Prognostic impact of left atrial function in heart failure with preserved ejection fraction in sinus rhythm vs. persistent atrial fibrillation
Abstract
Aims
We sought to determine the prognostic impact of left atrial (LA) size and function in patients with heart failure with preserved ejection fraction (HFpEF) in sinus rhythm (SR) vs. atrial fibrillation (AF).
Methods and results
We enrolled consecutive HFpEF patients and assessed indexed LA volumes and emptying fractions (LA-EF) on cardiac magnetic resonance imaging. In addition, all patients underwent right and left heart catheterization, 6 min walk test, and N-terminal prohormone of brain natriuretic peptide evaluation. We prospectively followed patients and used Cox regression models to determine the association of LA size and function with a composite endpoint of heart failure hospitalization and cardiovascular death. A total of 188 patients (71% female patients, 70 ± 8 years old) were included of whom 92 (49%) were in persistent AF. Sixty-five patients reached the combined endpoint during a follow-up of 31 (9–57) months. Multivariate Cox regression adjusted for established risk factors revealed that LA-EF was significantly associated with outcome in patients in SR [adjusted hazard ratio 2.14; 95% confidence interval (1.32–3.47) per 1-SD decline, P = 0.002]. In persistent AF, no LA imaging parameter was related to outcome. By receiver operating characteristic and restricted cubic spline analyses, we identified an LA-EF ≥ 40% as best indicator for favourable outcomes in patients with HFpEF and SR. Persistent AF carried a similar risk for adverse outcome compared with impaired LA-EF (<40%) in SR (log-rank, P = 0.340).
Conclusions
In HFpEF patients in SR, impaired LA-EF is independently associated with worse cardiovascular outcome, which is similar to persistent AF. In persistent AF, LA parameters lose their prognostic ability.
Introduction
Approximately 50% of patients with heart failure suffer from heart failure with preserved ejection fraction (HFpEF). Therapeutic options are still limited in HFpEF,1 thus the exploration of underlying pathomechanisms is of paramount importance for the understanding of the disease and development of new therapeutic targets. In the traditional model, HFpEF is characterized by stiffening of the left ventricular (LV) and diastolic dysfunction. This leads to left atrial (LA) pressure overload and remodelling, consecutive post-capillary pulmonary hypertension, and right ventricular (RV) and right atrial remodelling and dysfunction.2, 3 Comorbidity-driven systemic microvascular endothelial inflammation causing myocardial and skeletal-muscle inflammation and subsequent diffuse interstitial fibrosis has been proposed as one alternate driver of myocardial stiffening.2, 4 Findings of LA cardiomyocyte hypertrophy and LA microvascular dysfunction in early stages of HFpEF support the model of global myocardial remodelling.5 Impaired LA function is a hallmark of HFpEF and correlated with impaired cardiovascular outcome and pulmonary vascular disease.6, 7 Total LA-emptying fraction (EF) can be divided into passive conduit and active booster pump part.8 Impaired LA conduit function has been shown to be significantly associated with exercise intolerance9 and outcome10 in HFpEF patients in sinus rhythm (SR). Very recently, a close correlation of atrial transport function with fibrotic and arrhythmogenic LA tissue degeneration was shown.11 Subsequently, a substantial proportion of up to 60% of the HFpEF patient population are in atrial fibrillation (AF).12 Nevertheless, data on how LA size and function contribute to haemodynamic changes and cardiovascular outcome in HFpEF in SR vs. AF are still limited.13 In contrast to standard two-dimensional echocardiography, cardiac magnetic resonance (CMR) imaging enables excellent imaging of the LA wall14 and is also superior for LA volumetric assessment in both SR and AF.15
We conducted this study to assess CMR-derived LA functional parameters and their association with cardiovascular outcome. Furthermore, we tested correlations of LA phasic functional parameters with backward coupling to the pulmonary vasculature, antegrade coupling to LV filling pressures, CMR-derived extracellular volume (ECV) accumulation, and finally exercise capacity.
Methods
Study design
This study was performed at the Division of Cardiology at the Medical University of Vienna, a tertiary care centre with a high-volume cardiac catheterization laboratory and heart failure outpatient clinic. It was approved by its local ethical committee (EK No. 796/2010). From December 2010 to March 2017, consecutive patients were screened for HFpEF. All study participants gave written informed consent and were enrolled in a prospective observational fashion.
Clinical definitions
The diagnosis of HFpEF was based on guideline recommendation,16 including (1) signs or symptoms of heart failure, (2) preserved systolic LV ejection fraction (EF) (EF ≥ 50%), (3) elevated N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (>220 pg/mL), and (4) signs of diastolic dysfunction or structural changes such as LA enlargement or LV hypertrophy. In addition, we invasively confirmed elevated pulmonary artery wedge pressure to confirm the diagnosis of HFpEF. All patients underwent invasive coronary angiography. Reasons for exclusion were significant coronary artery disease on coronary angiography, significant valvular disease except tricuspid regurgitation, congenital heart disease, or signs of myocardial storage disease like amyloidosis. Screening for amyloidosis was performed according to current recommendations.17
Assessment techniques
Clinical and exercise capacity assessment
At baseline, all patients underwent clinical assessment regarding underlying comorbidities, New York Heart Association function class, laboratory values including NT-proBNP, and baseline electrocardiogram (ECG).
We performed a standardized 6 min walk distance (6MWD) on a 50 m indoor track for submaximal exercise capacity assessment.18
Right and left heart catheterization
For right heart catheterization, a 7F Swan-Ganz catheter (Baxter, Irvine, CA) was inserted via a jugular or femoral access. Pressures were documented as a digitized mean over the whole respiratory cycle including at least eight consecutive heart cycles using CathCorLX (Siemens AG, Berlin and Munich, Germany). Mean pulmonary artery wedge pressure as well as systolic, diastolic, and mean pulmonary artery pressures were documented. LV end-diastolic pressure was manually checked in each patient. Cardiac output was measured by thermodilution. Derived haemodynamic parameters were calculated with standard formulas. Following right heart catheterization, coronary angiography was performed in the same procedure.
Transthoracic echocardiography with tissue Doppler analysis
All patients underwent transthoracic echocardiography with tissue Doppler analysis performed by board certified physicians using General Electric Healthcare scanners such as GE Vivid 7 and Vivid S70. LV EF was evaluated by using biplane Simpson's technique. Pulsed wave Doppler of transmitral inflow and pulsed wave tissue Doppler of septal and lateral LV annulus wall motion were measured to further calculate E/e'.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance examinations were performed on a 1.5 T scanner (Avanto Fit, Siemens Healthcare GmbH, Erlangen, Germany). Patients with an estimated glomerular filtration rate of <30 mL/min/1.73 m2 were excluded.
Cardiac magnetic resonance examinations were performed according to standard protocols19 including late gadolinium enhancement imaging (0.15 mmoL/kg gadobutrol, Gadovist, Bayer Vital GmbH, Leverkusen, Germany) and T1 mapping using the modified Look-Locker inversion (MOLLI) sequence.20 For pre-contrast T1 mapping, electrocardiographically triggered MOLLI was applied using a 5(3)3 prototype (five acquisition heartbeats followed by three recovery heartbeats and further three acquisition heartbeats). For patients in SR during CMR study, retrospective ECG gating was performed and for patients in AF prospective gating. For post-contrast T1 mapping, a 4(1)3(1)2 prototype was used. T1 values from medial and lateral mid-cavity two-chamber and four-chamber view as well as mid short axis view were averaged. Regions of interest for T1 blood pool values were derived with sufficient distance to papillary muscles and the endomyocardial border, and MOLLI-ECV was then calculated using same day haematocrit values.
Left atrial chamber evaluation
- Conduit EF: passive atrial emptying starts with atrioventricular-valve opening.
- Booster pump EF: active contractile atrial emptying (which is lost in AF), ends with atrioventricular-valve closure.
Left atrial chamber evaluation was performed offline on a remote workstation with commercially available software (cvi42, Circle Cardiovascular Imaging Inc, Calgary, Alberta, Canada). The LA endocardial border and long axis diameter were drawn in a four-chamber and two-chamber view.11, 14 The maximum volume, minimum volume, and the volume before LA contraction (if in SR), each indexed for the body surface area (LAVi) and consecutive conduit; booster pump; and total LA-EF were then calculated using the biplane area-length formula. This process was repeated three times, and average values were calculated for further analysis.
Outcome measures
Study participants were followed by telephone calls and 6 months ambulatory visits including ECG. Primary outcome measure was a composite endpoint of hospitalization for heart failure and death from cardiovascular causes. Events were ascertained by follow-up visits and phone calls and adjudicated by an internal adjudication committee, who were blinded to patient characteristics as well as imaging and haemodynamic data.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or as median and interquartile ranges. Categorical variables are presented as numbers and per cent. Continuous variables were compared using the Mann–Whitney U-test, and for dichotomous variables, the χ2 test was applied. Spearman (ρ) test was used to find factors correlated with LA phasic function and volumetric analyses.
Parameters were divided into clinical, general, and specific LA CMR imaging and invasive haemodynamic categories. To define factors associated with AF, univariate logistic regression analysis was calculated for each parameter (Table 1).
| Variable | Sinus rhythm (n = 96) | AF (n = 92) | P value |
|---|---|---|---|
| Clinical parameters | |||
| Age (years) | 69 ± 9 | 72 ± 8 | 0.017 |
| Female sex, n (%) | 74 (77) | 59 (64) | 0.055 |
| Body mass index (kg/m2) | 31 ± 6 | 30 ± 6 | 0.576 |
| Paroxysmal AF | 30 (31) | 0 (0) | <0.001 |
| Persistent AF | 0 (0) | 92 (100) | <0.001 |
| Diabetes mellitus type II, n (%) | 37 (39) | 30 (33) | 0.364 |
| Hyperlipidaemia, n (%) | 57 (59) | 44 (48) | 0.107 |
| Arterial hypertension, n (%) | 89 (93) | 90 (98) | 0.497 |
| Heart rate (beats/min) | 69 ± 14 | 73 ± 13 | 0.014 |
| 6MWD (m) | 349 ± 108 | 313 ± 122 | 0.040 |
| Sleep apnoea, n (%) | 9 (9) | 11 (12) | 0.643 |
| COPD, n (%) | 25 (26) | 33 (36) | 0.101 |
| NYHA functional class, n (%) | 0.003 | ||
| II | 45 (47) | 26 (28) | |
| III | 49 (51) | 58 (63) | |
| IV | 2 (2) | 8 (9) | |
| NT-proBNP (ng/mL) | 0.52 (0.32 to 1.20) | 1.60 (0.99 to 2.42) | <0.001 |
| Glycated haemoglobin (%) | 6.1 ± 1.3 | 6.1 ± 0.7 | 0.583 |
| eGFR (mL/min/1.73 m2) | 60 ± 18 | 62 ± 19 | 0.736 |
| Gamma-glutamyl-transferase (U/L) | 27 (19 to 42) | 47 (28 to 94) | <0.001 |
| HMG-CoA reductase inhibitor, n (%) | 49 (51) | 35 (38) | 0.055 |
| Beta-blocker, n (%) | 67 (70) | 67 (73) | 1.000 |
| Diuretics, n (%) | 68 (71) | 74 (80) | 0.388 |
| ACE inhibitor, n (%) | 21 (22) | 31 (34) | 0.141 |
| AT II receptor antagonist, n (%) | 41 (43) | 34 (37) | 0.370 |
| Echocardiographic parameters | |||
| Interventricular septum (mm) | 13 ± 2 | 13 ± 2 | 0.337 |
| E/e' ratio | 16 ± 7 | 14 ± 5 | 0.482 |
| LV EF (%) | 60 ± 6 | 60 ± 7 | 0.529 |
| Systolic PAP (mmHg) | 55 ± 20 | 59 ± 15 | 0.112 |
| Cardiac magnetic resonance imaging parameters | |||
| LV end-diastolic diameter (mm) | 47 ± 6 | 47 ± 6 | 0.374 |
| RV end-diastolic diameter (mm) | 38 ± 7 | 43 ± 8 | <0.001 |
| Interventricular septum (mm) | 11 ± 2 | 11 ± 2 | 0.862 |
| LA diameter (mm) | 68 ± 8 | 75 ± 10 | <0.001 |
| LA area (cm2) | 30 ± 7 | 37 ± 9 | <0.001 |
| RA diameter (mm) | 61 ± 7 | 70 ± 10 | <0.001 |
| RA area (cm2) | 25 ± 6 | 35 ± 11 | <0.001 |
| LV EF (%) | 68 ± 9 | 61 ± 6 | <0.001 |
| LV global longitudinal strain (%) | −9.4 ± 3.4 | −5.7 ± 4.4 | <0.001 |
| LV end-diastolic volume (mL) | 128 ± 45 | 124 ± 43 | 0.793 |
| Cardiac output (L/min) | 5.5 ± 1.8 | 5.2 ± 2.9 | 0.145 |
| RV EF (%) | 56 ± 11 | 47 ± 10 | <0.001 |
| RV end-diastolic-volume (mL) | 144 ± 46 | 162 ± 65 | 0.035 |
| LGE present, n (%) | 33 (34) | 34 (37) | 0.623 |
| Amount of LGE (%) | 7 ± 3 | 8 ± 5 | 0.550 |
| MOLLI-ECV | 28.0 ± 3.2 | 31.0 ± 4.9 | <0.001 |
| LA volumetric analyses | |||
| Vi max. (mL/m2) | 55 ± 18 | 80 ± 27 | <0.001 |
| Vi pre A-wave (mL/m2) | 44 ± 16 | / | |
| Vi min. (mL/m2) | 35 ± 17 | 68 ± 26 | <0.001 |
| Conduit EF (%) | 20 ± 7 | / | |
| Booster pump EF (%) | 19 ± 9 | / | |
| Total EF (%) | 39 ± 11 | 16 ± 9 | <0.001 |
| Invasive haemodynamics | |||
| Systolic PAP (mmHg) | 52 ± 19 | 54 ± 18 | 0.225 |
| Diastolic PAP (mmHg) | 21 ± 8 | 23 ± 7 | 0.033 |
| Mean PAP (mmHg) | 33 ± 11 | 35 ± 10 | 0.154 |
| PAWP (mmHg) | 19 ± 6 | 21 ± 6 | 0.064 |
| LV end-diastolic pressure (mmHg) | 20 ± 7 | 20 ± 6 | 0.637 |
| TPG (mmHg) | 14 ± 7 | 15 ± 7 | 0.507 |
| Diastolic pressure gradient (mmHg) | 2.0 (−1.0 to 5.0) | 2.0 (−2.0 to 6.0) | 0.893 |
| CO thermodilution (L/min) | 5.5 ± 1.4 | 5.0 ± 1.2 | 0.020 |
| PVR (dyn-s-cm−5) | 198 (151 to 251) | 206 (141 to 293) | 0.283 |
- 6MWD, 6 min walk distance; ACE, angiotensin converting enzyme; AF, atrial fibrillation; AT II, angiotensin II; CO, cardiac output; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; max., maximum; min., minimum; MOLLI-ECV, modified Look-Locker inversion recovery sequence derived extracellular volume; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; TPG, transpulmonary pressure gradient; Vi, volume index.
- Values are given as mean ± SD or median and interquartile range or total numbers and per cent. Significant P values are in bold.
To assess associations with event-free survival, separate univariate Cox regression models were performed for all specific LA CMR parameters for patients in SR and in AF (Table 3). Adjusted hazard ratios (adj. HR) per 1-SD decline in conduit, booster pump, and total LA-EF or 1-SD increase in maximum and minimum LAVi were calculated. Adjustment was performed for age, 6MWD, NT-proBNP, and RV EF (Figure 1).
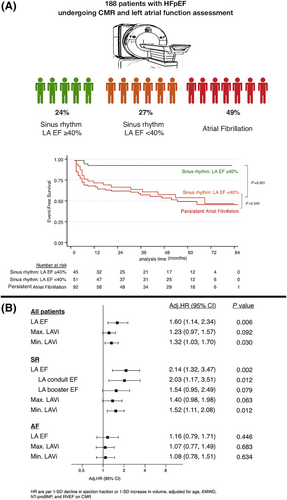
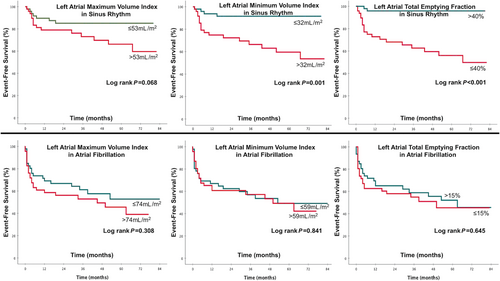
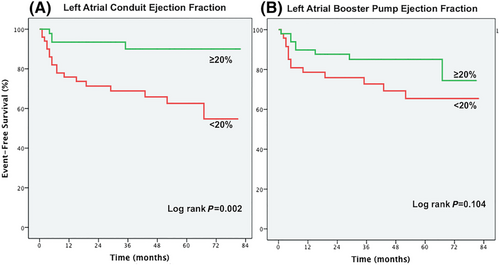
Kaplan–Meier plots (log-rank test) were applied to verify the time-dependent discriminative power of maximum and minimum LAVi, conduit, booster pump, and total LA-EF on cardiovascular outcome (Figures 1, 2, and 3).
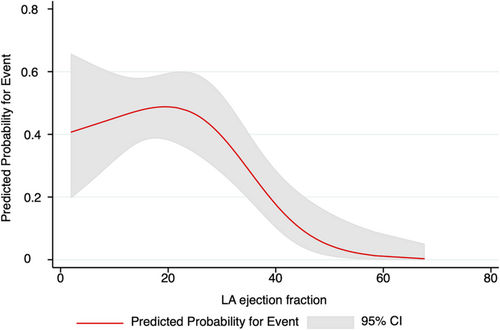
In an exploratory step, we performed restricted cubic splines (three knots) to assess the association between LA-EF and the composite endpoint and identify a potential cut-off value indicating higher risk (Figure 4). In addition, receiver operating characteristic analysis and the Youden Index were used to report a cut-off value in our cohort with best discriminative power for event prediction.
Statistical analyses were performed using dedicated software (IBM SPSS Version 21, SPSS Inc, Chicago, IL) and Stata 15.1 (StataCorp, College Station, TX) with significance level set to P < 0.05 for all tests unless stated otherwise.
Results
Study population
Between December 2010 and November 2017, a total 343 HFpEF patients were screened for enrolment. Fifty patients were excluded because of significant coronary artery disease (n = 18), NT-proBNP < 220 pg/mL (n = 16), cardiac amyloidosis (n = 15), and chronic thromboembolic pulmonary hypertension (n = 1). One hundred and five patients did not undergo CMR either due to contraindications (n = 46) or refusal (n = 52), and n = 7 (in AF during CMR examination) were excluded due to insufficient CMR image quality for LA function analysis. CMR analysis time was not significantly prolonged in AF patients.
The final study cohort comprised 188 patients. Table 1 displays clinical and CMR baseline characteristics stratified by ECG rhythm. Ninety-six patients (51%) were in SR and 92 (49%) in AF at the time of CMR. All AF patients had persistent AF. Thirty (31%) patients in SR at the time of CMR had a history of paroxysmal AF but did not differ from SR patients according to baseline characteristics (Supporting Information, Table S1). Patients in AF were older (P = 0.017), had a higher heart rate (P = 0.014), a shorter 6MWD (P = 0.040), and were in worse New York Heart Association functional class (P = 0.003). Furthermore, they had higher serum levels of NT-proBNP and gamma-glutamyl-transferase (each P < 0.001, respectively).
On CMR, patients with AF had markedly pronounced bi-atrial and RV dilatation and decreased bi-ventricular EF as compared with patients with SR (each P < 0.001 respectively, except for RV end-diastolic-volume P = 0.035). In addition, patients with AF presented with higher ECV on CMR (P < 0.001). No positive late gadolinium enhancement was found in any patient.
Left atrial volume and function in sinus rhythm vs. atrial fibrillation
All phasic volume parameters were significantly increased, and in contrast, total LA-EF significantly impaired in AF patients when compared with those in SR (Table 1; P < 0.001 for all).
Notably, among SR patients with and without a history of paroxysmal AF, no significant differences were observed (P > 0.05 for all; Table S1).
Left atrial function in correlation to functional capacity and backward and forward coupling
Table 2 summarizes the association of phasic LA function and markers of exercise capacity, LV diastolic function, and pulmonary vascular function. By correlation analysis, in patients with SR, LA conduit function (but not booster pump function) was significantly related with 6MWD (ρ = 0.24, P = 0.032). In addition, LA conduit function was significantly correlated with systolic pulmonary artery pressure (sPAP) (ρ = −0.22, P = 0.049) and pulmonary vascular resistance (ρ = −0.40, P = 0.001) as markers for backward coupling to the pulmonary vasculature. However, LA volumetric and functional parameters were not significantly correlated with LV EF (as evaluated by CMR) (Table 2). Furthermore, total LA-EF showed no significant correlation with LV global longitudinal strain in SR as well as in AF (ρ = −0.01, P = 0.975 and ρ = −0.11, P = 0.362, respectively). LA conduit function was not associated with NT-proBNP levels and LV end-diastolic pressure (ρ = −0.11, P = 0.319 and ρ = −0.02, P = 0.910, respectively). In contrast, LA booster pump EF, LA total EF, and all phasic LAVi were significantly correlated with NT-proBNP (each P < 0.001, respectively). In AF patients, LA functional parameters showed no significant correlations with 6MWD, sPAP, and pulmonary vascular resistance (P > 0.05 for all).
| 6MWD (m) | NT-proBNP (ng/mL) | LVEDP (mmHg) | sPAP (mmHg) | PVR (dyn-s-cm−5) | LV EF (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | P value | ρ | P value | ρ | P value | ρ | P value | ρ | P value | ρ | P value | |
| Sinus rhythm | ||||||||||||
| LA functional measurements | ||||||||||||
| Conduit EF (%) | 0.24 | 0.032 | −0.11 | 0.319 | −0.02 | 0.910 | −0.22 | 0.049 | −0.40 | 0.001 | −0.04 | 0.690 |
| Booster pump EF (%) | 0.03 | 0.776 | −0.46 | <0.001 | −0.02 | 0.967 | −0.01 | 0.859 | −0.10 | 0.441 | −0.03 | 0.817 |
| Total EF (%) | 0.17 | 0.125 | −0.43 | <0.001 | −0.11 | 0.396 | −0.20 | 0.080 | −0.27 | 0.025 | −0.04 | 0.698 |
| LA volumetric measurements | ||||||||||||
| Vi max (mL/m2) | <0.01 | 0.984 | 0.28 | 0.008 | 0.10 | 0.424 | 0.09 | 0.430 | −0.07 | 0.587 | −0.01 | 0.980 |
| Vi pre A (mL/m2) | −0.09 | 0.414 | 0.29 | 0.006 | 0.11 | 0.417 | 0.17 | 0.143 | 0.06 | 0.614 | −0.01 | 0.949 |
| Vi min (mL/m2) | −0.10 | 0.367 | 0.40 | <0.001 | 0.13 | 0.323 | 0.19 | 0.086 | 0.10 | 0.414 | 0.01 | 0.961 |
| Atrial fibrillation | ||||||||||||
| LA functional measurements | ||||||||||||
| Total EF (%) | 0.08 | 0.493 | −0.15 | 0.166 | 0.18 | 0.164 | −0.14 | 0.204 | −0.06 | 0.582 | −0.02 | 0.893 |
| LA volumetric measurements | ||||||||||||
| Vi max (mL/m2) | 0.01 | 0.907 | 0.09 | 0.421 | −0.07 | 0.600 | 0.01 | 0.908 | −0.12 | 0.262 | −0.01 | 0.956 |
| Vi min (mL/m2) | −0.00 | 0.987 | 0.11 | 0.297 | −0.12 | 0.351 | 0.05 | 0.644 | −0.08 | 0.495 | −0.01 | 0.968 |
- 6MWD, 6 min walk distance; ρ, Spearman correlation; EF, emptying fraction; LA, left atrial; LVEDP, left ventricular end-diastolic pressure; max., maximal; min., minimal; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary artery pressure; Vi, volume index.
- Significant P values are in bold.
Left atrial function and cardiovascular outcome
Patients were followed for 31 (9–57) months. Sixty-five (35%) patients reached the combined endpoint defined as hospitalization for heart failure (n = 60) or cardiovascular death (n = 5). Patients with persistent AF were at significantly higher risk to experience the composite endpoint as compared with patients in SR [HR 3.02, 95% confidence interval (CI) 1.77–5.16, log-rank, P < 0.001]. Table 3 summarizes details of the univariable Cox regression results.
| Univariate | ||||
|---|---|---|---|---|
| Variable | No event | Event | Hazard ratio (95% CI) | P value |
| Left atrial magnetic resonance imaging parameters in sinus rhythm | ||||
| (n = 74) | (n = 22) | |||
| LA volumetric analyses | ||||
| Maximum Vi (mL/m2) | 53 ± 15 | 62 ± 26 | 1.01 (0.99–1.03) | 0.084 |
| Vi pre A-wave, (mL/m2) | 41 ± 12 | 52 ± 24 | 1.02 (1.00–1.04) | 0.019 |
| Minimum Vi (mL/m2) | 31 ± 12 | 45 ± 24 | 1.02 (1.01–1.04) | 0.001 |
| Conduit EF (%) | 21 ± 6 | 16 ± 7 | 0.92 (0.86–0.98) | 0.008 |
| Booster EF (%) | 20 ± 9 | 16 ± 10 | 0.96 (0.92–1.01) | 0.087 |
| Total EF (%) | 42 ± 10 | 31 ± 10 | 0.94 (0.90–0.97) | <0.001 |
| Left atrial magnetic resonance imaging parameters in atrial fibrillation | ||||
| (n = 49) | (n = 43) | |||
| LA volumetric analyses | ||||
| Maximum LAVi (mL/m2) | 75 ± 21 | 80 ± 27 | 1.00 (0.99–1.02) | 0.675 |
| Minimum LAVi (mL/m2) | 63 ± 20 | 69 ± 24 | 1.00 (0.99–1.02) | 0.560 |
| LA total EF (%) | 17 ± 8 | 14 ± 6 | 0.98 (0.94–1.02) | 0.318 |
- 6MWD, 6 min walk distance; AF, atrial fibrillation; EF, ejection fraction; LA, left atrial; max., maximum; min., minimum; RV, right ventricular; sPAP, systolic pulmonary artery pressure; SR, strain rate; Vi, volume index.
- Values are given as mean ± SD or median and interquartile range or total numbers and per cent. Significant P values are in bold.
In patients with SR, several LA volumetric and functional parameters were related with outcome in the univariable regression analysis. In contrast, in patients with persistent AF, no LA parameter was related with outcome (Table 3 & Figures 2 and 3). However, these patients carried a similarly dismal prognosis when compared with patients in SR with impaired LA-EF (log-rank, P = 0.340) (Figure 1).
Figure 1 displays the association of LA volumetric and functional parameters with outcome adjusted for established risk factors, including age, 6MWD, NT-proBNP, and RV EF in SR vs. persistent AF. In SR, LA-EF is independently related with outcome (adj. HR 1.60, 95% CI 1.14–2.34, P = 0.006). This effect seems to be mainly driven by LA conduit EF (adj. HR 2.03, 95% CI 1.17–3.51, P = 0.012) (Figure 3). Furthermore, also minimum LAVi is independently related with outcome (adj. HR 1.52, 95% CI 1.11–2.08, P = 0.012). In patients in persistent AF, no LA parameter was related with outcome after adjustment for established risk factors (Figure 1).
In receiver operating characteristic analysis and Youden's J statistics demonstrated that an LA-EF cut-off of <40% carried a sensitivity of 90% and specificity of 65% (area under the curve 0.77; 95% CI 0.65–0.90) to predict an adverse outcome. This cut-off was further confirmed by visual assessment in a restricted cubic spline model demonstrating a stark increase in risk for events when LA-EF is lower than 40% (Figure 4).
Discussion
- Fifty per cent of HFpEF patients present in SR, among whom total LA-EF is independently associated with heart failure hospitalization and cardiovascular death. This effect is mainly driven by its passive emptying (conduit) function but not by its active emptying (booster pump) function. Moreover, LA conduit function is significantly correlated with pulmonary vascular function and exercise capacity.
- In HFpEF patients with persistent AF, LA size and function does not add prognostic information and is not related with pulmonary vascular function or exercise capacity.
- HFpEF patients in persistent AF do have a similar risk for worse cardiovascular outcome as compared with patients in SR with impaired LA-EF.
Prognostic value of left atrial volume and function
Left atrial enlargement has long been recognized as key feature in patients with HFpEF and reflects increased filling pressures due to diastolic dysfunction. Maximum LAVi is an established risk factor for the prediction of cardiovascular events.8, 21 More recent studies suggest that LA-EF and minimum LAVi are even more closely related with prognosis in the elderly and in HFpEF patients as compared with maximum LAVi.6, 8, 22, 23 These findings are in line with our results where minimum LAVi and total LA-EF outperformed maximum LAVi in predicting cardiovascular events (Table 3 & Figure 3), even after adjustment for established risk factors in HFpEF such as age, 6MWD, NT-proBNP, and RV EF (Figure 1).3, 18, 24, 25
Left atrial function in sinus rhythm and correlation to functional capacity and pulmonary vasculature
In SR, total LA-EF is put together by the passive conduit EF, which starts just after mitral valve opening and active booster pump EF, which represents active LA contraction.
- In our study cohort, LA conduit EF but no other LA function parameter was associated with 6MWD (Table 2), an established parameter of exercise capacity in HFpEF patients. These results complement recent findings of von Roeder et al.9 They found a close and independent association of LA conduit function and peak oxygen consumption in their CMR study on HFpEF patients. However, their findings were limited by small sample size (only 22 HFpEF patients) and lack of outcome data.
- Furthermore, only LA conduit EF showed a close correlation to sPAP (ρ = −0.28, P = 0.013) and pulmonary vascular resistance (ρ = −0.40, P = 0.001) as markers for pulmonary vascular performance (Table 2). Melenovsky et al.6 have recently described a correlation of LA function and pulmonary vascular function in HFpEF. Freed et al.27 confirmed this correlation.
In contrast to LA booster pump and total EF, LA conduit function did not show any association with NT-proBNP. Considering its close correlation with sPAP and pulmonary vascular resistance, it can be assumed that parameters of LA conduit function are rather reflecting pulmonary vascular than cardiac performance.28
Specific considerations concerning heart failure with preserved ejection fraction with persistent arterial fibrillation
Data regarding LA function in the context of AF in patients with HFpEF are limited.13 However, this is important as a substantial fraction of these patients present with AF.
Almost 65% of our study participants had a history of AF (16% paroxysmal & 49% persistent AF), which is in line with previous reports.12, 29 No difference regarding LA parameters was shown between patients with a history of paroxysmal AF and SR (Table S1), whereas patients in persistent AF presented with significantly increased LA volumes and relevant functional impairment (Table 1). LA parameters in AF patients were not associated with cardiovascular events, not even in the univariate analysis (Table 3 & Figures 2 and 3). This most likely was related to the fact that AF patients were at advanced stages of the disease, were older, and presented with shorter 6MWD, impaired RV performance, and higher LV ECV (Table 1).3, 18, 25, 30 Apparently, at this stage of disease, LA performance had lost its prognostic impact.
AF, and in particular persistent AF, is an established independent marker for adverse outcome in HFpEF.12, 29 Our data implicate that further risk stratification by evaluation of LA size and function is not expedient in this patient population. On the other hand, HFpEF patients in SR with an LA-EF < 40% have a similar risk for adverse outcome compared with HFpEF patients in persistent AF.
Limitations
This was a single-centre study; thus, a centre-specific bias cannot be ruled out although single-centre studies have several advantages such as homogenous patient selection, continuous workflow, and constant follow-up. Patients in AF during CMR study were deliberately not excluded from the trial as the proportion of HFpEF patients suffering from AF is up to 60%.29, 12 Furthermore, volumetric assessment of LA function in patients with AF during CMR image acquisition is well validated.15
However, it remains a limitation that LA volumetric assessment was not performed four-dimensional.
Also, image quality on CMR and scan times may be influenced in case of substantial arrhythmias. Although CMR studies with under average image quality were excluded from the analysis and LA volumetric analyses were performed as an average of three biplane measurements (equals six measurements), measuring errors cannot be fully excluded. As continuous ECG monitoring, for example, by implantable loop recorders was not performed in every patient, asymptomatic AF episodes may have been missed and real life burden of AF episodes might be higher. The cross-sectional observational study design limits conclusions about cause–effect relationships.
Conclusions
In HFpEF patients in SR, total LA-EF is independently associated with cardiovascular outcome. This effect is mainly driven by its passive emptying (conduit) function but not by its active emptying (booster pump) function. With disease progression including the development of persistent AF, LA functional parameters lose their prognostic ability. Our study promotes the use of LA-EF rather than the still widely used LA maximum volume for risk stratification in patients with HFpEF.
Clinical implications
For HFpEF patients in SR, assessment of LA-EF is superior to static LA volumetric measurements in predicting adverse events. Our data as well as several other studies support its major superiority over still widely used maximal LA expansion8 for defining HFpEF patients at risk to experience future cardiovascular events. Our results suggest an LA-EF value of <40% (in HFpEF SR) as the best predictive cut-off for adverse cardiovascular outcome. However, large-scale future studies are warranted to confirm this value.
Further studies on treatment of the underlying atrial myopathy in early stages of HFpEF including pharmacological intervention31 or AF ablation32 are warranted to possibly prevent further deterioration of LA function accompanied by the onset of permanent AF and pericardial restraint.13
Conflict of interest
The authors declare no disclosures.
Funding
This work was supported by the Austrian Society of Cardiology (to J.M. and F.D.).




