Are arrhythmias the drivers of sudden cardiac death in heart failure with preserved ejection fraction? A review
Abstract
In patients with heart failure with preserved ejection fraction (HFpEF), sudden cardiac death (SCD) accounts for approximately 25–30% of all-cause mortality and 40% of cardiovascular mortality in properly adjudicated large clinical trials. The mechanism of SCD in HFpEF remains unknown but thought to be driven by arrhythmic events. Apart from atrial fibrillation, which is prevalent in approximately 45% of HFpEF patients, the true burden of other cardiac arrhythmias in HFpEF remains undetermined. The incidence and risk of clinically significant advanced cardiac conduction disease with bradyarrhythmias and ventricular arrhythmias remain less known. Recommendations have been made for long-term cardiac rhythm monitoring to determine the incidence of arrhythmias and clarify mechanisms and mode of death in HFpEF patients. In animal studies, spontaneous ventricular arrhythmias and SCD are significantly elevated in HFpEF animals compared with controls without heart failure. In humans, these studies are scant, with a few published small-size studies suggesting an increased incidence of ventricular arrhythmias in HFpEF. Higher rates of clinically significant conduction disease and cardiac pacing are seen in HFpEF compared with the general population. Excepting atrial fibrillation, the predictive effect of other arrhythmias on heart failure hospitalization, all-cause mortality, and precisely SCD remains unknown. Given the high occurrence of SCD in the HFpEF population, it could potentially become a target for therapeutic interventions if driven by arrhythmias. Studies to address these knowledge gaps are urgently warranted. In this review, we have summarized data on arrhythmias and SCD in HFpEF while highlighting avenues for future research in this area.
Introduction
Heart failure resulting from several aetiologies is now considered a global pandemic, affecting more than 64 million people worldwide.1 In the Western world where ascertainment of cases is fairly feasible, the incidence and prevalence of heart failure is increasing, resulting in enormous and rising cost implications.2, 3 In the USA, for example, the annual median costs for heart failure care are estimated at ~$25 000 per patient.4 Heart failure is currently clinically classified based on a single measure of cardiac contractility, the left ventricular ejection fraction (LVEF). Heart failure with preserved ejection fraction (HFpEF) is defined with LVEF ≥50%, contrasted with heart failure with borderline or mid-range ejection fraction (HFmrEF) where LVEF is 41–49%, and heart failure with reduced ejection fraction (HFrEF) where LVEF is ≤40%.5, 6 HFpEF currently represents approximately 50% of all heart failure patients; the proportion of HFpEF is increasing and will soon become the predominant form of heart failure.6-8
Apart from atrial fibrillation (AF),9, 10 the true burden of arrhythmias in HFpEF remains unknown but is likely high and possibly contributing to increased morbidity and reduced survival in this patient group. The predictive effect of arrhythmias on heart failure hospitalization, all-cause mortality, and sudden cardiac death (SCD) in HFpEF also remains unknown. Identifying the specific HFpEF phenotypes at higher risk of life-threatening arrhythmias and SCD could lead to evidence-based interventions to reduce arrhythmia-induced morbidity and mortality in HFpEF. About a quarter or more of HFpEF patients suffer SCD as mode of death. Unlike HFrEF where most cases of SCD are due to ventricular arrhythmias, the proportion of patients dying from malignant arrhythmias among SCD in HFpEF patients remains unknown.11, 12 The mechanisms (arrhythmic vs. other causes) driving fatal events in HFpEF especially SCD are not well known. Mapping the natural history of HFpEF with respect to arrhythmias using longitudinal data appears to be urgently required. Death and hospitalizations resulting from arrhythmias would be a promising target for therapeutic intervention once we know their burdens and predictive effects on these hard outcomes. The objective of this review is to summarize the available data on arrhythmia in HFpEF especially with reference to SCD.
High morbidity and mortality in HFpEF including SCD
Although declining, the 30-day re-hospitalization rates following admission for heart failure are still approximately 20% in developed countries.6, 13-15 Despite improvements in survival, and irrespective of advances in therapy, the prognosis of heart failure remains poor with median survival of 2 years, and high 5-year absolute mortality rates of 50–75%, and significantly greater compared with heart failure-free individuals.2, 7, 16, 17 The prognosis of HFpEF subgroup is equally poor with a median survival of 2 years and 5-year mortality of ~75%.7 The significant morbidity with loss of economic productivity, the high mortality, and the astronomical cost implications associated with the management of heart failure have rendered it a high-priority area for policymakers, clinicians, and researchers.2, 5, 6 Therefore, novel clinical research perspectives are still warranted. All-cause and cardiovascular mortality are significantly elevated in HFpEF patients compared with matched controls without heart failure.18-20 The risk of all-cause mortality in HFpEF patients is two to four times that of controls without heart failure and normal left ventricular systolic function.21-23
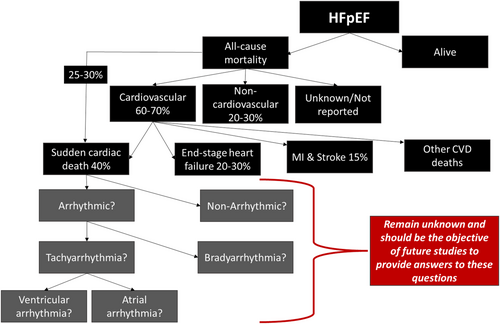
In randomized controlled trials (RTCs), majority of deaths in HFpEF patients were cardiovascular (~60–70% of total deaths), whereas 20–30% were due to non-cardiac causes. Observational epidemiological studies, however, report lower proportions of cardiovascular deaths (∼50–60%) in HFpEF patients when compared with RCTs.12 Case definition of mode of death in HFpEF patients is hampered by semantics as some studies use sudden death (SD), whereas others use SCD, but closer review revealed that the criteria used to defined SD and SCD in various clinical trials are similar.12 Therefore, SD and SCD are considered synonymous with respect to HFpEF, based on data from various studies. In this review, we will be using the term SCD. In HFpEF patients, SCD accounted for approximately 25–30% of all mortality and 40% of cardiovascular mortality, whereas worsening heart failure accounted for 20–30% of cardiovascular deaths, and MI and stroke accounted for a minority of cardiovascular deaths (each ~5–15%)12, 18, 20 (Figure 1). Conversely, in HFrEF patients, 80–85% of deaths are due to cardiovascular causes, with sudden death accounting for about ~45% of cardiovascular deaths and worsening heart failure ~25%.12, 20, 24 Cardiac dysrhythmias are responsible for majority of SCD in patients with HFrEF.25, 26 Ventricular arrhythmias are present at the time of death in 76% of SCD in patients implanted with an implantable cardioverter defibrillator (ICD).27 Unlike HFrEF where most of the cases of SCD are due to malignant ventricular arrhythmias [ventricular tachycardia (VT) and ventricular fibrillation (VF)], the proportion of HFpEF patients with SCD due to malignant arrhythmias remains unknown but is probably high.11, 12 The mechanisms driving fatal events in HFpEF especially SCD remain elusive. Mapping the natural history of HFpEF with respect to arrhythmias using longitudinal data is strongly required. Malignant tachy (or brady) arrhythmias may be important causes of SCD, but we just do not know. This is important as a high-risk group could exist where an ICD or a permanent pacemaker may be of benefit.
Treatment options in HFpEF are limited compared with HFrEF
Clinical trials have shown morbidity and mortality benefits of several pharmacotherapies and cardiac implantable electronic devices (CIEDs) like ICD and cardiac resynchronization therapy (CRT) implantations in HFrEF patients, leading to established guidelines for HFrEF management.5, 6, 28 Unfortunately, as shown in Table 1, major randomized controlled clinical trials have failed to show significant mortality beneficial effects of pharmacological therapy in HFpEF patients.29-47 Though observational studies seem to suggest some mortality benefit of renin–angiotensin blockade in HFpEF, RCTs did not show significant all-cause mortality benefit or reduction in heart failure hospitalization.40 Although beta-blockers are an established part of the pharmacological armamentarium in HFrEF, randomized clinical trials in HFpEF patients have not shown any significant reduction in all-cause mortality or heart failure hospitalization,36, 47 despite meta-analysis of observational studies suggesting some reduction in all-cause mortality associated with beta-blocker use in HFpEF.41, 48, 49 Mineralocorticoid receptor antagonists use did not significantly reduce the occurrence of the primary endpoint at intention-to-treat analysis of the TOPCAT trial or Aldo-DHF in HFpEF patients.38, 39 The DIG ancillary trial did not show any benefit of digoxin on HFpEF with respect to total mortality and all-cause mortality or cardiovascular hospitalizations when compared with placebo.32 In the PARAGON-HF trial, angiotensin receptor-neprilysin inhibitor (ARNI) did not significantly reduce hospitalizations for heart failure and death from cardiovascular causes in HFpEF.42
| Study | Year, size, and population | Randomization (study arms) | Median follow-up | Primary outcomes | Resultsa |
|---|---|---|---|---|---|
|
CHARM-Preserved Yusuf et al.29 |
2003 N = 3023, HFpEF |
Candesartan vs. placebo | 36.6 months | CVD death or HF hospitalization | HR 0·86 (0·74–1·00) P = 0.051 |
|
SENIORS Flather et al.30 |
2005 N = 2128, HFpEF |
Nebivolol vs. placebo | 21 months |
- All-cause mortality or CVD hospitalization - All-cause mortality |
- HR 0.86 (0.74–0.99) P = 0.039 - HR 0.88 (0.71–1.08) P = 0.21 |
|
PEP-CHF Cleland et al.31 |
2006 N = 850, HFpEF |
Perindopril vs. placebo | 2.1 years | All-cause mortality and HF hospitalization | HR 0.919 (0.700–1.208) P = 0.545 |
|
DIG ancillary trial Ahmed et al.32 |
2006 N = 988, HFpEF |
Digoxin vs. placebo | 37 months | HF hospitalization or HF mortality | HR 0.82 (0.63–1.07) P = 0.136 |
|
I-PRESERVE Massie et al.33 |
2008 N = 4128, HFpEF |
Irbesartan vs. placebo | 49.5 months | All-cause mortality or CVD hospitalization | HR 0.95 (0.86–1.050) P = 0.35 |
|
HK-DHF Yip et al.34 |
2008 N = 150, HFpEF |
Diuretics alone vs. diuretics plus irbesartan vs. diuretics plus ramipril | 1 year | Recurrent hospitalization | Rates were similar in all groups (12.2%, 11.1%, 11.4%) |
|
ALL-HAT Grimm et al.35 |
2009 N = 1479 with heart failure (LVEF >35%) |
- Lisinopril vs. chlorthalidone - Amlodipine vs. chlorthalidone - Doxazosin vs. chlorthalidone |
1 year | HF hospitalization |
- OR 1.33 (0.65–2.74) P =0.44 - OR 1.08 (0.53–2.21) P =0.83 - OR 1.33 (0.65–2.74) P =0.44 |
|
J-DHF Yamamoto et al.36 |
2013 N = 245, HFpEF |
Carvedilol vs. no carvedilol | 3.2 years | CVD death and HF hospitalization | HR 0.902 (0.546–1.48) P = 0.6854 |
|
RELAX Redfield et al.37 |
2013 N = 216, HFpEF |
Sildenafil vs. placebo | 24 weeks |
- Changes in VO2 max - Changes in 6MWT distance |
- Changes in VO2 max P =0.90 - Changes 6MWT P = 0.92 |
|
Aldo-DHF Edelmann et al.38 |
2013 N = 422, HFpEF |
Spironolactone vs. placebo | 12 months |
- Diastolic function (E/e′) - Peak VO2 (mL/min/kg) |
- −1.5 (−2.0 to −0.9) P = 0.001 - +0.1 (−0.6 to +0.8) P =0.8. |
|
TOPCAT Pitt et al.39 |
2014 N = 3445, HFpEF |
Spironolactone vs. placebo | 3.3 years | CVD death, aborted SCA, or HF hospitalization | HR 0.89 (0.77–1.04) P = 0.14 |
|
EMPA-REG OUTCOME Zinman et al.50 |
2015 N = 7020 (T2DM + CVD risk) |
Empagliflozin vs. placebo | 3.1 years | CVD death, non-fatal MI or non-fatal stroke | HR 0.86 (0.74–0.99) P = 0.04 for superiority |
|
Meta-analysis Bavishi et al.48 |
2015 N = 27 099 (17 studies), HFpEF |
Beta-blocker vs. placebo/no beta-blocker | At least 1 year |
Observational studies: - All-cause mortality - HF hospitalization |
- RR 0.81 (0.72–0.90) P < 0.001 - RR 0.79 (0.57–1.10) P > 0.05 |
|
RCTs only: - All-cause mortality - HF hospitalization |
- RR 0.94 (0.67–1.32) P = 0.72 - RR 0.90 (0.54–1.49) P = 0.68 |
||||
|
Meta-analysis Khan et al.40 |
2017 N = 17 284 (13 studies), HFpEF |
ACEI or ARB vs. placebo or standard therapy | 24.8 months |
All-cause mortality -RCTs only -Observational studies |
- RR 1.02 (0.93–1.11) P = 0.68 - RR 0.91 (0.87–0.95) P = 0.005 |
|
Meta-analysis Zheng et al.41 |
2017 N = 18 101 (25 RCTs), HFpEF |
- Beta-blocker vs. placebo - ACE-Is vs. placebo - ARBs vs. placebo - MRA vs. placebo |
- | All-cause mortality |
- RR: 0.78 (0.65–0.94) P = 0.008 - RR: 1.10 (0.85–1.43) P = 0.46 - RR: 1.02 (0.93–1.12) P = 0.71 - RR: 0.92 (0.79–1.08) P = 0.32 |
|
CANVAS trials Neal et al.51 |
2017 N = 10 142 (T2DM + CVD risk) |
Canagliflozin vs. placebo | 188.2 weeks | CVD death, non-fatal MI or non-fatal stroke | HR 0.86 (0.75–0.97) P < 0.001 (non-inferiority); P = 0.02 (superiority) |
|
PARAGON-HF Solomon et al.42 |
2019 N = 4822, HFpEF |
Sacubitril–valsartan vs. valsartan | 35 months | HF hospitalization and CVD death | Rate ratio 0.87 (0.75–1.01) P = 0.06 |
|
DECLARE-TIMI 58 Wiviott et al.52 |
2019 N = 17 160, T2DM + CVD risk (HF N = 1724) |
Dapagliflozin or placebo | 4.2 years |
- MACE - CVD death or HF hospitalization |
- Dapagliflozin non-inferior to placebo (P < 0.001) - HR 0.83 (0.73–0.95) P = 0.005 |
|
VITALITY-HFpEF Armstrong et al.64 |
2020 N = 789, HFpEF |
Vericiguat 15 and 10 mg/d vs. placebo | 24 weeks |
- KCCQ PLS least-squares mean difference - 6-MW distance mean scores |
- Vericiguat 15 and 10 mg/d vs. placebo (P = 0.47 and P = 0.80) - P = 0.45 (15 mg/d) and P = 0.81 (10 mg/d) |
|
SOLOIST-WHF Bhatt DL et al.59 |
2021 N = 1222 (T2DM + HF) (LVEF ≥50%, N = 256 patients) |
Sotagliflozin vs. placebo | 9.0 months | CVD death, HF hospitalization or urgent visit |
- All patients: HR 0.67 (0.52–0.85) P < 0.001 - LVEF ≥50%: HR 0.48 (0.27–0.86) |
|
SCORED Bhatt et al.60 |
2021 N = 10 584 (T2DM + CKD + CVD risk); (HF N = 3283, LVEF ≥50%, N = 1667) |
Sotagliflozin vs. placebo | 16 months | CVD death, HF hospitalization or urgent visit |
- All patients: HR 0.74 (0.63–0.88) P < 0.001 - LVEF ≥50%: HR 0.72 (0.52–0.99) |
|
Pooled data SOLOIST-WHF and SCORED, presented at ESC 2021 |
2021 N = 11 784 |
Sotagliflozin vs. placebo | 24 months | CVD death, HF hospitalization or urgent visit |
HFpEF: HR 0.73 (0.45–0.89) P = 0.009 All patients: HR 0.72 (0.63–0.82) P = 0.000002 |
|
EMPEROR-Preserved Anker et al.57 |
2021 N = 5988, HFpEF |
Empagliflozin (10 mg once daily) vs. placebo | 26.2 months |
- CVD death or HF hospitalization - CVD death - HF hospitalization |
- HR 0.79 (0.69–0.90) P < 0.001 - HR 0.91 (0.76–.09) - HR 0.73 (0.61–0.88) P < 0.001 |
|
NCT03030235 Nassif et al.65 |
2021 N = 324, HFpEF |
Dapagliflozin or placebo | 12 weeks |
- Improvement in KCCQ-CS - Improvement 6MWT |
- Improved KCCQ-CS (P = 0.001) - Improved 6MWT (P = 0.007) |
|
DELIVER Solomon et al.58 |
2022 N = 6263, HFpEF |
Dapagliflozin vs. placebo | 2.3 years |
- Worsening HF or CVD death - Worsening HF - CVD death |
- HR 0.82 (0.73–0.92) P < 0.001 - HR 0.79 (0.69–0.91) - HR 0.88 (0.74–1.05) |
- 6MMT, 6-min walk test; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; KCCQ-CS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid antagonist; OR, odds ratio; RR, relative risk; SCA, sudden cardiac arrest; T2DM, type 2 diabetes mellitus.
- a Results are effect size and 95% confidence intervals (in brackets).
Encouragingly, recent clinical trials of sodium-glucose cotransporter-2 (SGLT2) inhibitors50-54 have demonstrated significant reductions in cardiovascular mortality and heart failure hospitalization in type 2 diabetic patients and in the general heart failure population irrespective of diabetes, though the strongest benefits are seen in those with heart failure compared with those without heart failure53 and in HFrEF patients compared with HFpEF.54 Strong benefits associated with SGLT2 use are seen in HFrEF (DAPA-HF and EMPEROR-Reduced), depicting significant reduction in heart failure hospitalization or parenteral therapy for heart failure or cardiovascular death in HFrEF patients, irrespective of diabetes status.55, 56 Although the EMPEROR-Preserved and DELIVER trials of SGLT2 inhibitor use in HFpEF patients depicted a significant reduction in risk of composite outcome of cardiovascular death or hospitalization for heart failure, this was mainly driven by reduction in heart failure hospitalization in the SGTLT2 inhibitor group and not by mortality.57, 58 The SOLOIST-WHF and SCORED trials of combined SGLT2/SGLT1 inhibition in diabetic patients have shown some encouraging results (significant reduction in a composite of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure) in acutely decompensated heart failure patients, including a subgroup of patients with LVEF >50%.59, 60 There have also been reported favourable cardiovascular outcomes associated with the use glucagon-like peptide-1 (GLP-1) receptor agonists in type 2 diabetic patients, but no specific clinical trials have been done on the HFpEF subgroup.61-63 The above rubric indicates that beyond contemporary diuretics use for fluid retention and SGLT2 inhibitor use for morbidity benefit, specific therapeutic interventions with noteworthy prognostic significance for the management of HFpEF have remained elusive, and the search continues. Could tracking and treatment of arrhythmias be beneficial in HFpEF?
Possible impact of LVEF on treatment efficacy in HFpEF
HFpEF is a heterogeneous multisystem disease with a myriad of risks factors, potential aetiologies, pathophysiologic pathways, and multiple sub-phenotypes.66 There have been suggestions that LVEF is an effect modifier in treatment outcomes in HFpEF patients with declining benefit observed with increasing LVEF and specifically more benefit observed in patient with LVEF <60% compared with LVEF ≥60%.57 Earlier studies of pharmacological therapies in HFpEF patients that were negative for the overall effect of the intervention to the primary outcomes suggested some benefit in the patients with lower LVEF categories.42, 67, 68 For example, in the overall CHARM study, the risk of the primary outcome of cardiovascular death or HF hospitalization was significantly reduced in the candesartan compared with placebo in the HFrEF group (P < 0.001) and HMmrEF group (P = 0.02, but not HFpEF) (P = 0.57).67 In the TOPCAT study, stronger estimated benefits of spironolactone were observed at the lower end of the ejection fraction spectrum with respect to the primary endpoint.68 In the PARAGON-HF trial, there was significant benefit of treatment with sacubitril–valsartan compared with valsartan only in patients with LVEF ≤57% (median value), but in not those with LVEF >57% in subgroup analysis.42 More recently, in the EMPEROR-Preserved trial, empagliflozin compared with placebo significantly reduced the risk of the primary outcome in HFpEF patients with LVEF <60%, but not in patients with LVEF ≥60%, with the LVEF effect appearing to be a continuum with up-trending benefit observed with down-trending LVEF.57 However, in the DELIVER trial, it was observed that benefits of SGLT2 inhibitor dapagliflozin compared with placebo with respect to hospitalization for heart failure exacerbation or cardiovascular death were similar among patients with a LVEF ≥60% and those with LVEF <60%.58 Based on the above findings, it will seem rational in clinical settings to suggest dapagliflozin in HFpEF patients with an LVEF ≥60% and not the others.
AF burden in HFpEF
AF is present in ~45% of HFpEF patients, and the presence of AF in these patients is associated with an increased risk of all-cause mortality, heart failure hospitalization, and stroke.9, 10, 69-72 The prevalence and incidence of AF is higher in the HFpEF population compared with HFrEF and appears to increase proportionally with increasing LVEF in the setting of heart failure.9, 70, 71, 73 Possible explanations of high concurrent occurrence of HFpEF and AF is the fact they share similar risk factors like hypertension, diabetes, and obesity, as well as the fact that elevated ventricular filling pressure seen in HFpEF might lead to left atrial dilatation, fibrosis, and AF.73, 74 It is also possible that HFpEF and AF are parallel manifestations of some myocardial or systemic disease.74
Rhythm control with antiarrhythmic drugs and/or catheter ablation for AF in HFpEF
In HFpEF, left ventricular filling and preload are significantly dependent on atrial contraction to compensate for the poor ventricular compliance. The onset of AF results in loss of atrial systolic kick, increased left ventricular filling pressure, and thus setting the stage for possible decompensation of HFpEF.75 Antiarrhythmic drugs (AAD) are effective in treatment of AF in HFpEF patients for medium-term to long-term maintenance of sinus rhythm and mortality, with class III AAD more frequently used in majority of studies followed by class IC and then class IA drugs.75-78 Catheter ablation for AF is safe and appears to be effective in maintaining sinus rhythm in HFpEF patients similarly to what is seen in HFrEF patients,79, 80 and of equivalent efficacy to patients without heart failure.81 There is accruing evidence from observational studies that compared with medical therapy, catheter ablation in HFpEF is associated with significant reduction is AF recurrence,81-84 as well as heart failure hospitalization, the composite of heart failure hospitalization and mortality, and all-cause mortality alone.81-83 However, the efficacy of catheter ablation in HFpEF with respect to heart failure hospitalization and mortality is not universally observed.84 A subgroup analysis of heart failure patients (55% were HFpEF) enrolled into the EAST-AFNET4 trial showed that systematic early rhythm control therapy using AAD or catheter ablation compared with usual care was associated with significant reduction in the composite primary outcome of cardiovascular death, stroke, or hospitalization for heart failure or for acute coronary syndrome after median follow of 5 years.85 In RAFAS trial where median LVEF was 63% and 62% in the early rhythm arm and usual care arm respectively, early rhythm control was associated with significant reduction in recurrence of AF and occurrence of ischaemic stroke at 12 months follow-up, although no all-cause mortality or any-cause hospitalization benefit was observed.86 However, it is uncertain how many patients had underlying heart failure. More RCTs of AF ablation in HFpEF-specific patients with respect to survival outcome are warranted. Catheter ablation for AF in HFrEF patients has been shown to reduce mortality and heart failure hospitalization.87, 88
Malignant arrhythmias in HFpEF
Given that SCD properly adjudicated in large clinical trials accounted for 25–30% of total deaths in HFpEF patients,12, 18, 20 SCD could potentially become a target for therapeutic interventions if specific underlying arrhythmias are identified. The incidence of SCD due to ventricular tachyarrhythmias or significant bradyarrhythmias can be reduced with ICD or pacemaker implantation or antiarrhythmic medication or combination of these. Knowing the actual risk of ventricular arrhythmias in specific HFpEF phenotype(s) will be a prerequisite to consideration of trial of VT ablation for example and other therapies. Recommendations have been made for long-term cardiac rhythm monitoring to clarify mechanisms and mode of death in HFpEF patients.12, 18, 20 In animal studies, spontaneous ventricular arrhythmias and SCD have been observed to be significantly higher in HFpEF animals compared with controls without heart failure.11 In humans, these studies are scant, with a few published small-size studies yielding somewhat conflicting results.89-91
One recently published small sample size study of 113 patients (VIP-HF study) consisting of combined HFmrEF and HFpEF patients implanted with implantable loop recorders (ILR) to capture incident tachyarrhythmias and bradyarrhythmias showed 0.6, 11.5, and 3.2 per 100 person-years incidence of sustained VT, non-sustained VT, and bradyarrhythmia, respectively, during a median follow-up of 1.8 years.89 The incidence of sustained ventricular tachyarrhythmias in this HFmrEF/HFpEF population was low, whereas the number of clinically relevant bradycardias was more than expected, though the findings were limited by small sample size as the study recruited less than half of the intended sample size. Another small study of 125 patients that monitored arrhythmias through a 14-day ambulatory cardiac monitor or permanent pacemaker demonstrated that HFpEF patients might have a relatively high, and possibly under-appreciated burden of non-sustained ventricular tachycardia (NSVT), which conferred a higher risk of mortality.90 In a small retrospective study of patients who underwent ambulatory cardiac monitoring, VT was more prevalent in HFpEF patients compared with patients without heart failure (37% vs. 16%, P = 0.001) and increased QTc interval was associated with risk of VT.91 The findings of these two later small studies suggest that the HFpEF ventricular myocardium might be a substrate for elevated malignant arrhythmogenicity. QRS duration has been shown to be predictive of a composite of cardiovascular death, aborted cardiac arrest, or heart failure hospitalization in HFpEF patients.92
There are no large sample size human studies of incident ventricular arrhythmias or conduction disease or clear heart rhythm at time of death in pure HFpEF patients with LVEF ≥50%, and it remains uncertain how many of these patients die from preventable arrhythmias. Clinical studies in this area are urgently needed to fill to huge knowledge gap and guide future management of HFpEF patients. We are unaware of any current ongoing large study designed to determine the prevalence, incidence, and risks of arrhythmias and their predictive effect on mortality including SCD in HFpEF patients or the mechanisms of SCD in these patients. Without understanding the above, it might not be possible to design intervention clinical trials aimed at reducing SCD in HFpEF patients.
SGLT2 inhibitor and arrhythmias
In clinical trials of SGLT2 inhibitors vs. placebo, both atrial arrhythmias and SCD were significantly reduced in the SGLT2 inhibitor-treated group compared with placebo treatment group. In most of these trials, arrhythmias and SCD were reported as adverse clinical events in the safety profile section.93, 94 In a meta-analysis of 34 randomized trials with 63 166 patients in patients with type 2 diabetes or heart failure, SGLT2 inhibitors were associated with a significant reduction in the risk of incident atrial arrhythmias and the ‘SCD’ component of the SCD outcome compared with control, but not ventricular arrhythmias.93 Although majority of the studies included in this meta-analysis were HFrEF, it is possible that the morbidity benefits observed in the HFpEF SGLT2i trials could be linked to reduction in incident arrhythmias. Future studies with specific arrhythmia outcomes are required. However, the molecular mechanisms of the SGLT2 inhibitors in association with arrhythmia reduction remain uncertain.94
Predictors of SCD in HFpEF
Management of HFpEF is challenging given the heterogeneity of the disease and its multiple phenotypes. It will not be absurd to hypothesize that SCD is driven by malignant tachyarrhythmias or bradyarrhythmias. Determining the phenotype of HFpEF patients at highest risk of SCD and the mechanism(s) of SCD remain paramount prerequisites before the design and conduct of clinical intervention trials. Although prognostic scores and specific risk models for SCD have been identified in the HFpEF population,95-99 specific predictors that could be directly modified to reduce SCD have remained elusive. To compound the situation, these risk scores also included non-HFpEF patients, which raises the potential for non-generalizability to pure HFpEF patients. A multivariable risk prediction model for SCD consisting of age, gender, history of myocardial infarction, history of diabetes mellitus, presence of left bundle branch block on electrocardiogram, and NT-proBNP level in HFpEF patients was developed in a post hoc analysis of the I-PRESERVE trial in 201499 and recently validated in the TOPCAT trial.100 Another model with similar findings was still developed in the I-PRESERVE trial and validated in the CHARM-Preserved and TOPCAT trials.98 Although these models made progress by identifying patients with HFpEF who have a greater risk of SCD over time, none of these predictors can be intervened upon acutely to prevent SCD, and the mechanism of SCD (arrhythmic vs. other causes) in HFpEF still remains unknown. The I-PRESERVE, CHARM-Preserve, and TOPCAT also included patients with mid-range ejection fraction (HFmrEF),98, 99 which could slightly dilute the true HFpEF population defined as congestive heart failure with LVEF ≥50%. We hypothesize that the incidence of arrhythmias in HFpEF patients is significantly high and predictive of SCD and that arrhythmic mechanisms drive SCD. We also hypothesize that there is an identifiable risk model for specific HFpEF phenotypes at higher risk of SCD. Identified HFpEF patients at higher risk could be considered for clinical trials of electrophysiological (EP) study +/− cardiac implantable electronic device like an ICD or pacemaker and/or antiarrhythmic medications vs. conservative management as part of the search for efficacious evidence-based therapeutic interventions. Pulmonary hypertension is common and present in majority of HFpEF patients and predictor of mortality in these patients.101 Although isolated post-capillary pulmonary hypertension is likely a complication HFpEF, it possible that the combined post-capillary type could be part of the spectrum of the heterogeneous HFpEF disease. Pulmonary hypertension should be tested in future SCD risk prediction models in HFpEF. For a start, funding of future large studies to undertake long-term cardiac monitoring in HFpEF patients at high risk of SCD will be key. This high-risk group could be identified using the aforementioned multivariable risk prediction models for SCD,98-100 with the caveat that these models were developed in a study that also included HFmrEF patients and was not homogeneously classified as HFpEF. Therefore, there is the need for risk prediction models of SCD in pure HFpEF patients with LVEF of ≥50%, with possible consideration of testing parameters from cardiac MRI in future models.
Device therapies in HFpEF?
Prior studies have shown that HFpEF is significantly associated with chronotropic incompetence, which portends impaired aerobic capacity.102-106 For example, chronotropic incompetence was significantly more common in patients with HFpEF compared with matched healthy controls as measured by the percentage of the heart rate reserve used during maximal exercise (63% vs. 2%, P = 0.001) and percentage of predicted maximal HR (34% vs. 2%, P = 0.001).102 Based on these observations, it was felt that cardiac pacing may improve chronotropic function and thus overall functional capacity in HFpEF.102-104 However, there are no published randomized control trials of pacing compared with no pacing in HFpEF patients with observed chronotropic incompetence. The myPACE study is the first randomized controlled study currently enrolling to test the hypothesis that atrial pacing at moderately higher heart rates compared with the standard backup setting of 60 bpm might provide important benefits for pacemaker patients with HFpEF.107
The prevalence of permanent pacemaker (PPM) implantation in HFpEF varies between 8 and 18%.104, 108, 109 In one large registry of ~14 000 HFpEF patients (LVEF >40%), the prevalence of PPM was 18%. The incidence of new pacemaker implantation within 1 year after index hospitalization for HFpEF exacerbation was approximately 7%, and PPM use was more common among older patients, men, patients with AF, and patients with wider QRS duration (≥140 ms).104 These rates of pacemaker implantation in HFpEF are significantly higher than what is seen in the general adult population during routine clinical care,108, 110 suggesting a greater burden of conduction disease in these patients. Even though this might be true, there is also a chance that we observe a significant bias here as patients in need for pacemaker implantation are more likely to receive the diagnosis of HFpEF in contrast with the general population where the disease is largely underdiagnosed (especially elderly women).66 However, it has been observed that the presence of PPM in HFpEF is associated with adverse clinical outcomes driven mainly by HF hospitalization, but not mortality, raising the concern that right ventricular (RV) pacing leading to ventricular dyssynchrony might be detrimental in HFpEF.111 Albeit the non-availability of details about RV pacing percentage in the above study, as well as data on the indication and mode of pacing, suggestions have been made that in HFpEF patients with atrioventricular block who have a pacing indication, a CRT might be preferable. Nonetheless, there are no prospective randomized control trials that have tested this hypothesis and further studies in this area are warranted. In patients with ventricular pacing indication and/or anticipated high-percentage ventricular pacing and LVEF >35–50%, the BLOCK-HF trial showed that implanting a biventricular pacemaker compared with RV pacing significantly improved the primary endpoint, which was a composite of death, urgent care visit for heart failure, or a 15% increase in LV end-systolic volume index.112 Meanwhile, the BIOPACE study revealed no superiority of biventricular pacing compared with RV pacing in patients with atrioventricular block and any LVEF (mean LVEF was 55.4%).113
Currently, ICD implantation in HFpEF is only indicated for secondary prevention or high-risk infiltrative or hypertrophic cardiomyopathies, whereas in HFrEF there are clear established guidelines for routine clinical use.28 Also, in HFrEF, catheter ablation for VT is associated with a significant reduction in the odds of appropriate ICD therapies, appropriate ICD shocks, ventricular arrhythmia storms, and cardiac hospitalizations in patients with ischaemic cardiomyopathy.114 Similarly, in non-ischaemic cardiomyopathy, VT ablation for ventricular arrhythmias is associated with significant reduction in long-term VT recurrences and cardiac death.115 Data on the risk of ventricular arrhythmias in human HFpEF patients and their treatment are lacking. It is important therefore to determine the risk of ventricular arrhythmias in HFpEF as well as identifying a higher risk subgroup that could benefit from antiarrhythmic medications and possible device therapy. Studies in this area are highly encouraged. The effectiveness of catheter ablation for AF in HFpEF for morbidity benefit is now well known,85, 116 and a few observational studies suggest some mortality.81-83
Conclusion and future directions
The true burden of lethal and non-lethal (apart from AF) arrhythmias and their impact on morbidity and survival in HFpEF remain elusive.9, 10, 69-72 It remains unknown whether SCD that accounts for 25–30% of all deaths in HFpEF patients is driven by malignant arrhythmias, and thus potentially preventable.12, 18, 20 Studies aimed at determining the incidence and risk of arrhythmias as well as their predictive effect on mortality and heart failure hospitalization are urgently needed. The mechanisms of SCD are presently unexplored and unresolved, and development of novel therapies or re-adoption of existing treatment strategies to prevent SCD in HFpEF patients will most likely be predicated on understanding these mechanisms. This renders full comprehension of the mechanisms of SCD a prerequisite before design of clinical interventions studies aimed at reducing SCD in HFpEF. Studies designed with objective of determining the mechanism of SCD (arrhythmic or non-arrhythmic) are required. Given the prohibitive cost implications, if arrhythmia burden is found to significantly elevated and driving SCD, then a subgroup of HFpEF patients at elevated risk for SCD will need to be further identified for targeted treatment. Therefore, derivation of a risk prediction model for the phenotype of HFpEF at highest risk of malignant arrhythmias and SCD using the modern definition of HFpEF (heart failure with LVEF ≥50%), is also required. Exploration of new frontiers via randomized control trials like the utility of device therapy in HFpEF should be considered.
Conflict of interest
None.




