Fat-1 transgenic mice rich in endogenous omega-3 fatty acids are protected from lipopolysaccharide-induced cardiac dysfunction
Abstract
Aims
Cardiac malfunctions developing in result of sepsis are hard to treat so they eventually contribute to the increased mortality. Previous reports indicated for therapeutic potential of exogenous ω-3 polyunsaturated fatty acids (PUFA) in sepsis, but potential benefits of this compound on the malfunctional heart have not been explored yet. In the present study, we investigated whether the constantly elevated levels of endogenous ω-3 PUFA in transgenic fat-1 mice would alleviate the lipopolysaccharide (LPS)-induced cardiac failure and death.
Methods and results
After both wild type (WT) and transgenic fat-1 mice were challenged with LPS, a Kaplan–Meier curve and echocardiography were performed to evaluate the survival rates and cardiac function. Proteomics analysis, RT-PCR, western blotting, immune-histochemistry, and transmission electron microscopy were further performed to investigate the underlying mechanisms. Results showed that transgenic fat-1 mice exhibited the significantly lower mortality after LPS challenge as compared with their WT counterparts (30% vs. 42.5%, P < 0.05). LPS injection consistently impaired the left ventricular contractile function and caused the cardiac injury in the wild type mice, but not significantly affected the fat-1 mice (P < 0.05). Proteomic analyses, ELISA, and immunohistochemistry further revealed that myocardium of the LPS-challenged fat-1 mice demonstrated the significantly lower levels of pro-inflammatory markers and ROS than WT mice. Meaningfully, the LPS-treated fat-1 mice also demonstrated a significantly higher levels of LC3 II/I and Atg7 expressions than the LPS-treated WT mice (P < 0.05), as well as displayed a selectively increased levels of peroxisome proliferator-activated receptor (PPAR) γ and sirtuin (Sirt)-1 expression, associated with a parallel decrease in NFκB activation.
Conclusions
The fat-1 mice were protected from the detrimental LPS-induced inflammation and oxidative stress, and exhibited enhancement of the autophagic flux activities, associating with the increased Sirt-1 and PPARγ signals.
Introduction
Release of the bacterial endotoxin, lipopolysaccharide (LPS), frequently contributes to development of the life-threatening multi-organ dysfunctions in septic patients, admitted to the intensive care unit.1 Endotoxaemia also induces deterioration of the vital cardiac functions, leading to hypotension and other adverse clinical events.2 Regrettably, even adhering to the standards care protocols, recommend by the Surviving Sepsis Campaign, the mortality rate is approximately 10% in patients whose Sepsis-related Organ Failure Assessment (SOFA) score is greater than 2 and often exceeds 40% in ones with septic shock.3 Therefore, such distressing results stimulate the constant search for the new therapeutic approaches that would improve the ultimate outcomes of septic patients.
Importantly, a recent detection of the distinct changes in lipid metabolism of the critically ill patients, inspired further studies that eventually revealed that nutritional correction of the lipid profile, could be linked to the remarkable increase of their survival rate.4, 5 Especially valuable were results, indicating that even a short-term parenteral delivery of the ω-3 fatty acid fraction of the fish oil to patients with the acute respiratory distress syndrome6 and general critical illness,7 exerted the organ-protective effects and reduction of the systemic inflammation rate. While the general anti-oxidative and anti-inflammatory properties of ω-3 FA were justified in experimental studies, the putative beneficial effects of ω-3 FA on the cardiac dysfunctions have not been adequately investigated yet and their possible involvement in mechanisms controlling autophagy in relation to the development the cardiac complications remain not fully elucidated.8
The previously published data demonstrated that while the up-regulated autophagy contributes to the adaptive response in desmin-related cardiomyopathy,9 it may also deteriorate the clinical outcome under various hemodynamic stress conditions,10 such as diphtheria toxin-induced heart failure.11 It has been also reported that challenge of neonatal rat cardiomyocytes with LPS elicited their mitochondrial biogenesis and reduction of the autophagy-related cell death.12 Yet results of other studies suggested that rapamycin-stimulated autophagy may suppress the LPS-mediated ROS production, thereby alleviating injury of cardiomyocytes.13 Importantly, a recent report showing that treatment with ω-3 PUFA suppressed macrophage inflammasome activation, by enhancing autophagy has opened yet another path, worthy of further exploration.14
The relatively recent engineering of the transgenic mice, expressing the Caenorhabditis elegans fat-1 gene, encoding the ω-3 fatty acid desaturase created a very useful experimental model for studies of lipid metabolism. Importantly, these fat-1 transgenic mice produce ω-3 polyunsaturated fatty acids (PUFA) from ω-6 PUFA, thereby maintaining a low and well-balanced ratio of ω-6/ω-3 PUFA in their tissues.15 Because the availability of these transgenic mice offered the opportunity to address the molecular events underlying the beneficial impact of ω-3 PUFA.16 We now decided to use this experimental model in the present study to further explore mechanisms that would potentially protect against the LPS-induced cardiac dysfunction.
Consequently, our present study was designed to compare the progression of the LPS-induced pathological cardiac dysfunction in the wild type and in the fat-1 transgenic mice, characterized by the heightened expression of endogenous ω-3 PUFA. We have particularly explored whether the elevated level of this particular fatty acids in the fat-1 transgenic mice, would alleviate their LPS-induced septic cardiac dysfunction. We also aimed at the eventual elucidation of cellular and molecular mechanisms of such presumed effect. Importantly, the obtained results indicated that LPS-treated fat-1 transgenic mice produced less pro-inflammatory cytokines and ROS, that associated with enhancing autophagic flux activity, as well as with the augmenting expression of PPARγ and Sirt-1, when compared with their wild type counterparts, similarly challenged with the LPS. We therefore concluded that constantly elevated levels of endogenous ω-3 fatty acids in fat-1 transgenic mice protected them from the LPS-induced cardiac dysfunction.
Methods
Animal and experimental protocols
Transgenic fat-1 mice, provided by Dr. Kang from Massachusetts General Hospital, were created as previously reported and subsequently back crossed onto a C57BL background mice.17 Both fat-1 and wine type (WT) C57BL/6 mice were housed at temperature-controlled conditions and fed a special diet (10% safflower oil) high in ω-6 and low in ω-3 fatty acids until the desired age (8–10 weeks) and weight (22–25 g).
To induced sepsis associated cardiac dysfunction model, both fat-1 and WT mice were injected intraperitoneally with 10 mg/kg body weight LPS (Sigma-Aldrich, St. Louis, USA, Cat. NO. L2880) dissolved in sterile saline. The dosage of LPS was selected according to the earlier reports of significant cardiac dysfunction.18 All mice were sacrificed for further individual assessment 12 hours after LPS challenge. Mice in control group were administrated with an equivalent volume of saline. In a parallel experiment, both WT (n = 20) and fat-1 mice (n = 18) were given an intraperitoneal injection of Escherichia coli LPS at 30 mg/kg body weight and monitored for lethality every 6 h for up to 72 h based on Kaplan–Meier survival curve.19
All procedures involving experimental mice were performed in accordance with US National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication No. 85-23, revised 1996) and approved by Guangdong Provincial Hospital of CM on Research Animal Care (Guangzhou, China).
Echocardiographic assessment
Mice were anaesthetised with a mixture of 2% isoflurane/oxygen inhalation as described previously.20 Cardiac geometry and function were obtained in wild-type and fat-1 mice before and after LPS challenge, with the use of a 15-MHz scanning head (Visual Sonics, Toronto, Canada). Left-ventricular end-diastolic diameter (EDD) and end systolic diameter (ESD) were recorded in M-mode echocardiographic images.7 Fractional shortening (FS) and ejection fraction (EF) was calculated from using the method adopted by the American Society of Echocardiography.21
Proteomics profile assessments
For LC-MS/MS analysis, the myocardial tissue from fat-1 (n = 3) and WT mice (n = 3) treated with LPS were assessed. Following solubilization, 50 μg of protein from each tissue sample was denatured, alkylated, and then digested overnight at 37°C. Samples were resuspended and injected per LC-MS/MS run in triplicate on a Thermo Scientific LTQ ORBITRAP XL mass spectrometer connected to a Dionex Ultimate 3000 (RSLCnano) chromatography system. Each sample was loaded onto Biobasic Picotip Emitter (120 mm length, 75 μm ID) packed in house with Reprocil Pur C18 (1.9 μm) reverse phase media column and was separated by an increasing acetonitrile gradient. Survey full-scan MS spectra (300–2000 Da) were acquired in the Orbitrap, and the most intense ions from the preview scan were selected for MS/MS analysis.
Gene ontology analysis
To determine biological significance of differentially expressed protein, functional classification was performed using gene ontology/pathway analysis using DAVID bioinformatics resources v6.7. Gene ontology analysis gives relative representation of up-regulated and down-regulated genes in each function. To determine pathways associated with differentially expressed proteins, pathway analysis was performed as described previously.22
Histological analysis
The hearts of septic mice were harvested and embedded with OCT as described previously23; 5-μm thick left-ventricular sections were stained with haematoxylin and eosin (H&E).
After deparaffinization and blocking, the sections were incubated with a primary antibody against LC3 and myoglobin overnight. Subsequently, the sections were incubated with Alexa 568 and Alexa 488 for 60 min and then assessed and photographed under the Nikon Eclipse E1000 light microscope with the Nikon Digital Sight Camera (Nikon Instruments, Tokyo, Japan).
Transmission electron microscopy
The 1 mm3 fractions of left ventricle tissue were dissected and fixed with 2.5% glutaraldehyde/acrolein and 1% osmium tetroxide. After serial acetone dehydration, the samples were further processed to prepare the ultrathin sections, which were double-stained with uranyl acetate and lead citrate, and then qualitatively assessed using the FEI Tecnai 12 transmission electron microscope (Hitachi H-600, Tokyo, Japan).
Enzyme linked immunosorbent assay
The inflammatory cytokines in myocardium from endotoxin mice were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β, IL-6, MDA, and SOD (BioTeche, MN, USA). All spectrophotometric readings were performed with a microplate reader according to the instructions (Multiskan MK3, Thermo, USA).24
Immunoblotting analysis
Myocardial tissue samples from different experimental groups was homogenized and prepared in RIPA buffer using gentleMACS dissociator. Protein samples containing equal amounts of proteins (40 μg) were separated on SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes as previously described.25 The membranes were blocked with 5% milk and subsequently incubated over-night at 4°C with LC3A/B (#12741, CST, USA), anti-SQSTM1/p62 (#39749, CST, USA), anti-Atg7 (#12994, CST, USA), anti-IκB (#9242, CST, USA), anti-NFκB (#3031, CST, USA), anti-Sirt1 (#9475, CST, USA), and anti-PPARγ (#2443, CST, USA) antibodies. After immunoblotting, the film was scanned and the intensity of the immuno-detected bands was assessed with a BioRad calibrated densitometer.25 All experiments and densitometric quantifications were repeated at least three times.
Quantitative real-time PCR
Total RNA was extracted from the left ventricle tissue fragments with the RNAspin Mini Kit (GE Healthcare, Pittsburgh, PA, USA) and then reverse-transcribed by using the first-strand cDNA synthesis kit (Thermo Scientific, IL, USA) as previously described.26 Quantitative real-time reverse transcriptase-PCR analysis was performed for TNF-α, IL-1β, and IL-6, as well as for β-actin (as house-keeping gene) using a SYBR green real-time PCR kit (BioRad Inc. Hercules, Ca, USA).
Statistical analysis
Data that satisfied a normal distribution were presented as mean values and standard error of the mean (±SEM) from at least three independent experiments. Statistical significance (P < 0.05, two tailed) for each variable was estimated using a statistical software package (SPSS 13.0; SPSS Inc., Chicago, IL, USA).
Results
LPS-challenged fat-1 mice exhibited lower mortality rate than their WT counterparts
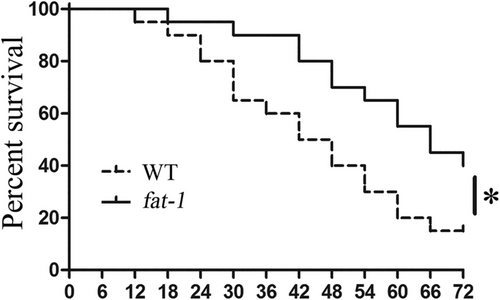
To evaluate the impact of endogenous ω-3 fatty acids on endotoxic shock and mortality, both WT and fat-1 mice were injected with LPS (30 mg/kg) and their survival rates were carefully monitored and evaluated using the Kaplan–Meier curve. We observed that LPS challenged mice from both groups displayed signs of endotoxic shock, such as, decreased physical activities, ruffled fur, diarrhoea, and ocular exudates. Remarkably, fat-1 mice exhibited a significantly lower mortality rate (30%) at 72 h after LPS challenge as compared with WT mice (42.5%; Figure 1). The median survival time in WT mice was significantly shorter than fat-1 mice (45 vs. 66 h, P = 0.0197), with hazard ratio 2.571 [95% CI of ratio, 1.163 to 5.683].
Fat-1 mice demonstrated a selective attenuation of LPS-induced myocardial injury
Echocardiographic measurement was performed to evaluate the cardiac structure and function of both WT and fat-1 mice at baseline and 12 h after their LPS challenge. As shown in Figure 2, there was no significant difference in left ventricular function between saline-treated WT and fat-1 mice. However, LPS administration significantly decreased cardiac function of WT mice (evidenced by the reduction of LVEF and LVFS) as compared with the saline-treated WT group (P < 0.05). Meaningfully, these LPS-induced cardiac dysfunctions were attenuated in fat-1 mice. In fact, LVEF and LVFS values of LPS-treated fat-1 mice were significantly better, as compared with LPS-treated WT mice (P < 0.05).
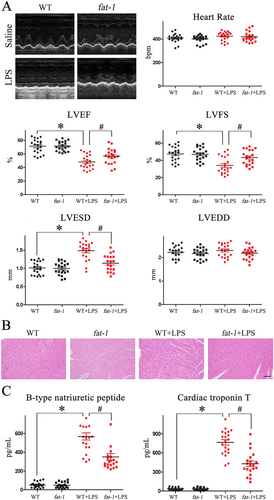
Moreover, H&E staining demonstrated that the myocardium from LPS-challenged fat-1 mice had a better preserved overall myocardial structure and lesser infiltration of inflammatory cell, as compared with the WT mice also challenged with LPS (Figure 2).
It has been previously established that myocardial BNP and cTnT secreted from atria and ventricles in response to stress are reliable predictors for the detection of myocardial pathological damage. Accordingly, we detected that expressions of myocardial BNP and cTnT were extremely increased in WT mice treated with LPS (P < 0.05). In contrast, both parameters were significantly decreased in LPS-challenged fat-1 mice (P < 0.05, Figure 2).
Proteomics revealed distinct modification of proteins expression profile in myocardium of LPS-challenged fat-1 mice
Results of the proteomics analysis of left ventricular tissue samples collected from fat-1 (n = 3) and WT (n = 3) mice at 12 h after LPS challenge, revealed altered expression profiles of 91 proteins (>2-fold change with a adjusted P value <0.05, Figure 3).
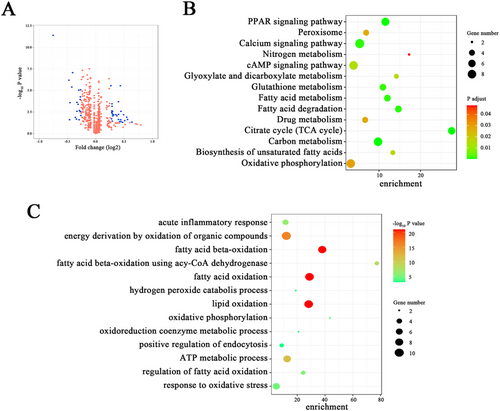
More precisely, the expression of 32 detected myocardial proteins were notably enlarged, and 59 proteins were markedly diminished. Furthermore, the DAVID analysis was additionally performed to reveal the possible functional meaning of the variable expressions of particular proteins in LPS-treated fat-1 mice. In fact, we identified that several canonical pathways were altered in fat-1 mice exposed to LPS. Their list includes PPAR signalling, cAMP signalling, fatty acid metabolism, carbon metabolism, and biosynthesis of unsaturated fatty acids pathways (Figure 3).
In addition, GO analysis aimed at clarification whether and how differentially expressed proteins would affect functions of cellular components engaged in basic biological processes, was also performed. Obtained results suggested that distinct modification of proteins expression profile in myocardium of LPS-challenged fat-1 mice would affect numerous biological processes, including acute inflammatory response, fatty acid oxidation, lipid oxidation, fatty acid beta-oxidation, and response to oxidative stress, ATP metabolic process functions (Figure 3).
Myocardium of LPS-challenged fat-1 mice revealed substantially reduced acute inflammatory damage then WT mice
Increasing evidence indicates that multiple proinflammatory mediators play key role in LPS-induced myocardial dysfunction.27 Thus, we further examined whether the endogenous ω-3 fatty acids in fat-1 mice would suppress pro-inflammatory cytokines stimulated by LPS. As shown in Figure 4, we found that expressions of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 (both mRNA and protein levels) were extensively elevated in myocardium of WT mice after LPS challenge. In contrast, detected levels of these LPS-induced cytokines were significantly reduced in myocardia of fat-1 mice (Figure 4).
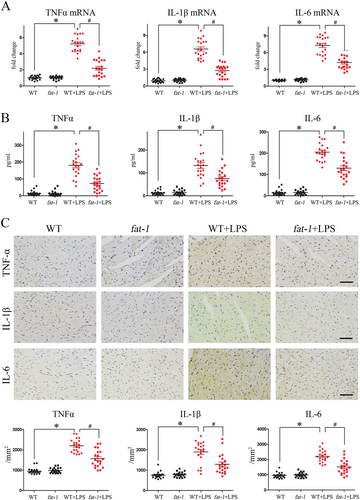
Results of the parallel immuno-histochemical detection of TNF-α, IL-1β, and IL-6 in the myocardial sections further supported the evidence that endogenous ω-3 fatty acids might eliminate the inflammatory factors (Figure 4).
Myocardium of LPS-challenged fat-1 mice did not manifest the evident oxidative injury
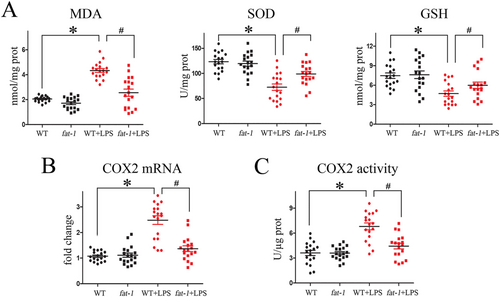
Previously accumulated data already suggested that oxidative stress constitutes the cornerstone of sepsis pathogenesis. To evaluate the putative antioxidant action of endogenous ω-3 fatty acids, during the LPS-induced endotoxaemia, we assessed the generation of ROS and O2− in samples of left-ventricular tissues using ELISA, PCR, and immune-fluorescence approaches, respectively. First, we have established that the LPS treatment of WT mice significantly enhanced generation of malondialdehyde (MDA) and lessened superoxide dismutase (SOD) and glutathione (GSH), thereby it contributed to development of the explicit oxidative stress of their myocardium. Meaningfully, we then documented that myocardium of transgenic fat-1 mice, rich in endogenous ω-3 FA, were practically spared from the LPS-induced oxidative injury (Figure 5). Consistently with these observations, we also found that the endogenous ω-3 fatty acids enrichment of transgenic fat-1 mice, also contributed to the reversal of the LPS-induced generation of myocardial COX2 mRNA and calmed down its activity (Figure 5).
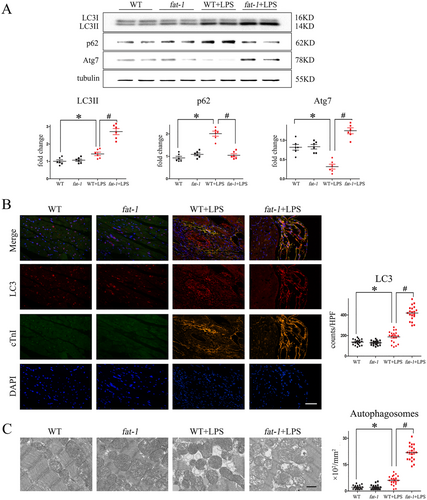
Myocardial autophagy in fat-1 mice amplified after LPS challenge
It has been previously demonstrated that myocardial autophagy may be actually beneficial for the maintenance of the overall cardiac functions and survival in sepsis.28 So now compared autophagic activity in myocardium from WT and fat-1 mice, in the presence and absence of LPS, by the western blot analysis, detecting protein markers of autophagy. As depicted in Figure 6, we found that myocardium of LPS-treated fat-1 mice showed a significantly increased levels ratio of LC3II/I and levels of Atg7, markers of autophagosome formation, as compared with WT mice. LPS treatment also significantly increased levels of the immuno-detected sequestosome 1 (SQSTM1)/p62. Interestingly, levels of immuno-detected p62 in LPS-treated fat-1 mice were significantly lower according to immunoblotting (Figure 6). Consistently, immunofluorescence with anti-LC3 antibody (Figure 6) and the transmission electron microscopy (Figure 6), both revealed the markedly decreased number of autophagosomes in fat-1 mice, as compared with the WT mice. These results suggest that LPS treatment induced the pathological accumulation of autophagosomes, only in WT mice but not the transgenic fat-1 mice.
The presence of endogenous ω-3 fatty acids considerably attenuated the LPS-induced activation of stress pathways
To investigate the potential role of LPS-induced signalling activation in fat-1 transgenic mice, the nuclear transcription factor κB (NFκB) and protein expression of Sirtuin (Sirt) 1, an essential regulator of lipid metabolism and stress response were examined in both WT and fat-1 mice respectively treated with or without LPS.
Obtained results revealed that LPS treatment substantially decreased the IκB levels, but enhanced activation of the NFκB pathway. Although ω-3 fatty acids enrichment itself did not affect the expression of NFκB, it abolished or significantly attenuated the LPS-induced activation of the downstream NFκB-dependent signalling pathway (Figure 7).
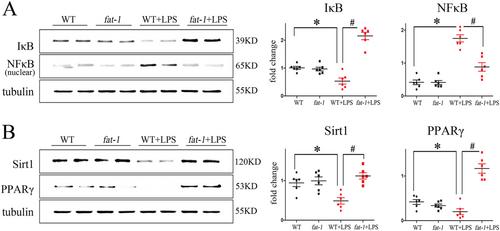
Moreover, the LPS treatment significantly down-regulated levels of Sirt1 and its downstream effector, the peroxisome proliferator-activated receptor gamma (PPARγ) which is an essential regulators of lipid metabolism and autophagy. Meaningfully, results of our proteomics analysis also detected the increased activation of the PPARγ signalling pathway. Immunohistochemistry and immunoblotting results further demonstrated that the enrichment in endogenous ω-3 fatty acids associated with the increased production of the relevant signalling proteins (Figure 7).
Discussion
It has been already documented that the persisted inflammation of various tissues, consistently induces generation of their free radicals, that eventually overwhelm a local antioxidants and lead to development of the septic shock.29 Results of previous, in vitro and in vivo studies, also demonstrated that inflammation plays a crucial role in the experimental LPS-induced cardiac dysfunction.30 Meaningfully, it has been also shown that experimental treatment with ω-3 fatty acids contribute to generation of the anti-oxidant defence system in the autoimmune disease prone mice, by increasing their production of anti-oxidant enzymes.31 Thus, it has been shown that ω-3 fatty acids may diminish release of several pro-inflammatory cytokines, from monocytes and macrophages infiltrating myocardium, thereby diminishing the cardiac dysfunction in the septic shock.32 Yet another studies demonstrated that dietary supplementation of healthy volunteers with ω-3 fatty acids (EPA and DHA) derived from vegetable or fish oils, subsided production of TNF-α, IL-1β, and IL-6 by theirs monocytes, stimulated in vitro with the LPS.33 More recently results relevant to the experimental model used in our study revealed that the fat-1 transgenic mice, generated less the NF-κB (p65/p50)-dependent secretion of IL-6 and TNF-α, following their LPS treatment, than a similarly treated WT mice.34 Therefore, these results suggesting that elevated production of endogenous ω-3 fatty acids would alleviate the detrimental effects of pro-inflammatory cytokines, inspired our present study, aimed at mechanistic explanation of this beneficial phenomenon.
Importantly, we were able to demonstrate that a constantly elevated production of endogenous ω-3 fatty acids by the fat-1 transgenic mice can be mechanistically linked to a significant attenuation of their myocardial dysfunction and improvement of their survival, during the LPS-induced endotoxaemia. Moreover, we have also established, for the first time, that the heightened levels of the endogenous ω-3 fatty acids in fat-1 transgenic, significantly attenuated the suppression of Sirt-1/PPARγ signalling pathway, induced by the LPS treatment. Consistently, these findings suggest that the ω-3 fatty acids, may also exert its anti (LPS-induced) inflammatory effects through the inhibition of cardiac IκBα phosphorylation and a subsequent NFκB activation. Because we also found a net up-regulation of the PPARγ gene expression in myocardium of fat-1 mice, we now suggest that the up-regulation of PPARγ expression, may reflect a yet another parallel mechanism, by which the ω-3 fatty acids exert their anti-inflammatory, anti-oxidative, and pro-autophagic effects.
It has been previously demonstrated that autophagy up-regulated under the initial stress also contributed to cardioprotection in sepsis, likely through a selective autophagic clearance of damaged cellular organelles and sequestering ROS, induced in cardiomyocytes by bacterial endotoxin LPS35, 36.37 Meaningfully, the relevant, data presented in our paper, also revealed heightened autophagic activity in the LPS challenged fat-1, as compared with the WT mice. They strongly indicated that increased autophagy represents yet another mechanism linked to the ω-3 fatty acids-elicited cardio-protection, especially through elimination of detrimental ROS, by removing of damaged mitochondria and by supporting biosynthesis of the antioxidant enzymes, that utilizes the free amino acids, proteolytically released from the cellular remnants. Thus, this set of our results suggest the engagements of indicating molecular signals, triggering a protective autophagy in LPS-induced toxaemia. However, further elucidation is still needed for better understanding the interplay between ROS production, autophagy of the injured cardiomyocytes and the ultimate myocardial dysfunction in sepsis.
It is now well documented that most of the pro-inflammatory genes are prominently up-regulated by nuclear translocation of NFκB following degradation of IκB.38 Relevantly to this phenomenon, it has been also proposed that ω-3 fatty acids might contribute to calming-down of the pro-inflammatory gene expression after the initial inhibition of the intracellular NFκB signalling pathways.39 We observed that LPS induced a significantly decrease in IκB level in the myocardia of WT mice, while the permanent overexpression of ω-3 fatty acids in the fat-1 transgenic mice significantly blocked LPS-induced phosphorylation and degradation of IκBα. These findings strongly support the existence of the important beneficial mechanism, in which the ω-3 fatty acids might protect harts against the LPS-induced pro-inflammatory genes expression, triggered through the inhibition of cardiac IκBα phosphorylation and the subsequent NFκB activation.
The above-mentioned results are mechanistically consistent with another report demonstrating that activation of Sirt-1 in C57/B6 mice challenged with LPS, associated with the inhibition of their NFκB activation, occurring after deacetylation its subunit p65 and with oxidative stress.40 Recently, the PPARγ activation has been also linked to prevention of the LPS-mediated sepsis-related cardiac dysfunction and to improved survival of experimental mice.41 The overexpression of PPARγ also associated with the increased resistance against the oxidative stress-induced cardiomyocyte apoptosis through the up-regulation of the Bcl-2 expression.42 On the other hand, it has been also shown that the ω-3 fatty acids deficiency related hepatic steatosis associated with the reduced protection against endoplasmic reticulum stress triggered by the reduced expression of PPARγ.43 Importantly, results of our present study indicate that up-regulation of PPARγ expression in myocardium of fat-1 mice contributes to the multilayer mechanisms by which the ω-3 fatty acids exert its anti-inflammatory, ant-oxidative and pro-autophagic capacity.
Conclusions
The presented study provides a new insight for our understanding of the mechanism, in which the ω-3 fatty acids might alleviate the sepsis-induced cardiac dysfunction. Collectively, the present findings suggest that constantly elevated levels of endogenous ω-3 fatty acids in fat-1 transgenic mice induce the heightened expressions of anti-inflammatory factors; LC3 I/II and Atg7, as well as a trigger a parallel activation of Sirt-1 and PPARγ signalling pathways contributing to protection of myocardium against the LPS-induced detrimental inflammation and oxidative stress. Myocardia of LPS-challenged fat-1 mice also displayed a more efficient autophagic clearance of the injured cardiomyocytes than their WT counterparts. Thus, we hope that the presented results obtained from the animal model strongly encourage further investigations aimed at determination of the strict ω-3 fatty acids-based pharmacological protocols, that would be recommended for treatment of patients afflicted with inflammatory and septic diseases, complicated with cardiac dysfunctions.
Authors' contributions
S. M. and A. H. drafted this manuscript; S. M., H. M., and P. P. C. performed the experiments; Y. L. made statistical analysis; M. Z. Z. and A. H. made a critical revision of the manuscript and contributed to the rationalization of the study. All authors read and approved the final manuscript.
Acknowledgements
We thank Prof. Jing Xuan Kang from Harvard Medical School for providing the fat-1 transgenic mice.
Conflict of interest
The authors declare no conflict of interest.
Funding
This study was funded by National Science Foundation of China (grant no. 81703877), Featured Innovative Project from Guangdong Provincial Universities (2019KTSCX029), Young Talents Support Project from China Association of Chinese Medicine (2019-QNRC2-C06), Youth Talent Development Program from Guangzhou University of Chinese Medicine (to Shuai Mao), and Team of Prevention and Treatment of Acute Myocardial Infarction with Chinese Medicine (2019KCXTD009). The sponsors have had no role in the project development, in the collection of data, in the preparation of this manuscript, nor the decision to publish.




