and (iii) advertisement asking for self-referral. Interested patients are contacted by or actively contact a recruiter who ascertains eligibility for the cohort study. Potentially eligible patients are asked for their written patient informed consent (PIC1) to participate in the screening process and in the cohort study. Consenting and eligible patients are rescreened 2–2.5 months later. In the meantime, they receive standard care for their conditions. As transient psychological problems can be expected to have resolved, this approach will ensure that only patients with more persistent symptoms are included into the cohort. Therefore, only patients eligible at both initial screening and rescreening remain in the study. These estimated 450 patients receive the set of baseline questionnaires (see Table 2) plus subsequent telephone or video interview. In addition, more details on their medical status will be obtained from the treating physicians (especially GP and/or hospital) and/or electronic medical records (EMR). After the baseline assessment, patients will be invited to participate in the embedded RCT and, if consenting, sign a second informed consent (PIC2). Those who decline RCT participation remain in the cohort. We will continue screening until we reach n = 300 patients in the RCT and approximately n = 150 in the cohort. Optimally, one informal carer per patient will additionally participate, recruited after the baseline visit has been performed.
| Instrument | Category of information | |
|---|---|---|
| Screening | Inclusion/exclusion criteria | |
| Current diagnoses (ICD) | ||
| Hospital Anxiety and Depression Scale (HADS, 14 items) | Anxiety, depression, distress | |
| Patient characteristics | Medical history and information extracted from medical records | |
| Demographic variables | ||
| Barthel index,63, 64 10 items | Physical/cognitive status, adherence, exercise | |
| Tests | MoCA blind (mild cognitive impairment, 15 items)65 | |
| 30-sec chair stand test (30s-CST, 1 item)66 | ||
| Self-report Questionnaires for patients | EQ-5D-5L (incl. EQ-VAS + verbal explanation, 6 items)67, 68 | Quality of life |
| Kansas City Cardiomyopathy Questionnaire (KCCQ-12, 12 items)69 | ||
| Global Perceived Effect on Quality of life,70, 71 1 item | ||
| Self-reported frailty72–5 items | Physical/cognitive status, adherence, exercise | |
| Subjective self-report questions on cognitive impairment,73 2 items | ||
| PROMIS Physical Function Short Form 4,74 4 items | ||
| Brief version of the International Physical Activity Questionnaire for the elderly (IPAQ-E, 7 items)75 | ||
| Medication Adherence Report Scale (MARS-5, 5 items)76 | ||
| Table of medical conditions (respecting and allowing calculation of comorbidity burden using the Charlson Co-morbidity Index77, 78 and incl. NYHA,79 39 items) | Medical conditions and symptoms incl. CMSAS | |
| Table of symptoms _ respecting Charlson Comorbidity Index (CMSAS, 21 items) | ||
| Mental health symptoms (Patient Health Questionnaire-4, PHQ-4, 4 items)80 | ||
| Medical Consumption Questionnaire (iMCQ)81 plus 5 items of Productivity Cost Questionnaire (iPCQ),29 36 items | Cost-effectiveness and cost-utility | |
| Questionnaire on perceived social support (ESSI, 5 items)30, 82 | Moderators: Resources and loneliness | |
| UCLA Three-Item Loneliness Scale,83, 84 3 items | ||
| General Self-Efficacy Scale (GSE, 3 items)85, 33 | ||
| Spirituality (FACIT Sp-12, 12 items)32 | ||
| BRIEF Health Literacy Screening Tool,31 4 items | ||
| Patient Acceptable Symptom State (PASS, 2 items)70, 71 | Moderators: Illness perception and expectations | |
| Illness Perception Questionnaire (Brief IPQ, 9 items)86 | ||
| Treatment expectations (TEX-Q short form, 6 items)87 | ||
| Multimorbidity Treatment Burden Questionnaire (MTBQ, 10 items)88 |
Stress and treatment burden |
|
| Perceived Stress Scale (PSS-4, 4 items)89, 90 | ||
| Quality indicators for quality of care, 12 items | ||
| Patient satisfaction questionnaire (custom), 4 items | Assessment of BCC-intervention aspects | |
| Working Alliance Inventory (WAI),91 12 items | ||
| Questionnaires for informal carers | Carer QoL 7D,92 7 items | |
| EQ-5D-5L (incl. EQ-VAS + verbal explanation, 6 items)67, 68 | ||
| Caregiver Burden Inventory (CBI, 24 items)93 | ||
| Carer Satisfaction with Treatment (custom) - 4 items | ||
| Satisfaction with Life Scale (SWLS, 5 items)94 | ||
| UCLA Three-Item Loneliness Scale, 3 items83, 84, 95 | ||
| Perceived emotional social support (ESSI, 5 items)30, 82 | ||
| Questions for GPs | Quality indicators for quality of care, via online survey, 12 items |
- EOT, end of treatment; FU, follow-up; GP, general physician; RA, research assistant; SN, study nurse.
- This table lists the data collected in the ESCAPE study and the instruments and means used for data collection.
- Care managers can do the baseline assessment; however, as they are not blinded they cannot do the EOT and FU assessments. Questionnaires will be sent to the patients, SN/RA will fill in the remaining points together with the patient at the phone/video visit.
To ensure timely recruitment and enhance recruitment efficiency, recruiters receive comprehensive training (see previous studies96, 97). Agreements about recruitment targets and milestones have been established with each recruiting site.
Study procedures
Patients randomized to the intervention group receive the ESCAPE BCC intervention in addition to their UC by physicians and others. Patients randomized to the control group and those remaining in the cohort receive UC for all their conditions and a leaflet summarizing their individual psychosocial, behavioural, and medical risk factor status but no other study-specific intervention. In addition, all patients in cohort and RCT receive access information to online health content on the ESCAPE website (see below) with Internet-based information (or directions) relevant for life with and treatment of their diseases.
Main assessments will take place for all patients at baseline, 9 months [end of treatment visit (‘EOT’)], and 18–33 months [follow-up (FU)], depending on date of recruitment (Table 3). Prior to the visits, patients are asked to fill in psychological questionnaires, online or mailed with paper/pencil, and to provide (possibly via GP or EMR) recent medical documents. The assessors ascertain completeness of the self-report data during the subsequent telephone interview belonging to the visit and ask for medical history, adverse events and healthcare utilization since the last visit. Assessment of medical status and risk factors at baseline is based on self-report and information from the GP, hospital, and/or EMR, as applicable.
| PERIODS | Prescreeninge and registration | Screening | Rescreening | Baseline ± randomization (‘V0’) | End of treatment (‘V-EOT’) | Follow-upf (‘V-FU’) |
|---|---|---|---|---|---|---|
| Time from baseline | −3 mo (± 2 weeks) | −14 days (± 2 weeks) | Day 0 | +9 mo (± 2 weeks) | +18 to 33 mo (±2 weeks) | |
| RCT | x | x | x | x | x | x |
| Cohort | x | x | x | x (no randomization) | x | x |
| Letter from GP to patient to initiate contact (subgroup only) | x | |||||
| PIC 1 - screening process and cohort | x | |||||
| PIC 2 - RCT | x | |||||
| PIC 3 - for informal carer | x | |||||
| Current diagnoses | x | xa | xa | xa | xa | xa |
| HADS, risk factors, inclusion and exclusion criteria | x | x | xb | |||
| Demographics of patient and informal carer | x | x | x | |||
| Medical history incl. cardiovascular and behavioural risk factors, treatment, and medication | x | xa | xa | |||
| Self-report questionnaires for patients incl. HADS | xc | xc | xc | |||
| Questionnaires for informal carer | x | x | ||||
| Randomization | x | |||||
| Adverse eventsd | x | x | x | |||
| Feedback on RCT (patient and carer) | x | x | ||||
- EOT, end of study treatment; FU, follow-up; GP, general practitioner; mo, month/s; PIC, patient-informed consent; RCT, randomized controlled trial.
- This table gives an overview over the time schedule of the ESCAPE study.
- a Full medical history incl. diagnoses and demographics will be obtained at baseline only. Only relevant changes from baseline will be documented during later assessments.
- b In case of a baseline visit >2 weeks after rescreen, the HADS risk factors and inclusion and exclusion criteria will be repeated.
- c Psychometric battery: see Table 2
- d Severe intercurrent medical events have to be documented as SAEs in the eCRF as outlined in section 16.
- e Prescreening on cardiology wards will be a personal contact visit, all other visits will be performed via phone or video call.
- f Data on intervention participation and process measures obtained during care management (intervention group only) will not be documented in the eCRF but in a separate pseudonymized registry. At the end of the study, these data will be converged with the trial database.
Additionally, informal carers of all patients, if possible, are asked for their informed consent and assessed at baseline and month 9 (EOT).
For patients who withdraw their consent for study participation, the study site or the initiator of the trial will request information on the survival status and—if applicable—date of death from the GP, a relative, or the residents' registration office at the end of the trial.
BCC intervention
The ESCAPE intervention is based on the BCC concept: a patient-centred team-based treatment strategy that promotes proactive, timely follow-up and support by a trained care manager (CM). The ESCAPE BCC strategy combines the framework from US trials20, 22, 98, 25 with own experiences from German multiprofessional psychosomatic treatment,26 family medicine,27 and cardiac rehabilitation.28 It also incorporates experiences from the ongoing TEACH trial,34, 35 testing a BCC intervention in German patients with coronary heart disease, elevated distress levels, and insufficiently controlled cardiovascular risk factors. Before the beginning of the trial, we involved patients and their informal carers and patient-representative organizations based on a participatory design in the development of trial protocol, choice of questionnaires, and the BCC intervention36, 37 and tested the practicability in a small feasibility study.
After the patient's automated randomization to the intervention group, an electronic record is automatically created in the care management ‘registry’ with all relevant medical, psychological, and sociodemographic information collected at the RCT baseline assessment. To prevent unblinding of blinded personnel (study physician, follow-up assessors), the sites CM(s) receive(s) an email to be informed about the allocation. The CM subsequently contacts the patient to explore their treatment preferences and barriers. All CM–patient conversation takes place via telephone or video- call. Based on a meta-algorithm for multi-morbidity (MAM),23 the CM collects any pertinent medical information that is then sent to the patient's GP who corrects, expands, and confirms the information and suggests a treatment plan considering current guidelines, the patient's medical history, preferences, values, and life goals. Critical signs or symptoms for severe health conditions, that is, so-called ‘red flags’, as, for example, angina pectoris, ankle oedema, or breathlessness, which should be regularly monitored, are highlighted. Subsequently, the CM and the patient agree in a shared decision manner38 on two to three goals in alignment with the treatment plan, for example, increase physical activity and self-monitoring of weight gain. During the next months, the CM regularly contacts the patient to educate them about the conditions and treatment and monitor bothersome symptoms and ‘red flags’. They provide support in goal attainment and integration of health behaviour and self-management into the patient's daily routine crucial for secondary prevention. They impart skills to cope with psychological burden, communicate across providers, and may encourage using community resources. CMs ensure that treatment agreements are followed and patients make progress with respect to their goals. If the patient so wishes, CMs also include informal carers in the discussion and support of patients' goals. CMs collaborate closely with their patients' GPs and update them on their patients' progress at regular intervals.
The CMs contact their patients as part of the intervention over 9 months, approximately two calls per month during the first 3–4 months, later one call per month. They alert GPs immediately if any concerning or safety issues arise and update the GPs on the patients' progress regularly. At each patient contact, the CM enters relevant patient information to document progress and critical medical changes (e.g. red flags, worsening of symptoms, hospitalizations, changes in medications, vital symptoms, or lab results) into the registry (see below), which serves to guide CMs through their patient contacts. The registry is also used to document MAM feedback by the GP and to incorporate all relevant medical information, treatment plans, and personalized goals and to facilitate the standardization of CM-patient encounters across all sites.
A clinical specialist team for each country consisting of a GP, cardiologist, pharmacist/clinical pharmacologist, and a mental health specialist (psychologist/ psychosomaticist/psychiatrist) supervises the CMs and makes treatment recommendations. During regular (online) case review meetings, the CMs present their patients to the clinical specialist team using the care management registry. The clinical specialist team (i) oversees that patients receive evidence-based treatment for all their conditions and distress and the initial patient-centred treatment plan is followed; (ii) assists CMs in addressing treatment barriers and supporting the patient and carer with their behavioural changes; and (iii) makes guideline-based treatment recommendations and monitors for possible negative effects of polypharmacy or drug–drug interactions. After a discussion, one or more recommendations are formulated that may pertain to (i) suggestions for clinical clarification, (ii) adjustments in pharmacotherapy according to STOPP/START2 criteria,39 and (iii) any interventions directly delivered or encouraged by the CM. Afterwards, the CM discusses the recommendations with the patient and carer and informs the GP. The GP remains responsible for the patient's care and can decline or modify the specialist team's recommendations. An international specialist team will meet once a month with a designated representative from each local specialist team to discuss specific medical, psychological, or procedural and protocol questions. In addition, CMs and specialist teams can communicate to discuss with the international specialist team to ensure consistency across all sites.
CMs have an officially recognized training as healthcare professionals with experience in patient care in general practice, mental health services, or cardiology. They must be able to work in a team. At the beginning of the trial, all CMs participated in several training sessions where they were instructed in the components of care management, communication skills including shared decision-making, motivational interviewing, and problem-solving techniques40 and the use of the MAM and the registry.
Furthermore, there will be regular refresher trainings that incorporate frequent questions, barriers, and reinforcement of communication skills. Before the start of the trial, we developed a standardized intervention manual and CM training materials adapted from materials published with earlier studies18, 20 or developed during our own studies22, 34, 35 and an ESCAPE feasibility trial. The intervention manual adhering to TIDieR guidelines41 will be published separately including detailed standard operating procedures (SOPs) and training materials for CMs and treatment teams available in all languages. The materials will be regularly updated based on the requirements of the various healthcare systems and necessary adaptions during the intervention phase to ensure cohesiveness across clinical practices and to allow for later widespread implementation.
To ensure the fidelity of the BCC intervention, we will use the National Institute of Health Behaviour Change Consortium Framework (NIH BCC)42, 43 that includes five domains of treatment fidelity (Study Design, Training, Delivery, Receipt, Enactment). Hence, for each patient, we will collect detailed information on type and content of treatment, length of contact, duration of contact over time, etc. This information will be combined with the effectiveness data to (i) identify moderators and mediators of treatment effectiveness, (ii) update and validate quality indicators for multi-morbidity care, (iii) identify barriers to treatment, and (iv) inform implementation across various healthcare systems.
UC
Due to the international nature of the ESCAPE trial, UC varies across the sites. For all sites, UC is based on national and/or European guidelines for all relevant conditions. Typically, UC entails follow-up at the GP, though some patients are also seen by specialists in private practice or in the hospitals' specialist clinics or departments.
Measures
Baseline and follow-up medical information is obtained from detailed patient interviews and medical reports, from which diagnoses and disease severity information (such as echocardiographic measures of systolic and diastolic cardiac function, natriuretic peptide levels, etc.) are documented if available. During the visits, we question the patients with regard to their NYHA class.
All self-rating scales have been validated in at least one language. Validated translations are used as far as possible and will be scored objectively by established algorithms reported in the literature. For rating scales without validated local-language version, translations were made using the EU-translator programme44 combined with review of the translation by the local investigators and a test of the translated version with two to three native speakers to assure correct understanding (translated versions are available from the first author on request).
HRQoL is the primary outcome. HRQoL is a comprehensive patient-centred measure of subjective health, covering the overall impact of somatic and mental health conditions on patients' lives. HRQoL is recommended as a core outcome for several chronic conditions and constitutes a main component of the Core Outcome Set for Multimorbidity Research.45 It is particularly well suited for evaluating the BCC approach in ESCAPE that addresses the whole range of multi-morbidity that is not reflected sufficiently in any single biomarker or specific symptom scale.
The EQ-5D-5L67, 68 will be used to measure overall HRQoL. The EQ-5D-5L index (primary outcome) covers the dimensions mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. It has been used in numerous studies across Europe, including in multi-morbid46, 47 and elderly48, 49 populations. It has been validated in numerous languages, shows acceptable sensitivity to change, and can also be used to evaluate cost-effectiveness. The EQ-5D-5L also includes a visual analogue scale (EQ-VAS, 0–100) to measure the global subjective health status, which will be used to determine a study-specific cut-off for minimal important change for specific scores and estimate the number of patients per group that has experienced a clinically important improvement.70, 71 To assess QoL aspects specifically related to life with HF, we will use the Kansas City Cardiomyopathy Questionnaire (KCCQ-12).69
Using the Hospital Anxiety and Depression Scale (HADS),50, 51 we will assess patients' levels of psychological distress. The HADS has been used in numerous medical studies, including patients with heart disease52 and yields separate scores for anxiety and depression. The total score (range 0–42) serves as accepted measure of overall psychological distress; a score >12 is expected to detect at least mild levels of distress shown to be prognostically relevant.
The secondary outcomes will cover objective medical endpoints, resource use and costs (for analysis of cost-effectiveness and cost utility), patient- and carer-reported outcomes covering specific areas of functioning, activity and well-being, and information about mediating and moderating factors. For details, see Table 2 and Appendix S2.
Outcome variables from the clinical study will be used to validate the quality indicators describing the quality of care for patients with multi-morbidity proposed by the MULTIqual project.53-55 The indicators capture whether and to what extent care processes with relevance to multi-morbidity have been implemented.
Trial status
Between September 2021 and June 2022, nine patients participated in a feasibility study in Göttingen involving the work groups responsible for organizing the RCT and for developing the BCC intervention, the local CM, and the German specialist team. Qualitatively, the study procedures appeared feasible, and the patients reported they benefited from the intervention.
Recruitment for the ESCAPE main study started at the coordinating site in Göttingen, Germany, in April 2022 with other sites following in subsequent months. The first patient was randomized in July 2022.
Patient data storage and protection
Documentation of patient data will be handled according to the regulations outlined in the CONSORT statement.56 Patient and informal carer data necessary for study evaluation are entered through an electronic case report form (eCRF) and stored in a GCP-compliant secuTrial® database. For details, see Appendix S2.
The intervention is supported by the imergo® e-health Integrated Care Platform (ICP) developed by the Digital Health department of the Fraunhofer Institute for Applied Information Technology FIT. For an impression of imergo®, see Figure 2; for more details, see Appendix S2.
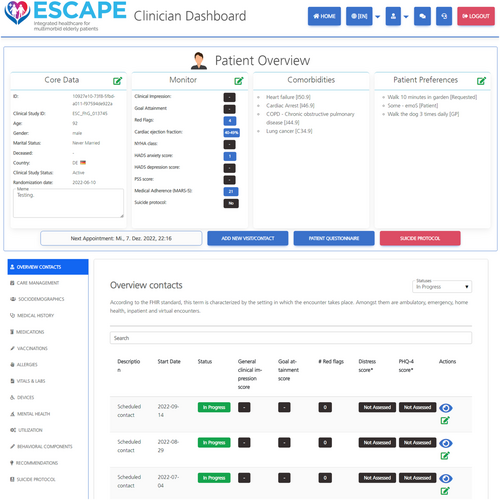
Quality control and monitoring
A Data Safety and Monitoring Board (DSMB) monitors the course of the study and can give a recommendation for study discontinuation, modification, or continuation. The principles for the DSMB are ethical and safety aspects for patients.
Quality control and quality assurance are ensured through monitoring according to ICH/GCP and internal SOPs following a risk-adapted and study specific monitoring strategy. For complete information, see Appendix S2.
Ethical and regulatory requirements
The study is conducted according to the Declaration of Helsinki. All patients and informal carers receive written information about the study describing the cohort study and the RCT and will only be included into the trial upon their written consent. The informed consent forms (PIC1: consent for screening and cohort; PIC2: consent for RCT; PIC3: consent for carer assessments) and the study protocol (current version in Göttingen V1.2/16 February 2022, No. 02853) have been approved by the responsible ethics committees of all participating study sites before inclusion of the first patient.
Adverse events and risks of participation in the trial
BCC as addition to standard care has been tested in several RCTs, and implementation studies in the USA have demonstrated safety and acceptance.20, 21 No differences in SAE or re-hospitalization rates between BCC and UC groups have been found in previous studies.16, 20 Therefore, we do not anticipate relevant study-specific risks for the patients. For details, see Appendix S2.
Statistical analysis
Patients are allocated to intervention using a 1:1 allocation ratio. The randomization procedure is stratified by gender, frailty, and country and based on permuted blocks of varying length and implemented within the secuTrial® platform.
Sample size calculation
A sample size of 130 patients per group yields a power of 80% at the usual two-sided significance level of 5% given a standardized treatment difference (Cohen's d) of 0.35. The assumed treatment difference is supported by a meta-analysis of RCTs testing collaborative care for depression in patients with heart disease that found the interventions effective for improving depressive symptoms [standardized mean difference (SMD) = −0.31], anxiety (SMD = −0.36), and mental QoL (SMD = +0.23). Furthermore, the only RCT using the EQ-5D as measure of global HRQoL in a collaborative care trial with cardiac patients57 found an effect size of 0.34. Adjusting for premature discontinuation of the study by about 15% of the patients over a period of 9 months from randomization, we aim to recruit a total of 300 patients into the RCT. Based on the literature and experiences from clinical trials conducted at the participating sites, we estimate that about 40% of the patients in the initial cohort will be eligible with about two-thirds of those eligible after the second screening deciding to participate in the RCT.34, 58, 59 Therefore, we anticipate that we need an initial cohort size of about 750 patients with approximately 450 patients remaining for long-term follow-up, including 300 patients willing to be randomized into the RCT.
Methods of analysis
The primary analysis of the RCT will follow the intention-to-treat (ITT) principle. The primary endpoint will be analysed using an analysis of covariance (ANCOVA) with baseline assessment of the primary outcome as covariate and stratification variables of the randomisation as factors. Missing data will be dealt with by multiple imputation. The analyses of the secondary endpoints will follow the same lines as the analysis of the primary endpoint, where appropriate. Recurrent events, such as hospitalizations, will be modelled by negative binomial regression accounting for between-patient heterogeneity and varying follow-up length of up to 30 months. Time to event outcomes such as death will be analysed using Cox proportional hazards models. Pre-planned subgroup analyses will include analyses by gender and frailty status. Sensitivity analyses will explore potential clustering (by CM) effects in hierarchical (multilevel) models.
Because the RCT is embedded in a cohort study, the external validity of the RCT can be assessed in terms of both baseline characteristics and outcomes.60 In addition, the cohort will allow the extrapolation of the intervention effect to the ‘real-world’ cohort reflecting routine care using the framework of a Bayesian hierarchical model.61 Furthermore, the cohort provides an opportunity to investigate prognostic factors for the natural disease course using regression models appropriate for the outcome scale. Details of the statistical analysis will be prespecified in a statistical analysis plan, which will be finalized prior to end of recruitment.
For the health economic evaluation, objective outcomes of interest will be verified by patients' medical records, health insurance data or self-report questionnaires for patients (iMCQ). A cost-effectiveness analysis (CEA) and a cost utility (CUA) will be performed with sub-group analyses for (i) depression vs. no depression and (ii) cardiovascular disease (CVD) vs. no CVD co-morbidities. Because benefit of the BCC approach in terms of HRQoL might not be reflected in a gain of quality-adjusted life years, country-specific CEA will be performed for countries with data on costs for a sufficient number of patients per group. The incremental cost-effectiveness ratios (ICER) will be calculated from a payer perspective. For patients with symptoms of depression, the costs per depression-free days (DFD) will be calculated with DFDs obtained using the iMCQ and HADS. For all patients, cost-effectiveness will also be measured in costs per avoided hospital admission. CUA will be calculated for specific countries using costs per QALY obtained from EQ-5D-5L taking into account different valuation algorithms.
Discussion
ESCAPE has developed an adapted biopsychosocial BCC intervention for older patients with somatic–mental multi-morbidity and will examine its impact of patients's HRQoL compared with UC. The intervention extends research on HF case management62 or BCC interventions for patients with cardiac–mental co-morbidity16, 20 to a highly vulnerable patient group in advanced age and with multiple physical and mental conditions. By fostering collaborations between patients and their informal carers, GPs, and treating medical specialists, ESCAPE offers a framework for addressing the complexity of multi-morbidity and its treatment in a team-based, patient-centred approach that overcomes paralleled single condition care. A recently developed meta-algorithm for multi-morbidity will support a targeted treatment plan developed through shared decision-making and integrating guideline-based treatment targets and patient preferences. The specifically designed imergo® patient registry with CM dashboard provides an infrastructure for handling the complex medical and psychosocial data needed for personalized recommendations and real-time support and monitoring of the treatment progress. Regular case reviews by multidisciplinary clinical specialist teams guarantee guideline adherence, goal attainment, and treatment fidelity. By adding pharmacological expertise to the specialty team, inappropriate prescribing and drug–drug interactions can be minimized. An overarching BCC approach will harmonize the work of local specialist teams including a pharmacologist and add expertise in additional medical areas.
Patients will thereby receive integrated treatment advice based on the best available evidence and their personal preferences, values, and life goals. They will receive reliable support for living with the physical and mental challenges of multi-morbidity, empowering them for improved self-management, disease coping, and utilization of community resources. Formal and informal carers are supported in caring for the complex needs of the patients and communicating with each other.
Taken together, the ESCAPE approach is intended to improve the quality of life of both patients and informal carers and the quality of care delivered by the treating physicians, leading to improved health outcomes and potentially cost savings to society. If proven successful, ESCAPE will provide recommendations and pathways adaptable for routine care, which could guide updates of existing guidelines to facilitate implementation across Europe. Our results could serve as a model for the treatment of various multi-morbidities. Nevertheless, varying UC across sites due to the international nature of the ESCAPE trial, an inhomogeneous population due to various recruitment pathways in different countries and varying compositions of diseases in multi-morbid patients will challenge the proof of concept. This challenge however has a great potential and the chance to develop a differentiated team-based treatment approach for patients with multi-morbidity and their carers, which may be useful for broad patient populations across various countries and healthcare systems.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The following authors declare conflicts of interest: Christoph Herrmann-Lingen, Susanne S. Pedersen, Søren T. Skou, Christian Albus, Dagmar Lühmann, Tim Friede, Niels E. Bruun, Jens Søndergaard. For details, see Appendix S2.
The following authors declare they have no conflicts of interest: Christine Zelenak, Jonas Nagel, Kristina Bersch, Lisa Derendorf, Frank Doyle, Birgit Herbeck Belnap, Sebastian Kohlmann, Carlos A. Velasco, Thomas Asendorf, Christian Axel Bang, Margarita Beresnevaite, Matthew M. Burg, Sussi Friis Buhl, Peter H. Gæde, Anna Markser, Klaudia Vivien Nagy, Chiara Rafanelli, Sanne Rasmussen, Jan Sørensen, Adrienne Stauder, Stephanie Stock, Stefano Urbinati, Diego Della Riva, Rolf Wachter and Florian Walker.
See Appendix S2 for information on role of funders and legal sponsor in study design, trial governance, trial registration, data safety and monitoring board, ethics advisory board, availability of data and materials, and declaration of interest.
Funding
 This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 945377 (ESCAPE). This output reflects the views of the authors, and the European Commission is not responsible for any use that may be made of the information contained therein.
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 945377 (ESCAPE). This output reflects the views of the authors, and the European Commission is not responsible for any use that may be made of the information contained therein.
The University of Göttingen Medical Center serves the legal role of a study sponsor. We acknowledge support by the Open Access Publication Funds of the Göttingen University.




