Clinical outcomes and structural remodelling after ablation of atrial fibrillation in heart failure with mildly reduced or mid-range ejection fraction
Abstract
Aims
The efficacy of catheter ablation (CA) on clinical outcomes and cardiac structural remodelling in atrial fibrillation (AF) patients with HF with mildly reduced or mid-range ejection fraction (HFmrEF) remains unclear. We aimed to compare the efficacy of CA with medical therapy (MT) in AF patients with HFmrEF.
Methods and results
We retrospectively screened a total of 36 879 patients with AF between 2005 and 2020. Patients who were initially diagnosed with echocardiography-proved HFmrEF and had follow-up echocardiography were enrolled. After applying propensity score matching in a 1:1 ratio, 72 patients treated by CA (Group 1) and 72 patients receiving MT (Group 2) were taken into further analysis. The co-morbidities were similar between the two groups, except for hyperlipidaemia. After a mean follow-up duration of 58.9 ± 42.6 months, Group 1 had a lower HF hospitalization and all-cause mortality compared with Group 2 (hazard ratio (HR), 0.089 [95% confidence interval (CI), 0.011–0.747]; P = 0.026 and HR, 0.121 [95% CI, 0.016–0.894]; P = 0.038, respectively). As for cardiac structural remodelling, the Group 1 had a better improvement in left ventricular ejection fraction (LVEF) and a more decreased left atrium (LA) diameter than Group 2 (+25.0% ± 18.0% vs. +6.2% ± 21.6%, P = <0.0001 and −1.6 ± 4.7 mm vs. +1.5 ± 8.2 mm, P = 0.008, respectively).
Conclusions
In patients with HFmrEF and AF, CA of AF could reduce both HF hospitalization and all-cause mortality as compared with those with MT. A significant improvement in LVEF and decrease in LA diameter were also observed in the CA group. Early rhythm control with CA should be taken into consideration in patients with HFmrEF and AF.
Introduction
In 2016, the concept of heart failure with mildly reduced or mid-range ejection fraction (HFmrEF) between 40% and 49% was established in the European Society of Cardiology (ESC) guideline,1 which accounts for 10% to 24% of patients with heart failure (HF).2 As one of the phenotypes of HF, HFmrEF not only resembles HF with reduced ejection fraction (HFrEF) regarding age, sex, ischaemic aetiology, and systolic pressure but also has a similar clinical prognosis and pathophysiological mechanism.2, 3 So far, there is still no efficient treatment with robust evidence to reduce morbidity and mortality in the patients with HF with preserved ejection fraction (HFpEF) and HFpEF4 even though they made up nearly half of HF hospitalization.5
Atrial fibrillation (AF) is the most common arrhythmia in patients with HF with an average prevalence of 25%6 and associated with higher morbidity and mortality.7 AF and HF often coexist and influence each other, which develops an endless vicious cycle towards further worsening HF symptom and therefore lead to unpleasant clinical outcomes.8 The majority of therapy for AF targeted at the population with HFrEF and the benefit of catheter ablation (CA) to lower mortality in the same population has also been established in several small randomized studies including the seminal CASTLE-AF trial, AATAC trial, and the subgroup analysis of the CANABA trial.9-11 Although patients with increasing left ventricular ejection fraction (LVEF ≥ 40%) have a higher prevalence of AF than HFrEF12 and are associated with similar adverse event rates including death, HF hospitalization, and stroke.13 The effective therapeutic strategies for AF focusing on the patients with HFmrEF as compared with medical therapy (MT) are still lacking. In this study, we aimed to investigate the clinical outcomes and cardiac structural remodelling in patients with HFmrEF undergoing CA of AF, and compared with those with MT.
Method
Study population
Between 15 June 2005 and 18 February 2020, we retrospectively screened a total of 36 879 patients with AF documented in 12-lead electrocardiogram or 24 h Holter recordings who were admitted to Taipei Veterans General Hospital or follow-up in our clinics. The exclusion criteria were (1) congenital heart disease; (2) severe valvular disease; (3) patients with LVEF < 40% or ≥50%; and (4) patients lacking follow-up echocardiography after CA or MT. According to the previous meta-analysis of AF ablation in HF, the HF hospitalization was 16% with CA and 39% with MT.14 With these anticipated incidences, the minimum number of subjects was 58 for each group for a 1:1 ratio propensity score matching with 5% type I error and 80% power.15 Propensity score analysis was used to adjust for three confounding factors, including age, gender, and LVEF. They were matched with a 1:1 ratio, which resulted in two balanced groups (72 patients in Group 1 that received CA of AF and 72 patients in Group 2 that received MT). The selection process is depicted in Figure 1. AF was defined according to the statement from the 2017 Heart Rhythm Society Expert consensus document.16
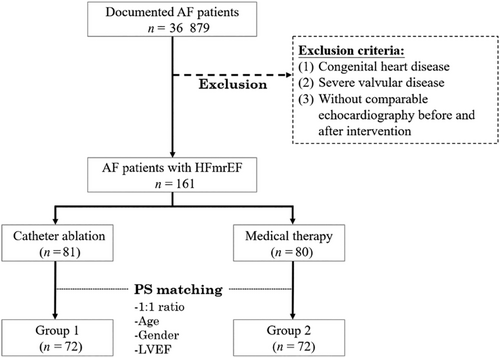
Study design
This retrospective study was conducted at the Taipei Veterans General Hospital in Taipei after approval by the institutional review board of the Taipei Veterans General Hospital (IRB: 2021-11-015BC). The investigation conforms with the principles outlined in the Declaration of Helsinki (Br Med J 1964; ii: 177). Both baseline data and echocardiography parameters were collected from the medical records of each patient.
Transthoracic echocardiogram
The baseline echocardiography was performed within 1 year before CA, and the follow-up echocardiography was performed after CA at least 3 months later in Group 1. Two-dimensional images were got by using an EPIQ CVx (Philips Healthcare, Andover, USA) or Vivid™ E95 (GE Healthcare, Horten, Norway) with a 2.5–5 MHz Doppler transducer. According to the American Society of Echocardiography recommendations,17 left ventricle wall thickness and cardiac chamber dimensions including inter-ventricular septal thickness in diastole (IVSd), left ventricular posterior wall thickness in diastole (LVPWd), left ventricular internal diameter in diastole (LVIDd), and left atrium (LA) diameter were measured during M-mode in the parasternal long-axis view. Modified Simpson's rule was used for LVEF calculation, and the septal ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity(E/e′) was collected to represent LV diastolic function. The RV systolic pressure (RVSP) was measured by adding the TR pressure gradient, which comes from the TR jet and right atrial pressure. The right atrium pressure was measured considering the diameter of the inferior vena cava diameter and determined collapsibility.
The procedure of catheter ablation
Electrophysiological study
An electrophysiological study and CA in the fasting state were performed on each patient after obtaining informed consent. All antiarrhythmic drugs (AADs), except for amiodarone, were discontinued for at least five half-lives before initiation of the procedure. Amiodarone was held 2 weeks before the procedure.
Catheter ablation of paroxysmal and persistent AF
The stepwise procedure of catheter ablation of paroxysmal and persistent AF was achieved, as described in our previous studies.18 For paroxysmal AF, we performed radiofrequency ablation [wide antral pulmonary vein (PV) isolation] or cryoballoon ablation [wide antral pulmonary vein (PV) isolation]. If the AF persisted after PV isolation, sinus rhythm was restored using electric cardioversion.
For non-paroxysmal AF, wide antral PV isolation was performed. If AF is still sustained after PV isolation, additional linear ablation, CFAE ablation, or posterior box isolation was performed based on the operator's decision. The details had been described in our previous studies.19 If AF terminated during the linear ablation across the CFAE sites, complete linear ablation to an anatomic obstacle or the nearest line was performed to prevent subsequent arrhythmia. If AF still did not stop after the above procedures, SR was restored by external cardioversion. We then tried to identify the non-pulmonary vein (NPV) focus after the restoration to SR during any step of the ablation procedure or after cardioversion. The endpoint of the non-PV trigger ablation was the disconnection between the superior vena cava and RA as well as between the coronary sinus and RA, and the elimination of other non-PV ectopic beats with the negative provocation of AF.19
A right atrial CTI ablation was routinely performed at the end of the paroxysmal or non-paroxysmal AF procedure. Bidirectional conduction block of linear ablation was demonstrated during sinus rhythm.
AADs uses after catheter ablation
After ablation, the AADs were kept during the blanking period and discontinued them after the blanking period if no AF recurrence. AADs may be continued after blanking period based on the operator's concerns about AF recurrence or patient's symptom even no documented AF recurrence.
Outcome assessment
The primary endpoint is HF hospitalization. Other secondary endpoints are all-cause mortality, new stroke, sinus rhythm maintenance, and changes of echocardiography parameters including LVEF and LA diameter. The data on clinical outcomes were obtained from the records of each patient carefully. The beginning of the follow-up period was defined as the point when the final treatment strategy for AF was determined. In Group 1, all patients were followed-up at 2 weeks after CA and every 1–3 months thereafter. The post-ablation follow-up included resting surface 12-lead electrocardiogram, 24 h Holter recordings, and/or cardiac event recording with a recording duration of 1 week performed regularly every 3 months for 1 year and an additional check-up when patients reported clinical symptoms. This was done to evaluate recurrence as suggested by the Heart Rhythm Society Task Force Consensus.20 Transthoracic echocardiography was scheduled after 6 and 12 months of AF ablation. One year after procedures, patients received regular follow-up visits over half a year. As for Group 2, patients treated with MT had regular clinic follow-up with resting surface 12-lead electrocardiogram every 3 months. Transthoracic echocardiography was scheduled after 6 and 12 months of medical treatment, and 24 h Holter monitoring was scheduled to avoid bradycardia.21 An additional checkup would be arranged if there has been a deterioration in clinical status.
Statistical analysis
Patient characteristics are expressed as mean ± standard deviation for continuous variables, and as frequency (percentage) for categorical variables. Continuous and categorical variables were compared using the Student's t-test and the χ2 test with Yates' correction, respectively. Proportions were compared using the χ2 test or Fisher's exact test, as appropriate. One-way analysis of variance was used to compare data among two groups. To reduce the treatment-selection bias and potential confounding, we adjusted for age, sex, and LVEF with propensity-score (PS) matching.22 The propensity score was obtained using logistic regression. The HF hospitalization-free and all-cause mortality survival curves were examined using the Kaplan–Meier method with the log-rank test. Cox proportional hazards regression was also used to compare the risk among the two groups, with results expressed as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS 20 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics and demographics
The baseline characteristics and demographics of the two groups (Group 1 vs. Group 2) are shown in Table 1. The age (59.49 ± 7.5 vs. 61.53 ± 6.8 years; P = 0.089), female sex (20.8% vs. 33.3%; P = 0.091), BMI (25.16 ± 3.9 vs. 24.37 ± 4.2 kg/m2; P = 0.730), CHA2DS2-VASc score (2.52 ± 1.4 vs. 2.96 ± 1.5 points; P = 0.056), AF type (persistent AF 58.3% vs. 63.9%; P = 0.494), and the use of HF medications/ADDs were similar between the two groups. Only hyperlipidaemia was more common in the Group 1 (27.8% vs. 9.7%; P = 0.006). In Group 1, three patients received cryoballoon ablation and the rest received CA (68 with traditional low-power long-duration ablation and 2 with high-power short-duration). In addition to successful PV isolation, additional ablation strategies including linear, trigger, and CFAE ablation were performed in 26.4%, 15.3%, and 18.1% of patients in Group 1, respectively.
| Variable (mean ± SD or %, N = 144) | |||
|---|---|---|---|
| Group 1 (CA) (N = 72) | Group 2 (MT) (N = 72) | P | |
| Age (year) | 59.49 ± 7.5 | 61.53 ± 6.8 | 0.089 |
| Female (%) | 15 (20.8) | 24 (33.3) | 0.091 |
| BMI (kg/m2) | 25.16 ± 3.9 | 24.37 ± 4.2 | 0.730 |
| Underlying disease | |||
| HTN (%) | 44 (61.1) | 35 (48.6) | 0.132 |
| DM (%) | 8 (11.1) | 11 (15.3) | 0.460 |
| CAD (%) | 11 (15.3) | 15 (20.8) | 0.386 |
| Hyperlipidaemia (%) | 20 (27.8) | 7 (9.7) | 0.006* |
| CVA (%) | 8 (11.6) | 8 (11.6) | 0.928 |
| COPD (%) | 2 (5.6) | 3 (4.2) | 0.758 |
| Thyroid disease (%) | 11 (15.3) | 9 (12.5) | 0.630 |
| Persistent AF (%) | 42 (58.3) | 46 (63.9) | 0.494 |
| CHA2DS2_VASc | 2.52 ± 1.4 | 2.96 ± 1.5 | 0.056 |
| HF and AAD medication | |||
| ACE-I/ARB/ARNI | 44 (61.1%) | 36 (50.0%) | 0.180 |
| Ivabradine | 1 (1.4%) | 0 (0.0%) | 0.316 |
| Beta-blocker | 27 (37.5%) | 34 (47.2%) | 0.238 |
| Amiodarone/dronedarone | 31 (43.1%) | 33 (45.8%) | 0.737 |
| Nondihydropyridine CCB | 10 (13.9%) | 18 (25.0%) | 0.092 |
| Adjuntive ablation strategies beyond PVI | |||
| Linear ablation | 19 (26.4%) | N/A | - |
| Trigger ablation | 11 (15.3%) | N/A | - |
| CFAE ablation | 13 (18.1%) | N/A | - |
- Abbreviations: AD, antiarrhythmic drug; ACE-I, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; CA, catheter ablation; CAD, coronary artery disease; CCB, calcium channel blockers; CFAE, complex fractionated atrial electrograms; COPD; chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetic mellitus; HTN, hypertension; MT, medical therapy; PVI, pulmonary vein isolation.
- * P-value < 0.05.
Baseline echocardiography parameters
The echocardiography parameters of the two groups are shown in Table 2. After similar echocardiography follow-up duration (18.95 ± 8.8 vs. 17.67 ± 14.1 months; P = 0.517), baseline LV thickness, LV size, mean left ventricular ejection fraction (45.51% ± 2.7% vs. 45.07% ± 2.7% ± 9%; P = 0.329), E/e′ ratio (13.64 ± 7.3 vs. 14.40 ± 6.6; P = 0.521), and RVSP (28.71 ± 7.9 vs. 32.68 ± 12.9; P = 0.058) were similar between the two groups except for baseline LA diameter, which was larger in the Group 2 (42.88 ± 6.19 vs. 46.30 ± 9.84 mm; P = 0.014).
| Variable (mean ± SD or %, N = 144) | |||
|---|---|---|---|
| Group 1 (CA) (N = 72) | Group 2 (MT) (N = 72) | P | |
| Echocardiography follow-up duration | 18.95 ± 8.8 | 17.67 ± 14.1 | 0.517 |
| Pre-LVEF | 45.51 ± 2.7 | 45.07 ± 2.7 | 0.329 |
| Pre-LA diameter | 42.88 ± 6.1 | 46.30 ± 9.8 | 0.014* |
| IVSd | 10.14 ± 1.8 | 10.47 ± 2.3 | 0.350 |
| LVPWd | 10.11 ± 1.9 | 10.13 ± 1.8 | 0.935 |
| LVIDd | 49.76 ± 6.6 | 52.16 ± 8.2 | 0.058 |
| Septal E/e′ | 13.64 ± 7.3 | 14.40 ± 6.6 | 0.521 |
| RVSP | 28.71 ± 7.9 | 32.68 ± 12.9 | 0.058 |
- Abbreviations: CA, catheter ablation; IVSd, inter-ventricular septal thickness in diastole; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diameter in diastole; LVPWd, left ventricular posterior wall thickness in diastole; MT, medical therapy; RVSP, right ventricle systolic pressure.
- * P-value <0.05.
Primary endpoint
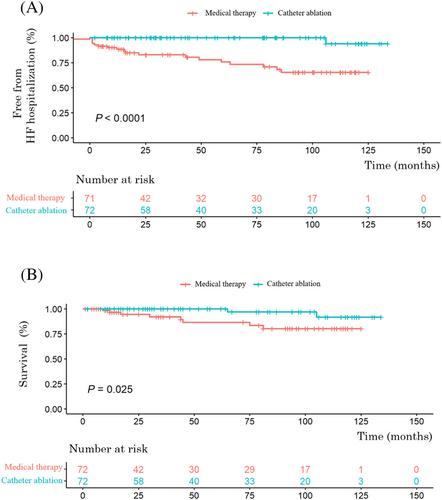
After a mean follow-up duration of 58.9 ± 42.6 months, 19 patients (13.2%) had hospitalizations for HF. Kaplan–Meier curve analysis revealed that more patients in Group 1 were free from HF hospitalizations than in Group 2 significantly (log-rank P < 0.0001) (Figure 2). In the multivariable cox regression analysis adjusting for age, sex, CHA2DS2-VAS, type of AF, hyperlipidaemia, thyroid disease, and baseline LA diameter, the Group 1 was still associated with a lower HF hospitalization (HR, 0.089 [95% CI, 0.011–0.747]; P = 0.026) (Table 3).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Events (N = 19) | Non-events (N = 125) | P-value | Exp(B) | 95% CI | P-value | ||
| Lower bond | Upper bond | ||||||
| Age, years | 63.16 ± 5.0 | 60.10 ± 7.4 | 0.029* | 1.009 | 0.906 | 1.125 | 0.867 |
| Female, % | 47.4 | 24 | 0.033* | 0.948 | 0.300 | 2.998 | 0.928 |
| BMI, kg/m2 | 25.13 ± 5.4 | 24.72 ± 3.9 | 0.683 | ||||
| CHA2DS2_VASc | 3.37 ± 2.1 | 2.63 ± 1.30 | 0.037* | 1.514 | 1.022 | 2.243 | 0.039* |
| Persistent AF | 36.8 | 64.8 | 0.020* | 0.502 | 0.171 | 1.478 | 0.211 |
| HTN, % | 47.4 | 56.0 | 0.481 | ||||
| DM, % | 21.1 | 12 | 0.277 | ||||
| CAD, % | 10.5 | 19.2 | 0.360 | ||||
| Hyperlipidaemia, % | 0 | 21.6 | 0.025* | ||||
| CVA, % | 21.1 | 9.8 | 0.152 | ||||
| Thyroid disease, % | 7 | 13 | 0.002* | 10.245 | 2.187 | 47.985 | 0.003* |
| Pre-LVEF, % | 44.68 ± 3.0 | 45.38 ± 2.6 | 0.299 | ||||
| Pre-LA diameter, mm | 50.44 ± 12.0 | 43.73 ± 7.3 | 0.032* | 1.052 | 0.997 | 1.112 | 0.066 |
| Septal E/e′ | 17.49 ± 9.3 | 13.49 ± 6.4 | 0.088 | ||||
| AF Ablation, % | 5.9 | 58.8 | 0.000* | 0.089 | 0.011 | 0.747 | 0.026* |
- Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; COPD; chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetic mellitus; HTN, hypertension; LA, left atrial; LVEF, left ventricular ejection fraction.
- * P-value <0.05.
Secondary endpoints
During follow-up, there are 10 fatalities. Kaplan–Meier curve analysis also revealed that more patients in Group 1 were free from death significantly (log-rank P = 0.025) (Figure 2). The benefit of CA on all-cause mortality in Group 1 remained significant after adjusting age, sex, baseline LA diameter, baseline septal E/e′, CHA2DS2-VAS, type of AF, and DM (HR, 0.121 [95% CI, 0.016–0.894]; P = 0.038) (Table 4). As for new-onset stroke, there is no significant difference between the two groups (log-rank P = 0.273).
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Events (N = 10) | Non-events (N = 134) | P-value | Exp(B) | 95% CI | P-value | ||
| Lower bond | Upper bond | ||||||
| Age, years | 65.00 ± 6.5 | 60.17 ± 7.1 | 0.040* | 1.087 | 0.922 | 1.280 | 0.320 |
| Female, % | 60 | 24.6 | 0.015* | 0.710 | 0.153 | 3.288 | 0.661 |
| BMI, kg/m2 | 24.08 ± 2.7 | 24.82 ± 4.1 | 0.581 | ||||
| CHA2DS2_VASc | 4.20 ± 2.1 | 2.61 ± 1.3 | 0.046* | 0.916 | 0.551 | 1.522 | 0.734 |
| Persistent AF | 30 | 63.4 | 0.036* | 0.401 | 0.091 | 1.767 | 0.227 |
| HTN, % | 50 | 55.2 | 0.749 | ||||
| DM, % | 40 | 11.2 | 0.009* | 1.103 | 0.142 | 8.593 | 0.925 |
| CAD, % | 40 | 16.4 | 0.061 | ||||
| Hyperlipidaemia, % | 10 | 19.4 | 0.462 | ||||
| CVA, % | 30 | 9.9 | 0.054 | ||||
| Thyroid disease, % | 20 | 13.4 | 0.562 | ||||
| Pre-LVEF, % | 45.70 ± 2.9 | 45.26 ± 2.7 | 0.625 | ||||
| Pre-LA diamter, mm | 38.40 ± 5.5 | 45.03 ± 8.3 | 0.015* | 0.908 | 0.805 | 1.024 | 0.116 |
| Septal E/e′ | 18.70 ± 5.2 | 13.67 ± 7.0 | 0.028* | 1.193 | 1.047 | 1.360 | 0.008* |
| AF Ablation, % | 20 | 52.2 | 0.049* | 0.121 | 0.016 | 0.894 | 0.038* |
- Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; COPD; chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetic mellitus; HTN, hypertension; LA, left atrial; LVEF, left ventricular ejection fraction.
- * P-value <0.05.
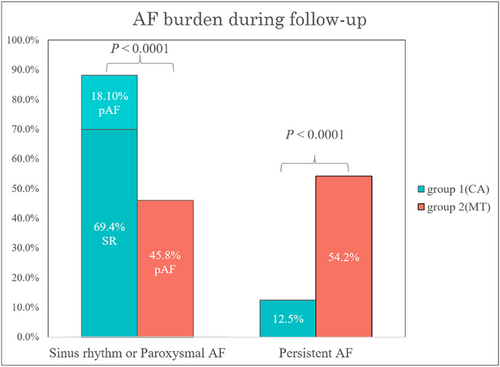
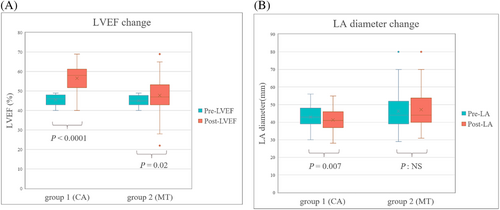
After a mean follow-up duration of 22.1 ± 17.1 months, the patients in Group 1 had a sinus rhythm maintenance rate of 69.4% and a lower percentage of persistent AF than the patients in Group 2 (12.5% vs. 54.2%, P < 0.0001) (Figure 3). Echocardiographic parameters before and after the indexed date of enrolment with a mean interval of 16.3 ± 9.3 months were compared, and we observed a significant improvement in LVEF (25.0% ± 18.0% vs. 6.2% ± 21.6%, P = <0.0001) and a significant decrease in LA diameter (−1.6 ± 4.7 mm vs. 1.5 ± 8.2 mm, P = 0.008) in the Group 1 (Figure 4 and 4) compared with Group 2. The changes in LV thickness, LV size, E/e′ ratio, and RVSP have no significant difference between the two groups.
Discussion
The main findings of this study were as follows: (1) In patients with HFmrEF undergoing CA of AF, there was less event rate of HF hospitalization than those with medical therapy. (2) Patients undergoing CA of AF had lower all-cause mortality than those with medical therapy. (3) After the CA of AF, the LVEF improved and LA diameter decreased significantly as compared with those on medical therapy.
The strategy of rhythm or rate control for patients with AF and HFmrEF
HFmrEF was first introduced in the 2016 ESC guideline as one of the HF phenotypes that shed the light on patients in this grey area who may benefit from the research targeted at this population with different aetiology, demographics, co-morbidities, and responses to treatment.1 Like HFpEF, there is no treatment has yet been shown to reduce mortality in patients with HFmrEF. As for the effect of treatment on HF hospitalization in HFmrEF patients with concomitant AF, beta-blockers are ineffective, either angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors is inconclusive, and digoxin has not yet been studied. However, a meta-analysis studying the efficacy of beta-blockers in HFrEF patients has demonstrated the reduction in all-cause mortality presented only in patients with sinus rhythm (HR 0.73, [95% CI, 0.67–0.80]; P < 0.001) but not in patients with AF, which highlights the importance of sinus rhythm maintenance to reduce mortality.23 In the present study, we observed a decreased AF burden after CA with a sinus rhythm maintenance of 69.4% and a lower percentage of persistent AF (12.5% in Group 1 vs. 54.2% in Group 2, P < 0.0001). The advantages of sinus rhythm maintenance have not only been found in the patients with HFrEF but have also been seen in the patients with HFpEF.24 In the present study focusing on a population with HFmrEF, rhythm control by the CA was also associated with a lower risk of HF hospitalizations and all-cause mortality than medical therapy significantly (log-rank P < 0.0001 and 0.025, respectively).
Comparison between the strategy of CA and MT for patients with AF and HFrEF
Similarly, several studies including the ARC-HF and CAMERA-MRI trials have shown that rhythm control by CA is more effective than rate control in improving quality of life, LVEF, and B-type natriuretic peptide in patients with HFrEF and persistent AF.25 However, the AF-CHF trial did not prove the superiority of pharmacological rhythm control versus rate control to lower CV death.26 The benefits of rhythm control on mortality may be masked by the side effect of the AADs but unveiled by CA. The superiority of rhythm control by CA versus AADs has been demonstrated in the multicenter randomized AATAC trial, in which HFrEF patients with persistent AF receiving CA were significantly more likely to be free from AF recurrence(70% vs. 34%, P < 0.001) and have lower mortality rate(8% vs. 18%) than the patient receiving amiodarone.9 Even with similar AF burden reduction, patients receiving AADs did not benefit from low AF burdens like patients receiving CA who had a significant reduction of hard clinical outcomes such as death and rehospitalizations.27 Based on the confirmations of CA efficacy on mortality reduction from larger randomized control trials—CASTLE-AF trial and subgroup analysis of the CABANA trial—CA has been given a Class IIA indication for AF patients with HFrEF in the 2020 ESC AF and 2021 ESC HF guidelines.4
The rationale for CA in patients with AF and HFmrEF
In agreement with previous randomized control trials targeted at HFrEF,9, 10 we observed substantial improvement in all-cause mortality and HF hospitalization in patients with HFmrEF and paroxysmal or persistent AF after CA. In contrast, the AMICA trial is a negative trial comparing CA and medical therapy in HF patients with persistent AF, and a mean of LVEF of 24.8 to 27.6% indicated that not all patients with AF and HFrEF will profit from a CA despite restoration of sinus rhythm.28 This is also proved by the subgroup of the CASTLE-AF trial, in which the beneficial effect of CA was not observed in the patients with advanced HF symptoms (New York Heart Association functional class III) and very severe reduced LVEF(<25%) (HR, 1.36 [95% CI, 0.69–2.65]; P = 0.01).10 It seems that the benefit of CA is more prominent in patients with relatively better LVEF and less advanced HF stage. This idea was also supported by Fujimoto et al. who found that compared with patients with HFrEF, patients with HFmrEF or HFpEF had a lower composite of all-cause death, HF hospitalization, and stroke or systemic embolism (HFrEF, HFmrEF vs. HFpEF, 31.6%, 12.2% vs. 10.4%, log-rank P < 0.001).29
Reviewing the trials of HFpEF,4 patients with HFmrEF comprise 11.7% of the CABANA trial, which is the first large RCT to describe an important mortality benefit from CA in patients mostly with preserved systolic function (HR, 0.57 [95% CI, 0.33–0.96] for all-cause mortality). However, the benefit of CA on mortality could only be seen in the subgroup with LVEF ≥ 50% in the post hoc analysis (HR, 0.40 [95% CI, 0.18–0.88]).11 In contrast, our study demonstrated that the ablation effect was still associated with lower all-cause mortality (HR, 0.121 [95% CI, 0.016–0.894]; P = 0.038). The discrepancy between the CABANA trial and our study may be due to 27% missing data of baseline EF in the CABANA trial. It could lead to underestimation of the benefit from CA in this specific population with HFmrEF by using the statistical method of multiple imputations to fill up missing values and due to an insufficient number of patients with LVEF <50% to reach normal distribution.12
Other independent predictors of long-term outcomes
In addition to CA, we also found that higher CHA2DS2-VASc scores were associated with higher HF hospitalization (HR, 1.514 [95% CI, 1.022–2.243]; P = 0.039, and higher septal E/e′ ratio was associated with higher all-cause mortality in the patients with AF and HFmrEF (HR, 1.193 [95% CI, 1.047–1.360]; P = 0.008). The CHA2DS2-VASc score is not only a well-known predictor of stroke caused by AF but also a proven independent predictor of HF in patients with non-valvular AF.30 Interestingly, in the study conducted by Koeda et al., a higher CHA2DS2-VASc score significantly predicts HF event in patients with HFpEF which was defined as LVEF ≥45% (HR, 1.46 [95% CI, 1.13–1.87]), whereas no significant differences were shown in patient with EF < 45%.30 Unlike HFrEF, HFpEF are generally older, more hypertensive, obese, diabetic, and more likely to display AF, and these co-morbidities are also major compositions of CHA2DS2-VASc score.31 The difference in pathophysiology of HFpEF and HFrEF may explain why CHA2DS2-VASc score is only a stronger predictor of HF in the population with HFpEF.
As for septal E/e′, a retrospective study included a total of 12 421 eligible HFrEF patients with or without AF has also demonstrated the E/e′ ratio is not just fleeting haemodynamic transients with elevated filling pressures but is independently and incrementally linked to long-term mortality (HR, 1.21 [95% CI, 1.07–1.37]; P = 0.003 for E/e′ > 20 and HR, 1.15 [95% CI, 1.02–1.29]; P = 0.02 for E/e′ > 14 to 20).32 This finding warrants the regular echocardiographic follow-up for evaluating the diastolic function, which is essential for risk stratification of patients with HF and strategy adjustment to upgrade medical therapy or consider new interventions.
Cardiac structural reverse remodelling after CA of AF in patients with HF
Favourable structural remodelling after CA including improvement in LVEF and decreases in LAD and LVEDD has been demonstrated in a retrospective cohort that included a total of 153 patients with 59% HFmrEF and 41% HFrEF.33
Left atrial reverse remodelling
Similarly, LA diameter reduction and LVEF improvement were observed in the patients receiving CA in our study. LA diameter is an important index of diastolic dysfunction. Although the mechanism of HFmrEF remains unclear. HFmrEF and HFpEF share things in common about some aspects of the pathophysiology mechanism which is a complex, synergistic interplay between AF, HF, and LA dysfunction.3 Unlike HFrEF, which is more likely to have ischaemic aetiology and left bundle branch block,2 HFpEF usually has diastolic dysfunction, which was triggered by abnormalities in excitation-contraction coupling and ventricular stiffness.34 In the spectrum of different LVEF, the HFmrEF group is closer to the HFrEF group concerning age, sex, systolic blood pressure, and ischaemic aetiology,2 but HFmrEF was found to associate with higher fibrosis-related biomarkers, including C-terminal propeptide of procollagen type I (PICP) and N-terminal propeptide of procollagen type III (PIIINP) and a higher ratio of PICP/PIIINP, which shifts the equilibrium towards the type I collagen synthesis and results in cardiac fibrosis.35
Interestingly, AF also plays a role in the formation of cardiac fibrosis by the natural evolution of tachycardia-mediated cardiomyopathy36 and the severity of cardiac fibrosis which is proportional to the AF burden.37 The study of Ling et al. included over 50 patients with paroxysmal or persistent AF undergoing cardiac magnetic resonance imaging and revealed that the presence of persistent AF was independently predictive of diffuse LV fibrosis, regardless of ejection fraction.37 It raises concern that the patients with HFmrEF may get benefit from the rhythm control targets to decrease AF burden.
AF is not only one cause of HF but also can drive clinical heart failure symptoms by a significant cardiac output decrement due to loss of efficient atrial systole and impaired LV diastolic function at higher heart rates during AF attack.38 Increased LA volume during HF could start another round of vicious circle by enhancing structural changes within the LA, namely, atrial fibrosis, which may not only increase AF burden but also cause a profound dysfunction in LA mechanics. Ultimately, further worsening of HF could be expected in patients with HFmrEF. In the present study, there is a proportional trend for increased baseline LA diameter and HF hospitalization but did not reach statistically significant after adjustment (HR, 0.40 [95% CI, 0.18–0.88], P = 0.066).
Left ventricular reverse remodelling
Finally, LVEF, as an important prognostic factor in patients with HF, our studies observed a significant increment in the CA group. The LVEF increased from 45.5 ± 2.7% to 56.6 ± 7.1% (P < 0.001) after ablation. Different prognoses were noted in patients with HFmrEF based on the changes of LVEF including improved, stable, and deteriorated levels that have been demonstrated in previous studies.39 In the patients receiving CA for AF, Yazaki et al. revealed that LVEF transition from HFmrEF to HFrEF had more poor outcomes (HF hospitalization or death).40 In this study, 14 of 144 patients had LVEF transitions from HFmrEF to HFrEF, and most of them had medication control (MT 85.7% vs. CA 14.3%, P = 0.005). Having a unique situation that could be either converted to HFpEF or HFrEF, patients with HFmrEF are worth interfering with a more aggressive therapeutic strategy to reach LVEF improvement for a better prognosis. To the best of our knowledge, this is the first study focusing on patients with HFmrEF and AF to compare clinical outcomes and cardiac structural reverse remodelling between CA and medical therapy.
Limitation
First, the case–control methodology was used to minimize the need for case numbers in this study. Further prospective randomized studies with a larger sample size are required to investigate the exact role of CA in patients with HFmrEF. Second, propensity score matching was applied to balance age, gender, and LVEF between the two groups, because we wanted to reflect target patients' characteristics in the real-world data and minimize the propensity score matching paradox. The baseline LA diameter per se is an independent predictor for major adverse cardiac events, which is higher in the MT group than in the CA group significantly. Thus, results should be interpreted cautiously in light of potential selection bias. Although there was no difference in the AADs between the two groups, this could be due to the small sample size in this study. Third, the CA group was younger and had lower baseline RVSP, LVIDd, and CHA2DS2_VASc but did not reach statistical significance. These potential confounding factors could be masked by the small sample size in this study. Although the schedule used for 12-lead electrocardiogram and echocardiography was similar between the two groups, the follow-up Holter in MT group was not regular. The inconsistent follow-up Holter between the two groups in this retrospective study may also be a potential confounder. Finally, sinus maintenance was determined by reviewing a 12-lead electrocardiogram and/or 24 h Holter recordings around the date the follow-up echocardiography was performed rather than continuous monitoring. And the EKG and/or 24 h Holter recordings were usually arranged for symptomatic patients during the follow-up period, which may underestimate the sinus rhythm maintenance rate in both groups.
Conclusions
In patients with HFmrEF and AF, CA of AF could reduce both HF hospitalization and all-cause mortality as compared with those with MT. A significant improvement in LVEF and decrease in LA diameter were also observed in the CA group. Early rhythm control with CA should be taken into consideration in patients with HFmrEF and AF.
Acknowledgement
This work is particularly supported by “Yin Yen-Liang Foundation Development and Construction Plan” of the College of Medicine, National Yang Ming Chiao Tung University.
Conflicts of interest
None declared.
Funding
This study was supported, in part, by research grants from the Taipei Veterans General Hospital (V108C-055, V109C-008, V110C-004, and V111C-075), Ministry of Science and Technology of Taiwan (MOST106-2314-B-010-035-MY3, 109-2314-B-010-063, 110-2314-B-A49A-542, and 111-2314-B-A49-007-MY3), Szu-Yuan Research Foundation of Internal Medicine (108010, 109024, 110008, and 111003), and “Yin Yen-Liang Foundation Development and Construction Plan” of the College of Medicine, National Yang Ming Chiao Tung University.




