Fibrosis-4 index reflects right ventricular function and prognosis in heart failure with preserved ejection fraction
Abstract
Aims
Fibrosis-4 index (FIB-4 index), calculated by age, aspartate aminotransferase, alanine aminotransferase, and platelet count, is a simple marker to evaluate liver fibrosis and is associated with right-sided heart failure. However, the clinical relevance of FIB-4 in patients with heart failure with preserved ejection fraction (HFpEF) remains unclear. We investigated the prognostic implication of the FIB-4 index regarding right ventricular dysfunction in patients with HFpEF.
Methods and results
This prospective study included 116 consecutive HFpEF patients (mean age 79 years, 43% male) hospitalized with acute decompensated heart failure. We evaluated the association of the FIB-4 index with right ventricular function determined by tricuspid annular plane systolic excursion (TAPSE) and tricuspid lateral annular systolic velocity (S′) before discharge. Cox regression analysis was performed to evaluate the association between the FIB-4 index and major adverse cardiovascular events (MACE) defined as the composite of cardiovascular death, readmission for heart failure, nonfatal myocardial infarction, and nonfatal stroke. FIB-4 index before discharge was significantly lower than that at admission (2.62 [1.92–3.46] and 3.03 [2.05–4.67], median [interquartile range], P < 0.001). Left ventricular ejection fraction, TAPSE, and S′ before discharge were 62.7 (55.9–68.6) %, 17.5 ± 4.6 mm (mean ± standard deviation), and 10.0 (8.0–12.0) cm/s, respectively. In multiple linear regression analysis, the FIB-4 index before discharge was inversely correlated with TAPSE (β minus;0.244, P = 0.014) and S′ (β −0.266, P = 0.009). During a median follow-up of 736 days, 37 MACE occurred. Multivariate Cox regression analysis revealed that a high FIB-4 index before discharge (per 1 point) was a significant predictor of MACE (hazard ratio 1.270, 95% confidence interval 1.052–1.532) after adjustment for male, serum creatinine, and haemoglobin. Receiver operating characteristic analysis indicated that the optimal cut-off value of FIB-4 index before discharge to predict MACE was 3.11. Kaplan–Meier survival analysis showed that patients with a FIB-4 index before discharge ≥3.11 had a significantly poorer prognosis than patients with FIB-4 index before discharge <3.11 (P = 0.029). Patients with an FIB-4 index ≥3.11 had a 2.202-fold (95% confidence interval 1.110–4.368) increased risk of MACE compared with those with an FIB-4 index <3.11 after adjustment for male, serum creatinine, and haemoglobin.
Conclusions
An increase in the FIB-4 index was associated with right ventricular dysfunction and a higher risk of future MACE in patients with HFpEF.
Introduction
Heart failure (HF) causes liver congestion and/or hypoperfusion and is occasionally associated with liver fibrosis.1 Liver biopsy was used to diagnose and assess the severity of liver fibrosis, but it is invasive and cannot be performed as a screening test.2, 3 Liver stiffness measured by transient elastography is a useful and simple method to assess liver fibrosis non-invasively and was reported to increase as central venous pressure increased and right ventricular (RV) function worsened.4, 5 Furthermore, an increase in liver stiffness was associated with a worse prognosis.6 Therefore, the assessment of liver stiffness is an alternative method to evaluate the severity of congestion and to predict the prognosis of patients with HF. However, transient elastography is difficult to use in daily practice because of the need for special equipment (echo machine), and it is time consuming and expensive. In addition, reliable assessment by transient elastography can be disturbed by ascites, narrow intercostal spaces, and morbid obesity.4 Thus, a simple index to evaluate liver fibrosis is needed.
The fibrosis-4 index (FIB-4 index) was reported as a simple index to evaluate liver fibrosis.7 The FIB-4 index is calculated from four parameters: age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count. The FIB-4 index was reported to reflect liver fibrosis associated with viral hepatitis of human immunodeficiency virus or hepatitis C virus infection and non-alcoholic fatty liver disease.7, 8 In patients with HF, the FIB-4 index increased as N-terminal pro-brain natriuretic peptide (NT-pro-BNP), right atrial pressure, and all-cause mortality increased.9-11 Currently, the FIB-4 index is thought to be a surrogate marker to evaluate the severity of venous congestion and prognosis in patients with HF. However, it remains unknown whether the FIB-4 index is related to the severity of RV dysfunction and whether a high FIB-4 index is associated with an increase in cardiovascular events in patients with HF with preserved ejection fraction (HFpEF), who are increasing in number because of ageing populations.
In this study, we investigated the relationship between the FIB-4 index and echocardiographic RV function in patients hospitalized for acute decompensated heart failure (ADHF) diagnosed as HFpEF. We compared the incidence of major adverse cardiovascular events (MACE) after discharge between patients with a high or low FIB-4 index.
Methods
Study patients
From February 2012 to December 2015, 165 patients admitted to the National Hospital Organization Iwakuni Clinical Center for ADHF and diagnosed as HFpEF were prospectively enrolled. A primary diagnosis of ADHF included signs and symptoms of fluid overload. Patients were eligible for inclusion in the trial if they had a left ventricular ejection fraction (LVEF) of 50% or more by echocardiography performed on admission. Patients who died before discharge (n = 3), with chronic liver diseases (hepatitis B, n = 4; hepatitis C, n = 12; primary biliary cirrhosis, n = 1; alcoholic liver disease, n = 3; non-alcoholic fatty liver disease, n = 1, history of hospitalization due to liver disorder of unknown cause, n = 1), and who underwent planned thoracic surgery and/or percutaneous coronary intervention within a half year after the onset of ADHF (n = 24) were excluded. Chronic liver diseases were diagnosed by a hepatologist along with blood examination, pre-existing liver disease, and history of treatment. Therefore, 49 patients were excluded from the study, and the remaining 116 patients were analysed. The investigation conforms to the principles outlined in the Declaration of Helsinki. This study was approved by our institution's human research committee, and all patients enrolled in the study provided written informed consent.
Fibrosis-4 index
Data of blood examinations on admission and before discharge were used. The FIB-4 index was calculated as follows: FIB-4 index = age (years) × AST (U/L)/[ALT (U/L)1/2 × platelet count (109/L)]. In addition, total bilirubin and serum albumin to evaluate liver function, haemoglobin, creatinine, HgbA1c, and NT-pro-BNP were measured because these parameters are thought to affect cardiovascular prognosis independently.
Echocardiography
A full echocardiographic assessment of cardiac functions was performed by experienced technicians using an IE33 echo machine (Philips Japan, Ltd., Tokyo, Japan) before discharge. The technicians who performed echocardiography were blinded to the FIB-4 index. From the apical approach, we measured the tricuspid annular plane systolic excursion (TAPSE) and tricuspid lateral annular systolic velocity (S′) to assess RV function. TAPSE was measured by the distance of the systolic excursion of the RV annulus along its longitudinal plane using M-mode presentation in an RV-focused apical 4-chamber view. S′ was measured by the velocity of tricuspid lateral annular using Doppler tissue imaging in an RV-focused apical 4-chamber view. The LVEF was measured by the modified Simpson technique using B-mode presentation in apical-2-chamber view and apical-4-chamber view. We also measured the peak early diastolic velocities (E), and the early diastolic myocardial velocities (e′) using general methods. The ratio of E and e′ (E/e′) was calculated to estimate LV filling pressures. The inter-observer variability of LVEF, TAPSE, and S′ was evaluated for 21 patients with heart failure independent of this study.
Endpoints and follow-up
All clinical events after discharge were obtained by medical records retrospectively until November 2016. The endpoint was defined as MACE, which is the composite outcome of death from cardiovascular causes, readmission for heart failure, nonfatal non-ST elevation/ST elevation, myocardial infarction, and ischaemic/non-ischaemic stroke.12
Statistical analysis
Categorical variables are shown as numbers (%). Continuous variables that were normally distributed are shown as the means ± standard deviation and were compared between groups using the Student's t-test. Continuous variables that were not normally distributed are shown as medians with interquartile ranges (IQR) and were compared using the Mann–Whitney U-test. The association of TAPSE and S′ with clinical variables including the FIB-4 index was examined using univariate and multivariate linear regression analyses. Continuous variables that were not normally distributed were turned into natural logarithmic values for linear regression analysis. In multivariate regression analysis, components of the FIB-4 index including age, AST, ALT, and platelet count were not included. Pearson linear regression analysis and Bland–Altman plots were performed to evaluate the inter-observer variability of the LVEF, TAPSE, and S′. Prognostic factors including the FIB-4 index, sex, serum creatinine, and haemoglobin were evaluated by multivariate Cox regression analysis. We evaluated the association with NT-pro-BNP instead of the FIB-4 index using the same model. To assess MACE, receiver operating characteristic (ROC) curve analysis was performed. The optimal cut-off value was defined as the point maximizing the Youden index (=max[sensitivity + specificity − 1]). To assess the prognostic value, the optimal cut-off values of the FIB-4 index were used to divide patients into two groups for Kaplan–Meier analysis, with event-free survival compared using a two-sided log-rank test. Statistically significant values were defined when P < 0.05. These analyses were performed using SPSS statistical software (version 25; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics and laboratory data
Table 1 shows the clinical characteristics of all patients. Study patients included 50 men (43%) with a mean age of 79 years. The median duration of hospitalization was 19 days (12.0–30.5 days), and the median follow-up period was 736 days (328–1103 days). Table 2 shows laboratory data from the day of admission and before discharge. The FIB-4 index before discharge was significantly lower than at admission (2.62 [1.92–3.46] and 3.03 [2.05–4.67], P < 0.001). AST, ALT, total bilirubin, and NT-proBNP before discharge were also lower than at admission (P < 0.001, P = 0.002, P < 0.001, and P < 0.001). Haemoglobin, platelet count, albumin, and serum creatinine did not differ between admission and discharge.
| Variables | n = 116 |
|---|---|
| Age, years | 79 ± 10 |
| Male | 50 (43) |
| Body mass index, kg/m2 | 21.9 ± 4.2 |
| Systolic blood pressure, mmHg | 121.8 ± 18.8 |
| Heart rate, b.p.m. | 69 ± 14 |
| Hypertension | 69 (59) |
| Diabetes mellitus | 32 (28) |
| Dyslipidaemia | 31 (27) |
| Atrial fibrillation | 60 (52) |
| Chronic heart failure | 38 (33) |
| COPD | 15 (13) |
| Prior PCI | 15 (13) |
| Prior CABG | 9 (8) |
| Medications on admission | |
| Beta-blockers | 45 (39) |
| ACEIs/ARBs | 63 (54) |
| Diuretics | 65 (54) |
| Echocardiographic data | |
| LVEF, % | 62.7 (55.9–68.6) |
| E/e′ | 13.6 (10.0–17.7) |
| TAPSE, mm | 17.5 ± 4.6 |
| S′, cm/s | 10.0 (8.0–12.0) |
- Data are presented as the number (%), mean ± standard deviation, or median (25th–75th percentile).
- ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; E/e′, early diastolic filling velocity/early diastolic velocity of the mitral annulus; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; S′, tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion.
| Variable | On admission | Before discharge | P value |
|---|---|---|---|
| Haemoglobin, g/dL | 11.4 ± 2.3 | 11.5 ± 1.7 | 0.450 |
| Platelet count, 109/L | 184.1 ± 68.9 | 194.2 ± 67.8 | 0.090 |
| AST, IU/L | 40 ± 37 | 26 ± 12 | <0.001 |
| ALT, IU/L | 31 ± 39 | 19 ± 15 | 0.002 |
| Fibrosis-4 index | 3.03 (2.05–4.67) | 2.62 (1.92–3.46) | <0.001 |
| Total bilirubin, g/dL | 0.8 (0.5–1.2) | 0.6 (0.5–0.9) | <0.001 |
| Albumin, g/dL | 3.6 ± 0.5 | 3.5 ± 0.6 | 0.213 |
| Serum creatinine, mg/dL | 1.0 (0.7–1.3) | 1.0 (0.8–1.4) | 0.419 |
| NT-proBNP, pg/mL | 3734 (1662–7658) | 1368 (630–2888) | <0.001 |
- Data are presented as the number (%), mean ± standard deviation, or median (25th–75th percentile).
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; HgbA1c, haemoglobin A1c; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Association of the fibrosis-4 index with tricuspid annular plane systolic excursion and S′
LVEF, TAPSE, S′, and E/e′ before discharge were 62.7 (55.9–68.6) %, 17.5 ± 4.6 mm, 10.0 (8.0–12.0) cm/s, and 13.6 (10.0–17.7), respectively. In terms of inter-observer variability, the measurements of LVEF, TAPSE, and S′ correlated well between the two technicians (r = 0.965 [P < 0.001], r = 0.899 [P < 0.001], and r = 0.826 [P < 0.001], respectively). The Bland–Altman analyses for LVEF, TAPSE, and S′ are shown in Figure 1(A–C).
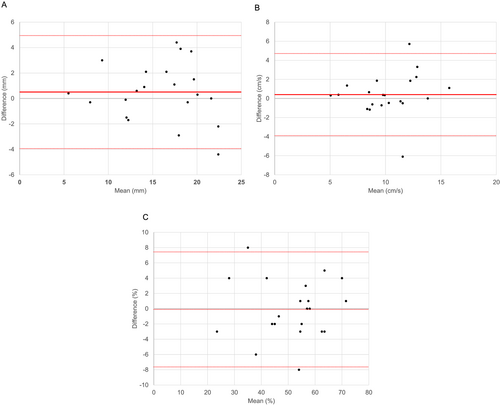
Table 3 shows the univariate and multivariate analyses of factors related to TAPSE and S′. TAPSE was significantly related to age (P = 0.003), systolic blood pressure (P = 0.004), platelet count (P = 0.011), and FIB-4 index (P = 0.001) by univariate analysis. Multivariate regression analysis revealed that TAPSE was significantly related to the FIB-4 index (P = 0.014). In addition, S′ was also significantly related to systolic blood pressure (P = 0.004), platelet count (P < 0.001), and FIB-4 index (P < 0.001) by univariate analysis. Multivariate regression analysis revealed that S′ was significantly related to the FIB-4 index (P = 0.009).
| TAPSE | S′a | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | P value | β | P value | β | P value | β | P value | |
| Age | −0.277 | 0.003 | −0.058 | 0.522 | ||||
| Male | 0.119 | 0.206 | 0.025 | 0.796 | ||||
| Body mass index | 0.158 | 0.096 | 0.007 | 0.944 | ||||
| Systolic blood pressure | 0.273 | 0.004 | 0.182 | 0.063 | 0.279 | 0.004 | 0.177 | 0.079 |
| Heart rate | −0.050 | 0.601 | −0.073 | 0.462 | ||||
| Haemoglobin | 0.004 | 0.969 | −0.173 | 0.075 | ||||
| Platelet count | 0.237 | 0.011 | 0.415 | <0.001 | ||||
| AST | −0.067 | 0.477 | 0.013 | 0.894 | ||||
| ALT | −0.040 | 0.668 | 0.078 | 0.452 | ||||
| Fibrosis-4 indexa | −0.300 | 0.001 | −0.244 | 0.014 | −0.333 | <0.001 | −0.266 | 0.009 |
| Total bilirubina | −0.019 | 0.857 | −0.141 | 0.190 | ||||
| Albumin | −0.166 | 0.138 | −0.161 | 0.161 | ||||
| Creatininea | 0.095 | 0.320 | <0.001 | 0.996 | ||||
| NT-proBNPa | −0.087 | 0.490 | −0.035 | 0.784 | ||||
| LVEFa | −0.016 | 0.867 | 0.114 | 0.238 | ||||
| E/e′a | −0.128 | 0.176 | −0.086 | 0.377 | ||||
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; E/e′, early diastolic filling velocity/early diastolic velocity of the mitral annulus; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; S′, tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion.
- a Values are logarithm transformed.
Relationship between the fibrosis-4 index and major adverse cardiovascular events
During the median follow-up of 736 days, 37 MACE occurred (two cardiovascular deaths, 30 readmissions for heart failure, and five strokes). As shown in Table 4, multivariate Cox regression analysis showed that the FIB-4 index on admission was significantly associated with the incidence of MACE (hazard ratio [HR] 1.124, 95% confidence interval [CI] 1.008–1.253, P = 0.035) after adjustment for male, serum creatinine, and haemoglobin. In addition, as shown in Table 5, the FIB-4 index before discharge was significantly associated with the incidence of MACE (HR 1.270, 95% CI 1.052–1.532, P = 0.013) after adjustment for male, serum creatinine, and haemoglobin. However, when NT-proBNP before discharge was used instead of FIB-4 in the same model, there was no significant association between NT-proBNP and the incidence of MACE (HR 1.000, 95% CI 0.999–1.000, P = 0.458).
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Fibrosis-4 index, per 1 point | 1.124 | 1.008 to 1.253 | 0.035 |
| Male | 0.829 | 0.395 to 1.741 | 0.620 |
| Creatinine, per 1 mg/dL | 1.512 | 1.184 to 1.931 | 0.001 |
| Haemoglobin, per 1 mg/dL | 0.950 | 0.765 to 1.179 | 0.641 |
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Fibrosis-4 index, per 1 point | 1.27 | 1.052 to 1.532 | 0.013 |
| Male | 0.999 | 0.494 to 2.019 | 0.997 |
| Serum creatinine, per 1 mg/dL | 1.467 | 1.158 to 1.859 | 0.002 |
| Haemoglobin, per 1 mg/dL | 0.930 | 0.752 to 1.152 | 0.507 |
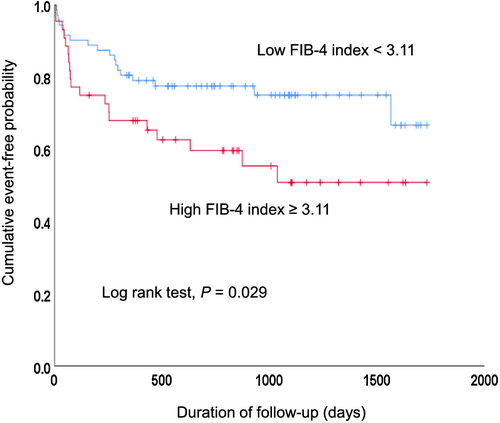
The ROC curve analysis showed that C-statics of the FIB-4 index before discharge to predict MACE were 0.573 (95% CI 0.462–0.685, P = 0.204), and the value maximizing the Youden index was 3.11. Figure 2 shows the Kaplan–Meier analysis of patients stratified according to the optimal cut-off value of the FIB-4 index before discharge. Patients with a higher FIB-4 index before discharge had a significantly poorer prognosis than patients with a lower FIB-4 index before discharge (P = 0.029). Patients with an FIB-4 index >3.11 had a 2.202-fold (95% CI 1.110–4.368) increased risk of MACE after adjustment for male, serum creatinine, and haemoglobin.
Discussion
This study investigated the potential relationship between the FIB-4 index and RV function assessed by TAPSE and S′ and to MACE after discharge in HFpEF patients hospitalized for ADHF. The high FIB-4 index group had a lower TAPSE and S′ and a higher incidence of MACE after discharge compared with the low FIB-4 index group. To the best of our knowledge, this is the first study to demonstrate that the FIB-4 index is a simple biomarker of the severity of RV dysfunction and is a poor prognostic marker for HFpEF patients.
Association of fibrosis-4 index and liver fibrosis
Liver biopsy remains the reference standard to assess the severity of liver disease. Currently, several non-invasive methods including serum biomarkers and imaging techniques are under investigation for the quantification of liver fibrosis. Among these methods, the FIB-4 index has been used as a surrogate marker of liver stiffness.7 A recent comparative study including chronic liver diseases such as non-alcoholic fatty liver disease and chronic hepatitis C demonstrated that the diagnostic performance of gadoxetate-enhanced MRI, transient elastography, and FIB-4 index to predict significant fibrosis was sufficiently accurate (area under the ROC: 0.78, 0.80, and 0.67, respectively).13 Thus, the FIB-4 index might be a marker of liver fibrosis in chronic liver disease.
Correlation of fibrosis-4 index with echocardiographic parameters of right ventricular function
The FIB-4 index is considered a simple biomarker of liver fibrosis in patients with liver diseases. However, the liver becomes stiff because of long-term hepatic congestion and accompanying fibrosis in patients with HF. Furthermore, liver stiffness measured by transient elastography increases as the central venous blood pressure increases.14 Pulmonary hypertension and right-sided heart failure occur more often in HFpEF patients than in heart failure with reduced ejection fraction (HFrEF) patients. RV dysfunction exacerbates right-sided heart failure and TAPSE and S′ are indexes of RV contraction in the longitudinal plane. The American Society of Echocardiography recommends using TAPSE and S′ to assess RV function because they show a correlation with other measures of RV global systolic function.15 Another study demonstrated that liver stiffness, measured by transient elastography, increased with a decrease in TAPSE.6 In this study, we demonstrated that a high FIB-4 index was associated with lower TAPSE and S′. Therefore, the FIB-4 index can be regarded as a biomarker for RV dysfunction in patients with HFpEF.
Prognostic impact of fibrosis-4 index in heart failure with preserved ejection fraction
The prognosis of patients with HF deteriorates as the RV function deteriorates and right heart failure progresses. Several studies indicated that RV dysfunction is an independent prognostic factor for HFpEF patients.16-18 Furthermore, a high FIB-4 index was associated with an increase in all-cause mortality in patients hospitalized for ADHF.11 In this study, we focused on patients with HFpEF and ADHF and also found that a high FIB-4 index was associated with an increase in the incidence of cardiovascular events. Our results indicate that the FIB-4 index is both a biomarker of RV function and a predictor of cardiovascular events in HFpEF patients. Thus, the FIB-4 index may provide important information on pathological changes in the liver in addition to RV dysfunction. There is a pathological link between the heart and the liver.1 HF causes liver congestion and hypoperfusion, and if it continues for a long time, liver fibrosis can occur, which reduces natriuresis and enhances fluid retention19 and arterial sclerosis, which impairs left ventricular diastolic function.20, 21
The cut-off value of the FIB-4 index to predict the prognosis of patients with HF has not been fully clarified. Sato et al. reported that in patients with decompensated HF, all-cause mortality in the second tertile (1.72 ≤ FIB-4 index < 3.01) and third tertile (3.01 ≤ FIB-4 index) groups was significantly greater than that in the first tertile (FIB-4 index < 1.72) group.11 Daichi et al. reported that an FIB-4 index of 2.15 was a cut-off value to predict the composite clinical outcome of all-cause death, readmission for HF, or left ventricular assist device implantation in patients with HF.10 This study showed the optimal cut-off value was 3.11 to predict MACE in patients with HFpEF. Larger prospective studies are needed to evaluate the most appropriate cut-off value of the FIB-4 index as a risk predictor of cardiovascular prognosis in HFpEF patients.
Interestingly, NT-pro-BNP was not an independent predictor of MACE in our patients with HFpEF, and there was no significant difference in NT-pro-BNP between the high and low FIB-4 index groups. These observations contradict those of previous studies.22, 23 Other studies showed that an increase in BNP or NT-pro-BNP was associated with a worse clinical outcome in patients with HFpEF, but that the level of BNP or NT-pro-BNP was significantly lower than that in patients with HFrEF.24, 25 Another study indicated that BNP or NT-pro-BNP was not likely to be increased in right-sided heart failure alone.26 Therefore, BNP or NT-pro-BNP may be insufficient to predict the prognosis of patients with HFpEF and a history of hospitalized for ADHF. In this situation, measurement of the FIB-4 index in addition to NT-pro-BNP may add independent and valuable information regarding future MACE in patients with HFpEF.
This study had several limitations. First, the study had a relatively small sample size and was a prospective cohort study at a single centre, which may be limited and underpowered. Second, chronic liver diseases were not completely ruled out because we did not perform additional examinations such as liver biopsy or computed tomography scans for evaluation. Third, we excluded patients who underwent thoracic surgery and/or percutaneous coronary intervention within half a year of hospitalization for ADHF, because these interventions might influence MACE. Consequently, some ischaemic cardiomyopathies or valvar diseases were excluded. Finally, we evaluated the FIB-4 index before discharge in this study; therefore, changes in the FIB-4 index during hospitalization or after discharge remain unclear.
The FIB-4 index, a marker of liver fibrosis, is associated with RV dysfunction and a high risk of a future cardiovascular event in patients with HFpEF.
Acknowledgement
We thank J. Ludovic Croxford, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
None declared.
Funding
This work was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.




