Comparison of left ventricular longitudinal systolic function parameters in the prediction of adverse outcome in heart failure with preserved ejection fraction
Abstract
Aims
Several different diagnostic parameters can be used to assess left ventricular (LV) longitudinal systolic function, but no studies comparing their predictive value have been conducted. We sought to compare the prognostic value of LV long-axis function parameters at rest and exercise using the population with heart failure with preserved ejection fraction (HFpEF).
Methods and results
Clinical and biochemical variables were collected at baseline in 201 patients with HFpEF. Echocardiography was performed at rest and immediately after exercise, with measurement of mitral annular plane systolic excursion, systolic tissue velocity (s′), global longitudinal strain (GLS), and global longitudinal strain rate (GLSR). Participants were followed for 48 (24–60) months for heart failure hospitalization and cardiovascular death. Seventy-four patients (36.8%) met the study endpoint. Cox regression analysis revealed that after adjustment for Meta-Analysis Global Group in Chronic Heart Failure risk score, brain natriuretic peptide (BNP), and peak VO2, heart failure hospitalization and cardiovascular death were significantly associated with GLS at rest [hazard ratio (HR) 0.91; 95% confidence interval (CI) 0.84–0.98; P = 0.016], GLS after exercise (HR 0.84; 95% CI 0.77–0.91; P < 0.001), and GLSR after exercise (HR 0.13; 95% CI 0.04–0.48; P = 0.002). The addition of each of the following: exercise GLS and GLSR and resting GLS to the base model including Meta-Analysis Global Group in Chronic Heart Failure, BNP, and peak VO2 improved predictive power for the study endpoint [net reclassification improvement (NRI) = 49%, P < 0.001; NRI = 42%, P = 0.004; and NRI = 38%, P = 0.009, respectively]. Exercise GLS was the only longitudinal parameter significantly improving c-statistics of the base model (0.68 vs. 0.73; P = 0.047).
Conclusions
Echocardiographic parameters of LV longitudinal function are not equipotential in predicting adverse outcomes in HFpEF. LV deformation indices, especially assessed with exercise, show the highest predictive utility independent from and incremental to clinical data and BNP.
Introduction
The longitudinal component of left ventricular (LV) mechanics represents an essential contributor to the overall cardiac performance. Abnormalities of longitudinal function appears early in the preclinical phase of myocardial impairment and then progressively increases over the symptomatic stages of disease.1-5 Derangements of LV longitudinal systolic function provide prognostic information that may be useful for defining patient management strategies in a variety of cardiovascular disorders, including heart failure (HF).6-13 Myocardial deformation, tissue velocities, and mitral annular displacement have provided different technical approaches to measuring LV longitudinal contraction. All have been effectively used for risk stratification,1, 14-17 but their comparative value remains unclear, as no studies comparing the predictive value of different longitudinal function measures have been reported to date.
Reductions in LV longitudinal function are predictive of adverse outcomes in HF with preserved ejection fraction (HFpEF18, 19). The measurement of LV longitudinal systolic reserve at exercise may outperform resting assessment in prognostic evaluation of this clinical syndrome.20, 21 Accordingly, the aim of this study was to compare the prognostic utility of four echocardiographically derived LV longitudinal systolic function parameters: global longitudinal strain (GLS) and global longitudinal strain rate (GLSR), mitral annular systolic velocity (s′), and mitral annular plane systolic excursion (MAPSE) measured at both rest and exercise using a well-characterized HFpEF population.
Methods
Patient selection
In this study, 201 patients satisfying the HFpEF criteria in place at the time of recruitment (i.e. between 2012 and 201522) were recruited from hospital clinics at a tertiary cardiology centre (University Hospital, Wroclaw, Poland). Each participant underwent cardiopulmonary exercise testing, resting and immediate post-exercise echocardiography, and blood sampling for laboratory assessments.
The major inclusion criteria comprised (i) signs and symptoms of HF (dyspnoea, fatigue, and exercise intolerance) consistent with New York Heart Association Functional Class II or III, with reduced exercise capacity (<100% of age-predicted and sex-predicted normal ranges for peak oxygen consumption); (ii) preserved LV ejection fraction (>50%); and (iii) evidence of diastolic dysfunction according to the 2009 European Association of Echocardiography/American Society of Echocardiography criteria available at the time of recruitment [reduced e′ (septal e′ <8 cm/s and lateral e′ <10 cm/s) and the presence of the following additional features: Grade I—E/A < 1, E/e′ (using average e′) <13, the time difference between atrial reversal wave of the pulmonary venous flow duration and mitral A-wave duration (ΔAdur) <30 ms, and ΔE/A during the Valsalva manoeuvre <0.5; Grade II—E/A 1–2 and at least two of the three abnormalities: E/e′ ≥13, ΔAdur ≥ 30 ms, and ΔE/A during the Valsalva manoeuvre ≥0.5; and Grade III—E/A >2.0 and at least one of the two abnormalities: E/e′ ≥13 and ΔAdur ≥30 ms].23
The exclusion criteria included (i) atrial fibrillation or flutter; (ii) ischaemic heart disease, defined by the presence of atherosclerotic lesions at coronary angiography or inducible ischaemia during exercise testing (because myocardial ischaemia could limit exercise capacity without involving diastolic dysfunction); (iii) moderate and severe valvular heart disease; (iv) established or suspected pulmonary disease (vital capacity <80% or forced expiratory volume in 1 s being <80% of age-specific and sex-specific reference values); (v) haemoglobin <11 mg/dL; and (vi) other significant co-morbidities, including malignancy, renal failure, infections, and autoimmune, skeletal, and thyroid illnesses.
All study subjects were informed of the purpose of the study and provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee.
Outcome
Patients were followed over a median period of 48 (24–60) months for the composite endpoint of HF hospitalization (defined as hospital admission due to HF worsening requiring intensification of diuretic therapy) or cardiovascular death. This event rate was verified by regular contact with patients or their proxies.
The current analysis focused on the comparison of prognostic abilities of LV longitudinal systolic function parameters. A comprehensive evaluation of clinical risk in the studied HFpEF population covering a shorter follow-up was presented in our previous paper.20
Echocardiography
Echocardiography imaging was performed using standard equipment (Vivid e9, General Electric Medical Systems, Milwaukee, WI, USA) with phased array 2.5 MHz multi-frequency transducer. Imaging data were analysed offline after being saved in the digital format on a secure server.
Conventional imaging
Cardiac dimensions and wall thicknesses were measured according to recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.24 LV volumes and left atrial volumes were estimated by the biplane Simpson and area-length methods, respectively. LV end-diastolic and end-systolic volumes in the apical four-chamber and two-chamber views were used for calculation of ejection fraction.
Strain and strain rate
The assessment of LV longitudinal deformation was performed using a semi-automated two-dimensional speckle tracking technique (Echopac PC Version 113, General Electric Medical Systems, Horten, Norway) analysing all six segments of the left ventricle in the three apical views (four-chamber, two-chamber, and long-axis views) with temporal resolution of 60–90 frames per second. After manually tracking the endocardial border and selecting appropriate wall thickness, the software automatically identified segments and tracked the motion of acoustic markers in each view. Segments with incorrect tracking were readjusted manually in the next step. LV deformation curves were automatically generated and displayed. The greatest negative values on the midmyocardial strain curve and on the strain rate curve during LV systole were measured, and averages from all analysable segments were displayed as GLS and GLSR, respectively.
The assessment of left atrial longitudinal deformation was carried out in the apical four-chamber and two-chamber views using the onset of QRS as the zero reference point. Left atrial reservoir strain was measured as the peak value during LV systole, and contractile strain was assessed as the value of strain at the onset of P wave in electrocardiogram. The final left atrial strain values were the averages from both apical views.
Tissue systolic velocity
Peak systolic tissue velocity (s′) was assessed by pulsed-wave tissue Doppler at the septal and lateral aspects of the mitral annulus, and an average value from both sides was used in the analysis.
Mitral annular plane systolic excursion
Measurements were performed by M-mode from the apical four-chamber view at the septal and lateral border of the mitral annular plane, with the M-mode cursor line being positioned parallel to longitudinal LV motion. In case of the scan line misalignment, anatomical M-mode was used to correct the insonation angle. The total displacement was measured from the lowest point at end-diastole to aortic valve closure (end of the T wave on the electrocardiogram). The value of MAPSE averaged from both sides of the mitral annulus was used in the analysis.
The acquisitions for LV longitudinal function assessment were performed as a first part of post-test imaging and accomplished within 60–90 s after exercise termination.
Assessment of left ventricular diastolic function
Left ventricular inflow parameters including peak early (E) and late diastolic flow velocity (A) and deceleration time of early diastolic flow wave (DT) were evaluated from the apical four-chamber view by pulsed-wave Doppler with the sample volume placed between the tips of the mitral leaflets. Analogous to s′, peak early diastolic tissue velocity (e′) was measured using pulsed-wave tissue Doppler at the septal and lateral segments of the mitral annulus. The ratio of mitral early diastolic velocity to the average e′ velocity obtained from the septal and lateral portions of the mitral annulus (E/e′) was calculated to approximate LV filling pressure.
All echocardiographic parameters were averaged over three consecutive cardiac cycles.
Cardiopulmonary exercise testing and blood assays
Each participant underwent symptom-limited treadmill exercise testing using a modified Bruce protocol with standard electrocardiogram and blood pressure monitoring. Ventilation, oxygen uptake, and carbon dioxide production were monitored continuously, and peak oxygen uptake (peak VO2) was calculated as the average oxygen consumption during the last 30 s of exercise. Exercise capacity was also assessed in metabolic equivalents on the basis of the peak exercise intensity. Previous pharmacotherapy was maintained unchanged.
Peripheral venous blood samples were obtained for laboratory measurements including brain natriuretic peptide (BNP).
The Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score (including the following variables: age, gender, ejection fraction, creatinine, diabetes mellitus, chronic obstructive pulmonary disease, systolic blood pressure, body mass index, New York Heart Association class, angiotensin-converting enzyme inhibitor use, beta-blocker use, HF duration, and current smoker) was calculated for each participant to assess prognosis on the basis of clinical parameters.25
Statistical analysis
Data are presented as mean ± standard deviation for normally distributed variables, as median (inter-quartile range) for skewed variables (BNP), and as counts and percentages for categorical variables. Between-group comparisons of clinical and echocardiographic features in patients with and without events were made using univariable Cox regression. The associations of LV longitudinal systolic function parameters with the study endpoint were assessed with Cox proportional hazard models adjusted for MAGGIC risk score, BNP, and peak VO2. A cause-specific competing risk approach was used for the outcome of interest (HF hospitalization or cardiovascular death) and the competing risk of non-cardiovascular death. Relative risks were expressed as hazard ratios with 95% confidence intervals (CIs). The incremental value of LV longitudinal systolic function parameters for the prediction of outcome was examined in nested Cox models by the addition of the parameter of interest (i.e. GLS, GLSR, s′, or MAPSE, each evaluated at rest and exercise) to a base model including the MAGGIC risk score, BNP, and peak VO2. The change in overall log-likelihood ratio χ2 was used to assess the increase in predictive power after the addition of LV longitudinal systolic function parameter. The generalized R2 was used to assess the explanatory power of each model. The relative quality of models was estimated by the Akaike information criterion. The c-statistic was used to evaluate model performance. The significance of change in c-statistic after the addition of LV longitudinal systolic function parameter to the base model was evaluated using the bootstrap method with 2000 iterations for each test. The net reclassification improvement, calculated on the basis of category-free approach, was used to evaluate the magnitude of reclassification achieved after the addition of LV longitudinal systolic function parameter to a base model. The reproducibility of GLS, GLSR, s′, and MAPSE measurements was assessed by the Bland–Altman method (mean difference and 95% CI) and intraclass correlation coefficient. To facilitate the interpretation of the reproducibility of parameters acquired by different techniques, the Bland–Altman metrics were indexed to the mean value of test and retest measurements. Accordingly, the difference between test and retest was calculated as a percentage of the mean value of these measurements and, analogous to the non-index values, presented as mean difference and 95% CI. All analyses were carried out with standard statistical software (Statistica Version 13, TIBCO Software Inc., Palo Alto, CA, USA, and R software Version 3.5.3 (http://cran.r-project.org/). Statistical significance was set at P < 0.05.
Results
Patient characteristics and events
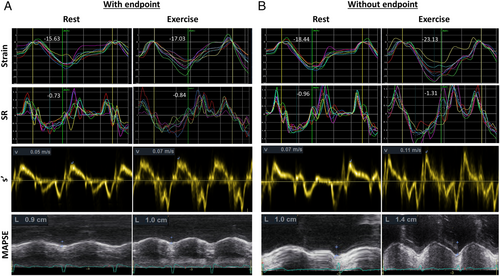
During a median follow-up of 48 months, the study endpoint of hospitalization due to HF worsening or cardiovascular death occurred in 74 patients (36.8%). The overall mortality rate was 10.9% (22 patients). The cause of death was cardiac in 13 cases, cerebral stroke in three cases, and haematological malignancy, pneumonia, and septic shock—each in one case, and in three cases, the reason was not established. The demographic, clinical, and echocardiographic characteristics of patients with and without the endpoint are presented in Table 1. Primary, infiltrative, or storage disease-associated cardiomyopathy was not diagnosed in any study participant. The subset with events was characterized by older age, higher prevalence of diabetes, lower estimated glomerular filtration rate and exercise capacity, higher BNP and MAGGIC risk score, and more impaired left atrial function (lower reservoir and contractile strain), LV diastolic function (higher E/e′ ratio at rest and exercise and larger left atrial size), and longitudinal systolic function (lower GLS, GLSR, s′, and MAPSE at both rest and exercise). Examples of LV longitudinal function parameters in patients with and without the study endpoint are presented in Figure 1.
| Without endpoint (n = 127) | With endpoint (n = 74) | P | |
|---|---|---|---|
| Age (years) | 62.7 ± 8.0 | 66.8 ± 8.3 | 0.001 |
| Male sex, n (%) | 33 (26%) | 20 (27%) | 0.74 |
| Diabetes, n (%) | 32 (25%) | 33 (45%) | 0.005 |
| Hypertension, n (%) | 118 (93%) | 69 (93%) | 0.74 |
| BMI (kg/m2) | 29.4 ± 4.2 | 30.0 ± 4.0 | 0.39 |
| NYHA Class II, n (%) | 103 (81%) | 53 (72%) | 0.08 |
| NYHA Class III, n (%) | 24 (19%) | 21 (28%) | |
| Haemoglobin (g/dL) | 13.8 ± 1.1 | 13.4 ± 1.0 | 0.06 |
| eGFR (mL/min/1.73 m2) | 68.4 ± 15.9 | 61.7 ± 14.5 | 0.008 |
| BNP (pg/mL) | 40 (23–74) | 59 (34–155) | <0.001 |
| Peak VO2 (mL/min/kg) | 16.0 ± 4.6 | 14.3 ± 4.7 | 0.03 |
| VE/VCO2 slope | 27.5 ± 5.3 | 29.0 ± 6.5 | 0.06 |
| AT VO2 (mL/min/kg) | 15.9 ± 6.8 | 14.3 ± 4.7 | 0.11 |
| METs per protocol | 5.9 ± 2.9 | 4.5 ± 2.6 | 0.004 |
| MAGGIC risk score | 13.8 ± 4.9 | 16.7 ± 4.9 | <0.001 |
| SBP exe (mmHg) | 166 ± 22 | 166 ± 21 | 0.90 |
| DBP exe (mmHg) | 67 ± 13 | 66 ± 11 | 0.47 |
| Beta-blockers, n (%) | 88 (69%) | 57 (77%) | 0.30 |
| Ca blockers, n (%) | 46 (36%) | 30 (40%) | 0.57 |
| ACEI/ARB, n (%) | 119 (94%) | 70 (95%) | 0.79 |
| Thiazides, n (%) | 60 (47%) | 40 (54%) | 0.30 |
| Loop diuretics, n (%) | 20 (16%) | 16 (22%) | 0.23 |
| LVMI (g/m2.7) | 53.3 ± 13.3 | 56.8 ± 13.5 | 0.07 |
| LAVI (mL/m2) | 32.4 ± 9.5 | 36.0 ± 9.6 | 0.02 |
| LA reservoir strain (%) | 28.6 ± 6.8 | 25.9 ± 6.0 | 0.007 |
| LA contractile strain (%) | 14.2 ± 4.1 | 12.9 ± 4.0 | 0.04 |
| RV free wall strain (%) | 27.9 ± 7.1 | 26.6 ± 8.1 | 0.31 |
| EF (%) | 72.4 ± 7.7 | 72.5 ± 9.9 | 0.85 |
| E/e′ rest | 10.8 ± 3.0 | 12.4 ± 4.2 | <0.001 |
| E/e′ exe | 14.4 ± 4.3 | 16.4 ± 5.5 | 0.005 |
| GLS rest (%) | 18.9 ± 2.9 | 17.6 ± 3.5 | 0.005 |
| GLS exe (%) | 20.9 ± 3.0 | 18.6 ± 3.4 | <0.001 |
| GLSR rest (%) | 0.93 ± 0.20 | 0.86 ± 0.18 | 0.02 |
| GLSR exe (%) | 1.12 ± 0.22 | 0.98 ± 0.22 | <0.001 |
| s′ rest (cm/s) | 7.4 ± 1.3 | 6.9 ± 1.2 | 0.003 |
| s′ exe (cm/s) | 9.9 ± 1.8 | 9.0 ± 1.7 | <0.001 |
| MAPSE rest (mm) | 12.6 ± 1.8 | 11.7 ± 1.9 | 0.002 |
| MAPSE exe (mm) | 14.3 ± 2.1 | 13.3 ± 2.1 | 0.003 |
- ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AT, anaerobic threshold; BMI, body mass index; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; E, early mitral inflow; e′, early diastolic mitral annular velocity; EF, ejection fraction; eGFR, estimated glomerular filtration rate; exe, after exercise; GLS, global longitudinal strain; GLSR, global peak systolic strain rate; LA, left atrial; LAVI, left atrial volume index; LVMI, left ventricular mass index; MAPSE, mitral annular plane systolic excursion; METs, metabolic equivalents; NYHA, New York Heart Association; rest, at rest; RV, right ventricular; s′, peak systolic mitral annular velocity; SBP, systolic blood pressure; VE/VCO2, ventilation vs. carbon dioxide production slope; VO2, oxygen uptake.
- Values are mean ± SD, median (inter-quartile range), or n (%).
Significant correlations with peak VO2 were found for exercise GLSR and exercise s′, but not for exercise MAPSE and exercise GLS (Table 2). The ability to discriminate peak VO2 < 20 mL/min/kg was highest for exercise GLSR (Table 2).
| Parameter | Association with peak VO2 | AUC for prediction of peak VO2 < 20 mL/min/kg | Optimal cutpoint | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Exercise GLSR | r = 0.27; P < 0.001 | 0.68 | 1.06 s−1 | 61% | 73% |
| Exercise s′ | r = 0.25; P < 0.001 | 0.64 | 9.5 cm/s | 55% | 77% |
| Exercise MAPSE | r = 0.14; P = 0.06 | 0.61 | 14.0 mm | 64% | 60% |
| Exercise GLS | r = 0.13; P = 0.08 | 0.59 | 22.0% | 84% | 37% |
- AUC, area under the curve; LV, left ventricular; other abbreviations as in Table 1.
Associations of left ventricular longitudinal systolic function parameters with outcome
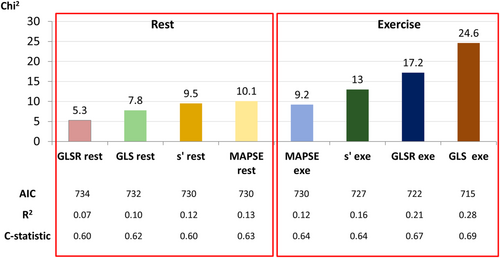
In a series of univariate Cox proportional hazard models, all the four LV longitudinal systolic function metrics, that is, GLS, GLSR, s′, and MAPSE, assessed at both rest and exercise, were significantly associated with the study endpoint (Figure 2). A significant association with the investigated outcome was also demonstrated for GLS at rest <16% (hazard ratio 1.81; 95% CI 1.11–2.96; P = 0.018). A series of Cox proportional hazards models adjusted for MAGGIC risk score, BNP, and peak VO2 revealed that the only resting LV longitudinal systolic function parameter significantly associated with the study endpoint was GLS. In the analogous models developed for LV longitudinal systolic function indices evaluated after exercise, two parameters—GLS and GLSR—proved to be significant predictors of HF hospitalization and cardiovascular death (Table 3 and Figure 3).
| Parameter | Unadjusted | Adjusted for MAGGIC risk score, BNP, and peak VO2 | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| GLS rest | 0.90 (0.83–0.97) | 0.005 | 0.91 (0.84–0.98) | 0.016 |
| GLSR rest | 0.24 (0.07–0.83) | 0.02 | 0.29 (0.08–1.03) | 0.056 |
| s′ rest | 0.74 (0.61–0.90) | 0.003 | 0.84 (0.69–1.03) | 0.106 |
| MAPSE rest | 0.82 (0.73–0.92) | 0.002 | 0.90 (0.79–1.02) | 0.106 |
| GLS exe | 0.82 (0.76–0.89) | <0.001 | 0.84 (0.77–0.91) | <0.001 |
| GLSR exe | 0.09 (0.03–0.30) | <0.001 | 0.13 (0.04–0.48) | 0.002 |
| s′ exe | 0.90 (0.83–0.97) | <0.001 | 0.86 (0.74–1.01) | 0.064 |
| MAPSE exe | 0.24 (0.07–0.83) | 0.003 | 0.92 (0.81–1.03) | 0.144 |
Incremental prognostic value of left ventricular longitudinal systolic function parameters
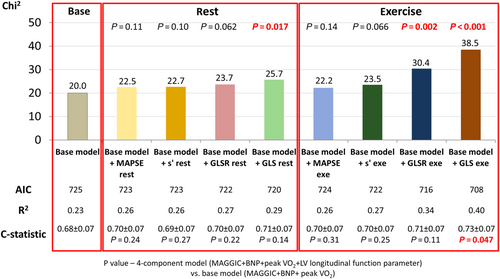
In the sequential Cox analysis, the addition of GLS and GLSR measured after exercise, as well as the addition of resting GLS to the model including MAGGIC risk score, BNP, and peak VO2, resulted in a significant increase in the χ2 values. On the other hand, a significant increase in c-statistics was noted only for exercise GLS. No significant incremental benefit was shown from adding GLSR assessed at rest, as well as MAPSE and s′ evaluated at both rest and exercise to the previously mentioned base model (Figure 3).
Reclassification improvement
The net reclassification improvement varied between 25% and 49% after addition of LV longitudinal systolic function parameters to the model including MAGGIC risk score, BNP, and peak VO2. Statistical significance in these analyses was demonstrated only for GLS measured at both rest and exercise and GLSR measured at exercise (Table 4).
| Parameter | NRI | P |
|---|---|---|
| Rest | ||
| GLS | 38% | 0.009 |
| GLSR | 28% | 0.060 |
| s′ | 26% | 0.078 |
| MAPSE | 25% | 0.089 |
| Exercise | ||
| GLS | 49% | <0.001 |
| GLSR | 42% | 0.004 |
| s′ | 27% | 0.066 |
| MAPSE | 26% | 0.070 |
- MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure; NRI, net reclassification improvement; other abbreviations as in Table 1.
Feasibility and reproducibility of echocardiography
During post-processing of the acquired data, 6% of all LV segments at rest and 11% after exercise were unsuitable for further analysis of myocardial deformation due to artefacts and signal noise. The degree of concordance of measurements of LV longitudinal systolic function parameters assessed in 15 randomly selected examinations is presented in Table 5.
| Bland–Altman | Indexed Bland–Altman | ICC | |||||
|---|---|---|---|---|---|---|---|
| Intraobserver | Intraobserver | Interobserver | Interobserver | Intraobserver | Interobserver | ||
| GLS | Rest | 0.1 (−0.4 to 0.5) | 0.3 (0.3 to 1.0) | 0.3 (−2.7 to 3.3) | 1.7 (0.8 to 4.1) | 0.94 | 0.91 |
| Exercise | 0.2 (−0.2 to 0.6) | −0.2 (−0.9 to 0.5) | 3.1 (−2.1 to 8.4) | −2.7 (−7.1 to 1.7) | 0.97 | 0.97 | |
| GLSR | Rest | 0.03 (−0.01 to 0.06) | 0.05 (−0.01 to 0.09) | 3.0 (−1.0 to 7.0) | 5.6 (−0.6 to 10.7) | 0.92 | 0.88 |
| Exercise | −0.01 (−0.06 to 0.08) | 0.05 (−0.04 to 0.14) | −0.1 (−7.3 to 7.2) | 3.7 (−3.8 to 11.2) | 0.96 | 0.91 | |
| s′ | Rest | 0.14 (−0.09 to 0.37) | −0.39 (−0.81 to 0.03) | 2.4 (−0.9 to 5.7) | −5.1 (−10.3 to 0.2) | 0.97 | 0.94 |
| Exercise | −0.07 (0.41 to 0.27) | −0.32 (−0.88 to 0.24) | −0.1 (3.4 to 3.2) | −3.3 (−9.4 to 2.8) | 0.98 | 0.92 | |
| MAPSE | Rest | 0.20 (−0.49 to 0.90) | 0.16 (−0.40 to 0.72) | 2.3 (−3.9 to 8.4) | 0.8 (−4.7 to 6.2) | 0.90 | 0.91 |
| Exercise | 0.28 (−0.24 to 0.81) | −0.62 (−1.25 to 0.01) | 1.8 (−2.1 to 5.6) | −4.9 (−9.7 to 0.1) | 0.94 | 0.89 | |
- ICC, intraclass correlation coefficient; LV, left ventricular; other abbreviations as in Table 1.
- Indexed Bland–Altman—mean difference and 95% confidence interval after indexation to the mean value of test and retest measurements.
Discussion
This study demonstrates that echocardiographic parameters of LV longitudinal systolic function are not equipotent in predicting adverse outcomes in HFpEF. LV deformation indices, especially assessed with exercise, show the highest predictive utility independent from and incremental to clinical data and BNP.
Left ventricular long-axis function contributes to both LV ejection and filling.14 It is governed mainly by the subendocardial myocardial fibres, which are particularly vulnerable to injury due to the increased wall stress and disproportion between oxygen demand and supply, resulting in the development of myocardial fibrosis.1 Several parameters reflecting global LV longitudinal shortening, derived by different echocardiographic techniques, such as M-mode, tissue Doppler, and speckle tracking, have proven useful for diagnostic purposes. Indeed, GLS, systolic tissue velocity, and MAPSE all have prognostic significance in the general population and in a plethora of pathological conditions including HF, valvular and congenital heart disease, cardiomyopathies, diabetes, hypertension, and ischaemic heart disease.8-10, 15, 26-28 Studies conducted in HF showed prognostic superiority of LV long-axis parameters over other echocardiographic measures of myocardial systolic function irrespective of the ejection fraction status.11-13, 18, 19
Despite the abundance of single parameter-based studies, the selection of the most appropriate prognostication tool remains a matter of debate, with no evidence favouring one approach over the others. Our review of available literature shows the lack of direct comparisons of the predictive value of echocardiographic indices referring to LV long-axis systolic function. We demonstrated that when the evaluated parameters were measured in resting conditions, GLS was most effective as a prognosticator. In addition, assessment under an exercise load provided more prognostic information than at rest and also revealed superiority of LV deformation, especially GLS, which added a statistically significant incremental prognostic value to clinical data and BNP as confirmed by c-statistics. Furthermore, only GLS and GLSR measured at both rest and after exercise afforded a significant improvement in the accuracy of clinical risk classification based on MAGGIC risk score, BNP, and peak VO2.
The superior prognostic value of LV deformation might be attributable to the fact that strain and strain rate are less susceptible to translational movement than s′ and MAPSE and therefore might be more specific markers of myocardial systolic function. In addition, the assessment of LV longitudinal deformation across the entire LV myocardium may provide more adequate information than the assessment of systolic descent of the mitral annulus by s′ and MAPSE. The prognostic relevance of LV longitudinal response to exercise in HFpEF underpins the notion that diminished LV systolic reserve might be more important than abnormalities of myocardial performance at rest. Accordingly, exertional measurements of LV longitudinal function might improve risk evaluation in this HF category.
It should be emphasized that the absolute differences in LV longitudinal function parameters between the subsets with and without the endpoint were small, which might be attributable to a moderately severe progression of cardiovascular pathologies at baseline. However, this might affect the ability of these indices to be effectively implemented in clinical practice, including determining usable cut-off values for worse prognosis, and warrants further investigation.
Among an increasing number of studies using cardiovascular magnetic resonance, little comparative data exist with respect to the prognostic assessment of LV long-axis function parameters.29-31 A very recent paper revealed that both lateral MAPSE and feature tracking GLS independently contributed to risk prediction in hypertensive patients, which implies that these indices may supply complementary prognostic information.32 We did not show any analogous relationship in our analysis, which might be explained by between-modality and patient clinical profile differences.
Limitations
First, the exclusion of patients with atrial fibrillation and myocardial ischaemia might limit generalization of our results to all subjects with HFpEF. Second, the recognition of HFpEF was based solely on the clinical data, with no verification by invasive measurements, which might limit in some cases a definite ascertainment of diagnosis. Third, the relatively low temporal resolution of exercise imaging might have affected the accuracy of GLSR measurements. Fourth, a single-centre patient enrolment might constrain the external validity of our findings. Fifth, the extrapolation of our results to other cardiovascular pathologies is uncertain, especially in conditions more affecting annular velocities and displacement than myocardial deformation, like mitral valve disease or annular calcifications. Because of the inter-modality differences, the results of this paper cannot be directly extrapolated to studies using CMR techniques. Finally, GLS as defined in the recent consensus paper was based on peak strain throughout the LV region of interest, rather than the averaged regional strain used in our work. We planned the latter approach for two reasons—first, to enable us to remove segments where tracking was inadequate, and in order to make our measurement of regional contraction reflective of function, irrespective of conduction disturbances.
Conclusions
The abilities of echocardiographic parameters of LV long-axis systolic function to stratify clinical risk in HFpEF are not equivalent. LV deformation seems to be the most reasonable prognostic marker of long-axis performance in this disease setting. Potential differences favouring the use of myocardial deformation indices are more apparent for measurements carried out under an exercise load. The improvement of predictive value of these parameters during the assessment at stress may provide a rationale for the inclusion of exercise echocardiography to prognostic evaluation in HFpEF.
Conflict of interest
None declared.
Funding
This work was supported by Wroclaw Medical University, Wroclaw, Poland (ST-678), and the Royal Hobart Hospital Research Foundation, Hobart, Tasmania, Australia (13-024).




