Association of longitudinal left atrial strain with mortality after tricuspid valve surgery
Dr. Seo currently is working at Kangwon National University Hospital.
Abstract
Aims
Tricuspid valve (TV) surgery for functional tricuspid regurgitation (TR) is becoming more common, but the associated mortality remains high. Therefore, we evaluated the clinical and echocardiographic parameters associated with all-cause mortality in patients with severe functional TR who underwent TV surgery.
Methods and results
A total of 286 patients with severe functional TR who underwent TV replacement or repair was analysed between January 2006 and December 2017. We assessed changes in conventional echocardiographic parameters and strain, such as peak atrial longitudinal strain (PALS). During a median follow-up period of 5.3 years, 71 (24.8%) patients died due to any cause. When comparing groups with and without all-cause deaths, there were no significant differences in terms of sex, co-morbidities, medication use, and surgery type. However, patients who died were older and more likely to have refractory atrial fibrillation (AF). With multivariate Cox modelling, age >65 years (adjusted hazard ratio [HR], 2.81, 95% confidence interval [CI], 1.59–4.96; P < 0.001), refractory AF (adjusted HR, 2.84, 95% CI, 1.36–5.94; P = 0.006), lower albumin level (adjusted HR, 0.50, 95% CI, 0.31–0.82), and reduced PALS (adjusted HR, 1.87, 95% CI, 1.06–3.33; P = 0.032) were significant determinants of all-cause mortality. PALS decline was associated with refractory AF (adjusted HR, 5.74, 95% CI, 2.81–11.7; P < 0.001) and the absence of a Maze procedure (adjusted HR, 2.95, 95% CI, 1.51–5.78; P = 0.002).
Conclusions
A reduction in PALS was significantly associated with all-cause mortality in our cohort of patients with severe functional TR who underwent TV surgery. This phenomenon is related to refractory AF and more aggressive intervention for AF is necessary concomitant with TV surgery.
Introduction
Functional tricuspid regurgitation (TR) usually occurs secondary to left-sided heart disease or atrial fibrillation (AF).1, 2 Historically, functional TR was believed to be benign and to resolve after resolution of left-sided heart disease.3 However, recent research has reported that functional TR is an ongoing process and can worsen if left untreated.4, 5 Therefore, the latest guidelines recommend that patients undergoing left-sided valve surgery also undergo tricuspid valve (TV) surgery if severe functional TR is present.6, 7 Additionally, although interest in isolated TV surgery is increasing, the associated mortality rate remains high.8 We evaluated the clinical and echocardiographic parameters associated with all-cause mortality in patients with severe functional TR who underwent TV surgery.
Methods
Study population
We identified all patients with severe functional TR who underwent TV replacement or repair between January 2006 and December 2017. Patients with organic TR, defined as congenital, lead- or device-related, or infective endocarditis, or who had undergone a previous TV surgery were excluded. Most patients underwent concomitant left-sided valve surgery, and 129 (45%) patients underwent the Maze procedure.
Data collection and outcomes
Baseline demographic, echocardiographic, laboratory, and follow-up clinical outcomes data were collected retrospectively through a medical records review. The presence of AF was evaluated during preoperative electrocardiography, regardless of onset time. Refractory AF was defined as the presence of AF at last follow-up. Residual TR was defined as at least moderate TR during follow-up. The primary study outcome was all-cause death; however, we also assessed changes in conventional echocardiographic parameters and strain. The study protocol was approved by the institutional review board of a single centre, and the need for informed consent was waived because of the retrospective nature of the study.
Echocardiography
Comprehensive transthoracic echocardiography was performed using commercially available equipment (Vivid 7, GE Medical System, Milwaukee, WI, USA; or Acuson 512, Siemens Medical Solution, Mountain View, CA, USA; or Sonos 5,500, Philips Medical Systems, Andover, MA, USA). Standard M-mode, two-dimensional, and colour Doppler imaging were performed in parasternal, suprasternal, substernal, and apical views with positional adjustment where appropriate. The first and last echocardiograms collected during the study period were analysed to compare changes in parameters, and any changes observed during the follow-up period are presented as Δ. The median interval of echocardiography was 3.3 years (interquartile range [IQR], 1.4–6.6 years). Anatomic measurements were performed according to the recommendations of the American Society of Echocardiography (ASE).9 Severe TR was defined as TR with a vena contracta width >0.7 cm, systolic flow reversal in hepatic veins, and an annulus dilatation >4 cm or inadequate cusp coaptation according to the current ASE guidelines.10
Strain analysis
Left atrial (LA) speckle-tracking analysis was performed using a customized software package (2D Cardiac Performance Analysis; TomTec, Munich, Germany) according to task force recommendations.11 At least three consecutive beats from three standard apical views were collected for analysis. Speckle-tracking analysis was not performed for patients with poor image quality, which was defined as at least one segment dropout, missing view, or significant foreshortening of the left ventricle (LV), right ventricle (RV), or LA. We used the ventricular cycle as the reference point to calculate LA strain. To generate LA strain curves, the LA endocardial border was traced manually in the apical four- and two- chamber views. We only measured LA reservoir strain (peak longitudinal LA strain = peak atrial longitudinal strain [PALS]) because every patient initially had AF. In addition to LA strain, we measured LV longitudinal strain (LV GLS) and RV free-wall longitudinal strain (RV FWLS). LV and RV strains were calculated from standard apical views acquired in routine practice including LV-focused apical 4-, 2-, and 3-chamber view for LV strain and RV-focused apical 4-chamber view for RV strain. The region of interest was adjusted to include the entire myocardium. The GLS was calculated by averaging the peak strains of all segments of the cardiac chamber. PALS was defined as the peak positive strain value (i.e. lengthening) during late ventricular systole on the averaged longitudinal strain curve. The average PALS was measured at the end of the reservoir phase with the tracking location marker placed at the end of the QRS wave (which was calculated from three beats in patients with AF, Supporting Information, Figure S1). One investigator, who was experienced with strain imaging (S. J. H.) and was blinded to the outcome and echocardiographic characteristics, evaluated atrial strain in all the study participants. We assessed measurement reproducibility in 20 randomly selected cases (including 10 patients with AF and 10 patients without AF) by repeated measurements (performed by S. J. H. and K. J. Y., who also has experience in strain analysis). The observers were blinded to one another's strain measurements and the clinical endpoints.
Statistical analysis
Continuous variables are reported as mean ± standard deviation and were compared using Welch's t-test or the Wilcoxon rank-sum test, as appropriate. Categorical variables were summarized by frequency or percentage and analysed with the Chi-square test or Fisher's exact test, as appropriate. A regression analysis using Cox proportional hazards modelling was performed to identify independent predictors of all-cause death after surgery. The cumulative incidence of clinical events was evaluated by Kaplan–Meier analysis. The level of significance was assessed with the log-rank test. All statistical tests were two-tailed, and P < 0.05 was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences version 25.0 software program (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
Baseline characteristics of the patient population according to all-cause death are summarized in Table 1. The median follow-up period after surgery was 5.3 years (IQR, 3.1–8.9 years). During the follow-up period, 71 (24.8%) deaths occurred due to any cause. Initially, all patients had AF. Long-standing persistent AF (>1 year) was found in 153 patients (53.5%), persistent AF (>1 week) in 118 (41.3%), and new-onset AF (<1 week) in 15 (5.2%). When comparing groups with and without all-cause deaths, there were no significant differences in terms of sex, co-morbidities (e.g. diabetes and hypertension), medication use, and surgery type. However, patients who died were older and more likely to have refractory AF. Regarding laboratory findings, it was determined that levels of haemoglobin, total cholesterol, and albumin and the estimated glomerular filtration rate (eGFR) were lower in the group with deaths. In patients who underwent isolated TV surgery, those who died showed lower haemoglobin, albumin, and eGFR (Supporting Information, Table S1). In those who underwent TV and combined left-sided valve surgery, those who died showed similar differences in laboratory tests (Supporting Information, Table S2).
| Total (n = 286) | Death (n = 71) | Survival (n = 215) | *P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 63 ± 11 | 69 ± 8 | 61 ± 11 | <0.001 |
| Male | 101 (35) | 31 (44) | 70 (33) | 0.090 |
| Body mass index, kg/m2 | 22.9 ± 3.5 | 22.2 ± 3.4 | 23.1 ± 3.5 | 0.083 |
| Medical history | ||||
| Diabetes | 42 (15) | 14 (20) | 28 (13) | 0.167 |
| Hypertension | 71 (25) | 23 (32) | 48 (22) | 0.089 |
| Smoking ever | 42 (15) | 13 (18) | 29 (14) | 0.320 |
| Ischemic heart disease | 17 (5.9) | 2 (2.8) | 15 (7.0) | 0.199 |
| Previous stroke | 41 (14) | 11 (16) | 30 (14) | 0.748 |
| Congestive heart failure | 163 (57) | 45 (63) | 118 (55) | 0.210 |
| Medications | ||||
| Antiplatelet | 36 (13) | 6 (8.5) | 30 (14) | 0.221 |
| ACE inhibitor or ARB | 105 (37) | 25 (35) | 80 (37) | 0.762 |
| Beta-blocker | 69 (24) | 17 (24) | 52 (24) | 0.967 |
| Diuretics, including MRA | 193 (68) | 47 (66) | 146 (68) | 0.790 |
| Statin | 25 (8.7) | 5 (7.0) | 20 (9.3) | 0.559 |
| Anticoagulation | 161 (56) | 41 (58) | 120 (56) | 0.776 |
| Antiarrhythmic agents | 83 (29) | 20 (28) | 63 (29) | 0.838 |
| Type of surgery | ||||
| Tricuspid valve replacement | 86 (30) | 22 (31) | 64 (30) | 0.846 |
| Tricuspid valve repair | 200 (70) | 49 (69) | 151 (70) | 0.846 |
| Combined left-sided valve surgery | 228 (80) | 57 (80) | 171 (80) | 0.892 |
| Aortic valve surgery | 17 (7.5) | 1 (2.0) | 16 (9.4) | |
| Mitral valve surgery | 162 (71) | 36 (63) | 126 (74) | |
| Aortic and mitral valve surgery | 48 (21) | 20 (35) | 28 (16) | |
| Pulmonic valve surgery | 1 (0.5) | 0 (0.0) | 1 (0.6) | |
| Combined Maze operation | 129 (45) | 29 (41) | 100 (47) | 0.405 |
| Refractory atrial fibrillation | 184 (64) | 57 (80) | 127 (59) | 0.001 |
| Residual tricuspid regurgitation | 27 (9.4) | 8 (11) | 19 (8.8) | 0.544 |
| Laboratories | ||||
| Haemoglobin, g/dL | 11.9 ± 2.2 | 10.9 ± 1.8 | 12.2 ± 2.3 | < 0.001 |
| Total cholesterol, mg/dL | 145 ± 35 | 137 ± 35 | 148 ± 35 | 0.035 |
| Total bilirubin, mg/dL | 1.0 (0.6, 1.5) | 1.0 (0.7, 1.4) | 0.9 (0.6, 1.6) | 0.829 |
| Albumin, g/dL | 4.0 ± 0.4 | 3.8 ± 0.5 | 4.1 ± 0.4 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 70.4 ± 23.5 | 61.2 ± 24.8 | 73.5 ± 22.2 | < 0.001 |
| CRP, mg/dL | 0.14 (0.06, 0.42) | 0.27 (0.10, 0.90) | 0.11 (0.05, 0.31) | 0.078 |
- ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist.
- Values are presented as mean ± standard deviation or number (%) or median (interquartile range). Statistical significance was defined as P < 0.05 by Welch's t-test (continuous variables) or the χ2 t-test (categorical variables). Bold formatting of values indicates the presence of statistical significance (P < 0.05).
- * P-value for death versus survival.
Echocardiographic parameters
Preoperative echocardiographic parameters and changes that occurred during the follow-up period are reported in Table 2. There were similar results for initial LV/RV sizes and function, LA volume, and parameters of diastolic function in patient groups with and without deaths. Patients who died tended to have greater tricuspid annular diameter at initial examination (44.3 ± 8.5 mm vs. 41.9 ± 7.8 mm; P = 0.023), greater increase in LV end-systolic dimension, and greater decrease in LV ejection fraction at the last follow-up. Further, changes in both PALS (−1.0 (−4.9, 1.7) vs. 0.4 (−3.0, 5.0); P = 0.003) and LV GLS (−1.9 (−4.8, 3.1) vs. –0.3 (−3.6, 2.8); P = 0.019) were significantly reduced among the group with deaths during follow-up. In patients who underwent isolated TV surgery only, there was a difference in PALS change; there was no such difference in those who underwent TV and combined surgery (Supporting Information, Tables S1 and S2).
| Total (n = 286) | Death (n = 71) | Survival (n = 215) | *P-value | |
|---|---|---|---|---|
| LVEDD, mm | 52.5 ± 8.9 | 53.8 ± 9.2 | 52.1 ± 8.8 | 0.167 |
| ΔLVEDD, mm | 0.4 (−3.4, 5.0) | 0.4 (−3.0, 5.3) | 0.4 (−3.6, 5.0) | 0.442 |
| LVESD, mm | 34.5 ± 7.6 | 35.6 ± 7.7 | 34.2 ± 7.6 | 0.193 |
| ΔLVESD, mm | 0.0 (−3.6, 3.8) | 2.0 (−2.9, 5.0) | 0.0 (−4.0, 3.0) | 0.010 |
| LVEF, % | 57.0 ± 9.5 | 57.6 ± 9.5 | 56.8 ± 9.5 | 0.535 |
| ΔLVEF, % | 1.0 (−5.0, 8.7) | −2.6 (−9.9, 4.0) | 2.0 (−4.0, 9.5) | <0.001 |
| LAVI, mL/m2 | 133.9 ± 105.4 | 159.1 ± 155.8 | 125.7 ± 81.6 | 0.091 |
| ΔLAVI, mL/m2 | −15.0 (−45.2, 9.8) | −9.8 (−45.7, 8.8) | −18.8 (−45.2, 10.9) | 0.556 |
| E, m/s | 1.58 ± 0.53 | 1.60 ± 0.52 | 1.58 ± 0.53 | 0.814 |
| ΔE, m/s | 0.02 (−0.28, 0.31) | 0.08 (−0.20, 0.46) | 0.00 (−0.29, 0.28) | 0.188 |
| e′, m/s | 0.084 ± 0.077 | 0.074 ± 0.022 | 0.089 ± 0.088 | 0.298 |
| Δe′, m/s | −0.021 (−0.039, −0.004) | −0.026 (−0.040, −0.010) | −0.020 (−0.038, −0.004) | 0.817 |
| E/e′ | 23.1 ± 12.8 | 24.5 ± 14.7 | 22.8 ± 12.1 | 0.338 |
| ΔE/e′ | 6.8 (−0.3, 15.5) | 10.6 (0.2, 16.7) | 5.3 (−1.2, 15.0) | 0.155 |
| RVD, mm | 48.1 ± 9.0 | 48.2 ± 9.8 | 48.0 ± 8.8 | 0.887 |
| ΔRVD, mm | −7.7 (−12.5, 2.0) | −5.6 (−11.9, −0.9) | −8.5 (−12.6, −3.0) | 0.180 |
| RAD, mm | 58.4 ± 13.1 | 57.1 ± 10.4 | 58.8 ± 13.8 | 0.340 |
| ΔRAD, mm | −7.0 (−13.0, 1.7) | −5.7 (−11.5, 1.6) | −8.1 (−13.7, −2.6) | 0.010 |
| TAD, mm | 42.5 ± 8.0 | 44.3 ± 8.5 | 41.9 ± 7.8 | 0.023 |
| ΔTAD, mm | −18.0 (−24.5, −11.3) | −20.6 (−26.0, −13.8) | −17.4 (−23.6, −10.6) | 0.069 |
| TAPSE, mm | 16.0 ± 4.1 | 17.4 ± 5.0 | 15.7 ± 3.9 | 0.120 |
| ΔTAPSE, mm | −3.4 (−7.4, −0.4) | −6.9 (−11.6, −0.6) | −3.0 (−6.2, −0.3) | 0.092 |
| S′, m/s | 0.11 ± 0.05 | 0.13 ± 0.10 | 0.10 ± 0.02 | 0.106 |
| ΔS′, m/s | −0.03 (−0.05, −0.01) | −0.04 (−0.06, −0.02) | −0.03 (−0.04, −0.01) | 0.078 |
| RVSP, mmHg | 49.7 ± 16.4 | 52.5 ± 15.4 | 48.8 ± 16.6 | 0.102 |
| ΔRVSP, mmHg | −13.0 (−23.7, −0.6) | −14.7 (−27.5, 6.9) | −12.7 (−22.5, −1.5) | 0.472 |
| PALS, % | 6.2 ± 5.1 | 6.8 ± 6.1 | 6.0 ± 4.7 | 0.236 |
| ΔPALS, % | −0.2 (−3.8, 4.3) | −1.0 (−4.9, 1.7) | 0.4 (−3.0, 5.0) | 0.003 |
| LV GLS, % | −11.3 ± 4.6 | −10.6 ± 5.3 | −11.5 ± 4.3 | 0.193 |
| ΔLV GLS, % | −0.5 (−3.9, 2.8) | −1.9 (−4.8, 3.1) | −0.3 (−3.6, 2.8) | 0.019 |
| RV FWLS, % | −11.3 ± 6.8 | −11.0 ± 6.2 | −11.3 ± 7.0 | 0.697 |
| ΔRV FWLS, % | −0.9 (−6.4, 4.1) | −2.8 (−7.2, 2.0) | −0.2 (−6.0, 4.9) | 0.111 |
- E, early diastolic mitral flow velocity; e’, mitral annulus early diastolic velocity; LAVI, left atrial volume index; LV, left ventricle; LVEDD, LV end-diastolic dimension; LVEF, LV ejection fraction; LVESD, LV end-systolic dimension; LV GLS, LV global longitudinal strain; PALS, peak atrial longitudinal strain; RA, right atrium; RAD, RA minor dimension; RV, right ventricle; RVD, RV base diameter; RV FWLS, RV free-wall longitudinal strain; RVSP, RV systolic pressure; S′, tricuspid annulus peak systolic velocity; TAD, tricuspid annulus diameter; TAPSE, tricuspid annular plane systolic excursion.
- Values are presented as mean ± standard deviation or median (interquartile range). Statistical significance was defined as P < 0.05 by Welch's t-test (continuous variables). Bold formatting of values indicates the presence of statistical significance (P < 0.05).
- * P-value for death versus survival.
Prognostic factors of all-cause mortality after TV surgery
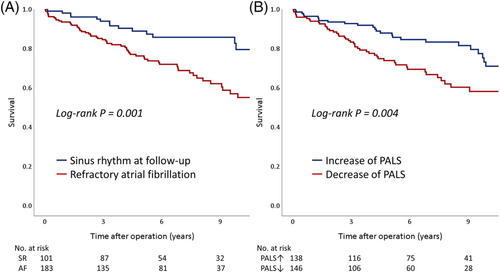
Table 3 shows the results of Cox proportional hazards modelling. During univariate survival analysis, age >65 years, hypertension, persistent AF, decrease in PALS, and reduction in RV FWLS showed significant associations with all-cause mortality. In multivariate analyses, age >65 years (adjusted hazard ratio [HR], 2.81, 95% confidence interval [CI], 1.59–4.96; P < 0.001), refractory AF (adjusted HR, 2.84, 95% CI, 1.36–5.94; P = 0.006), lower albumin level (adjusted HR, 0.50, 95% CI, 0.31–0.82), reduction in PALS (adjusted HR, 1.87, 95% CI, 1.06–3.33; P = 0.032), and reduction in RV FWLS (adjusted HR, 1.75, 95% CI, 1.04–2.94) were significant determinants for all-cause mortality among significant parameters in the univariate analyses. All-cause mortality was higher in patients with refractory AF than in those who remained in sinus rhythm (log-rank P = 0.001) (Figure 1). Patients who experienced a decrease in PALS had a higher mortality rate than did those who experienced an increase in PALS (log-rank P = 0.004) (Figure 1). The total number of hospitalizations was not significantly different between patients who experienced a decrease in PALS and those who experienced an increase in PALS (34 vs. 24; P = 0.193) (Supporting Information, Figure S2).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Old age (>65 years) | 3.79 (2.23–6.42) | <0.001 | 2.81 (1.59–4.96) | <0.001 |
| Diabetes | 1.61 (0.90–2.90) | 0.109 | ||
| Hypertension | 1.71 (1.04–2.81) | 0.035 | ||
| Congestive heart failure | 1.23 (0.76–1.99) | 0.405 | ||
| Combined left-sided valve surgery | 1.00 (0.56–1.79) | 0.990 | ||
| Combined Maze procedure | 0.76 (0.47–1.22) | 0.259 | ||
| Refractory atrial fibrillation | 2.61 (1.45–4.68) | 0.001 | 2.84 (1.36–5.94) | 0.006 |
| Haemoglobin, g/dL | 0.85 (0.79–0.92) | <0.001 | ||
| Albumin, g/dL | 0.38 (0.25–0.60) | <0.001 | 0.50 (0.31–0.82) | 0.006 |
| eGFR, mL/min/1.73 m2 | 0.98 (0.97–0.99) | <0.001 | ||
| Decrease of LAVI | 1.12 (0.69–1.84) | 0.646 | ||
| Initial PALS | 1.04 (0.99–1.09) | 0.068 | ||
| Decrease of PALS | 2.03 (1.25–3.31) | 0.005 | 1.87 (1.06–3.33) | 0.032 |
| Decrease of LV GLS | 1.43 (0.88–2.30) | 0.148 | ||
| Decrease of RV FWLS | 1.75 (1.08–2.85) | 0.024 | 1.75 (1.04–2.94) | 0.036 |
- CI, confidence interval; eGFR, estimated glomerular filtration rate; LAVI, left atrial volume index; LV GLS, left ventricular global longitudinal strain; OR, odds ratio; PALS, peak atrial longitudinal strain; RV FWLS, right ventricular free-wall longitudinal strain; TR, TV, tricuspid valve.
Prognostic factors of mortality after isolated TV surgery
Only reduction in PALS showed significant association with higher mortality in patients who underwent isolated TV surgery (adjusted HR, 14.0, 95% CI, 1.26–155; P = 0.032) (Table 4).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Old age (>65 years) | 1.49 (0.51–4.30) | 0.466 | ||
| Diabetes | 0.77 (0.10–5.93) | 0.803 | ||
| Hypertension | 1.71 (0.53–5.51) | 0.367 | ||
| Combined Maze procedure | 0.31 (0.07–1.41) | 0.131 | ||
| Refractory atrial fibrillation | 35.3 (0.26–4,771) | 0.154 | ||
| Haemoglobin, g/dL | 0.72 (0.52–0.99) | 0.041 | ||
| Albumin, g/dL | 0.38 (0.14–0.99) | 0.049 | ||
| Decrease of LAVI | 1.26 (0.43–3.64) | 0.676 | ||
| Initial PALS | 1.13 (1.02–1.24) | 0.017 | ||
| Decrease of PALS | 13.7 (1.78–105) | 0.012 | 14.0 (1.26–155) | 0.032 |
| Decrease of LV GLS | 2.45 (0.77–7.83) | 0.130 | ||
| Decrease of RV FWLS | 4.71 (1.05–21.1) | 0.043 | ||
- CI, confidence interval; LAVI, left atrial volume index; LV GLS, left ventricular global longitudinal strain; OR, odds ratio; PALS, peak atrial longitudinal strain; RV FWLS, right ventricular free-wall longitudinal strain.
Prognostic factors of mortality after TV and combined left-sided valve surgery.
In patients who underwent TV and combined left-sided valve surgery, age >65 years, persistent AF, and albumin level were significantly associated with all-cause mortality (Supporting Information, Table S3).
Variables associated with PALS decline
In laboratory and echocardiographic findings, patients with PALS decline had lower haemoglobin level, larger left atrial volume index, and greater reduction in LV GLS at the last follow-up (Supporting Information, Table S4). PALS decline was significantly associated with refractory AF (adjusted HR, 5.74, 95% CI, 2.81–11.7; P < 0.001) and absence of a Maze procedure (adjusted HR, 2.95, 95% CI, 1.51–5.78; P = 0.002) (Table 5).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Old age (>65 years) | 1.24 (0.78–1.98) | 0.365 | ||
| Diabetes | 1.06 (0.55–2.03) | 0.871 | ||
| Hypertension | 1.22 (0.71–2.09) | 0.472 | ||
| Congestive heart failure | 0.87 (0.54–1.39) | 0.549 | ||
| Combined left-sided valve surgery | 0.75 (0.42–1.34) | 0.334 | ||
| No Maze procedure | 5.77 (3.45–9.63) | <0.001 | 2.95 (1.51–5.78) | 0.002 |
| Refractory atrial fibrillation | 9.06 (5.07–16.2) | <0.001 | 5.74 (2.81–11.7) | <0.001 |
| Decrease of LAVI | 0.92 (0.56–1.51) | 0.738 | ||
| Decrease of LV GLS | 2.12 (1.32–3.40) | 0.002 | ||
| Decrease of RV FWLS | 1.07 (0.67–1.70) | 0.792 | ||
- CI, confidence interval; LAVI, left atrial volume index; LV GLS, left ventricular global longitudinal strain; OR, odds ratio; PALS, peak atrial longitudinal strain; RV FWLS, right ventricular free-wall longitudinal strain.
Discussion
In this study, we evaluated clinical and echocardiographic parameters according to all-cause mortality in patients with severe functional TR who underwent TV surgery. The major findings of this study were as follows. (i) There were no significant differences in co-morbidities or surgery type between groups with and without deaths, but patients who experienced death were older and had more frequent refractory AF. (ii) A decrease in PALS was significantly associated with a higher all-cause mortality rate after TV surgery, especially for isolated TV surgery. (iii) A decline in PALS was significantly associated with refractory AF and absence of a Maze procedure. These findings suggest that longitudinal PALS could be a useful imaging marker for predicting all-cause mortality in patients with functional TR and AF who undergo surgery.
Functional TR with normal structural TV leaflets and chordae is often secondary to long-standing persistent AF.1, 12 Increased mortality among patients with TR and AF has led to enhanced interest in various therapeutic strategies.7 The optimal timing for surgical intervention in cases of functional TR remains controversial. However, delays in surgery must be avoided given risks for irreversible RV damage, organ failure, and poor results with later surgical intervention.13 Previous studies have demonstrated that clinical factors of age, male sex, liver cirrhosis, a lower glomerular filtration rate, lower haemoglobin level, and the presence of severe symptoms (e.g. dyspnoea) are associated with adverse outcomes after TR surgery.13-15 Additionally, AF-associated TR resulted in reduced leaflet coaptation, which increases the recurrence risk of both AF and TR.16, 17 This study showed that higher mortality was associated with older age and refractory AF. Nevertheless, only 45% of all study participants underwent the Maze procedure. Addition of the Maze procedure was associated with a reduction in thromboembolic complications and improvement in haemodynamic performance among patients undergoing mechanical valve replacement.18 Also, the degree of residual TR was not different between the death and survival groups. These results suggest a need for earlier and more aggressive intervention for AF in patients with severe functional TR.
Significant functional TR was independently associated with adverse outcomes in patients with AF, although LV ejection fraction was preserved.19 Functional TR is most often caused by significant left-sided valvular and myocardial disease, which leads to increased left-sided pressures, pulmonary hypertension, greater RV afterload, and RV remodelling.20, 21 However, there is little research on the role of LA as the key parameter in patients with severe TR and AF.22 LA has an important role in modulating LV filling and is an important biomarker of cardiovascular disease and adverse cardiovascular outcomes.23, 24 LA strain has been applied mainly to cases of left-sided heart diseases, such as heart failure or mitral regurgitation.25-27 In this study, there was no difference in initial PALS between groups with and without deaths; however, changes in PALS were decreased significantly among the patient group experiencing death. Notably, the decrease in PALS was significantly associated with all-cause mortality in patients with severe functional TR who underwent TV surgery. However, PALS decline was a significant prognosticator in isolated TV surgery but not in TV or combined left-sided valve surgery. This finding suggests that PALS is closely related to AF, and there are some limitations on the additional role of PALS in non-AF patients or those who underwent multi-valve surgery. Also, PALS decline was significantly associated with absence of a Maze procedure. The Maze procedure reverses most of the negative effects of AF at ventricular, atrial, and valvular levels.28 Therefore, in patients undergoing TV surgery, performance of the Maze procedure should be encouraged.
Study limitations
This study has several limitations. First, this was a retrospective study; thus, strain measurements were collected from stored images using dedicated software, and success in measuring strain was dependent on the quality of these images. Additionally, we only measured PALS, which does not reflect overall LA function. Second, most patients underwent left-sided valve surgery, which could affect LA function. Third, intraobserver variability was possible because the PALS value is small in the AF-rhythm population, which has a relatively large atrium. Fourth, AF was confirmed by electrocardiogram at initial and follow-up points only, not by continuous monitoring. Fifth, LA measurements were not performed in LA-focused views, so we could not avoid foreshortening of the LA. Finally, because PALS is closely related to AF, it might be difficult to regard PALS as an independent prognostic factor in patients with functional TR undergoing valve surgery.
Conclusions
PALS decline showed a significant association with all-cause mortality in patients with severe functional TR undergoing TV surgery. This is related to refractory AF, and more aggressive intervention for AF is necessary concomitant with TV surgery.
Conflict of interest
None declared.




