Pump thrombosis and dynamic outflow graft compression: complications in left ventricular assist device therapy
Abstract
Over the past decade, left ventricular assist device (VAD) therapy has become more prevalent and increasingly safe. Severe complications, such as VAD pump thrombosis and outflow graft obstruction, are rare, yet still associated with high morbidity and mortality. Clinical presentation, VAD alarm and log files, laboratory analysis, and non-invasive cardiac imaging are crucial for establishing the correct diagnosis and determining clinical management. Early intervention is critical to prevent adverse cardiac remodelling or VAD pump failure.
Case report
A 67-year-old man with a history of ischaemic heart disease and bypass grafting presented to the cardiology department with ongoing low-flow alarms of his left ventricular assist device (LVAD).
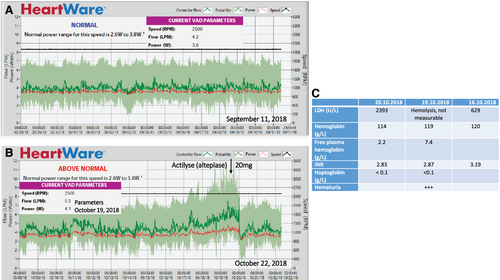
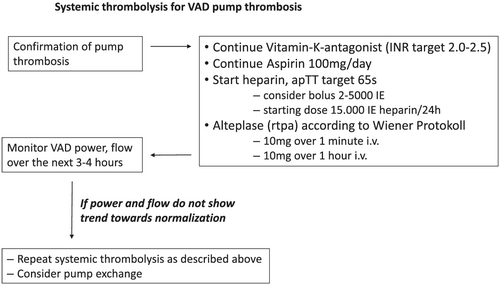
Two years prior to presentation a HeartWare™ HVAD™, LVAD was implanted via an anterolateral thoracotomy with an outflow graft anastomosis to the descending aorta. This access route was chosen because of the repeat-surgery situation after coronary artery bypass grafting with prior sternotomy. The descending aorta was easy to reach without needing a second incision over the ascending aorta. After uneventful post-operative recovery and improvement from New York Heart Association Class IV to I–II, the patient presented with haematuria 4 months after LVAD implant. Auscultation suggested obstructed LVAD flow (Supporting Information, Audio S1). Laboratory analysis showing haemolysis in combination with a flow and power increase in the LVAD log files1 confirmed ventricular assist device (VAD) pump thrombosis (Figure 1). Haematuria being the only symptom, systemic thrombolysis was performed with 20 mg alteplase (Figure 2), which successfully restored LVAD function with normalization of VAD flow and power and resolution of haematuria (Supporting Information, Audio S2 and Figure 1). After recurrent pump thromboses 6, 12, 13, and 14 months after LVAD implantation—each of the episodes was responsive to systemic thrombolysis—the decision to exchange the LVAD pump was made.
Because of the low incidence of pump thrombosis in the MOMENTUM 3 trial,2 the HeartWare HVAD was replaced with a HeartMate 3 VAD. After disconnecting the HeartWare by loosening the sewing ring screw, the sewing cuff of the heartware was completely removed and replaced by the apical cuff of the HeartMate 3 with the core-then-sew technique. Each cuff has a different, non-compatible, closure system. Intraoperatively, the different sizes of the outflow grafts of the HVAD and HeartMate 3, the mobility of the new device, and the ingrown, static old outflow graft made it difficult to assess to ideal position and outflow graft length of the newly implanted HeartMate 3. To allow reattachment, the HeartMate 3 outflow graft was shortened, thereby removing most of the bend relief. The old outflow graft to the descending aorta was preserved and shortened accordingly by removing the bend relief of the HeartWare system. The larger size of the HeartMate 3 and intraoperative low-flow alarms caused by external compression of the outflow graft necessitated intraoperative resection of two ribs. As repeated low-flow alarms were encountered during initial patient mobilization, a third rib, causing a hypomochlion, was resected on the first post-operative day. Thereafter, the post-operative recovery remained uneventful without reoccurrence of low LVAD flow.
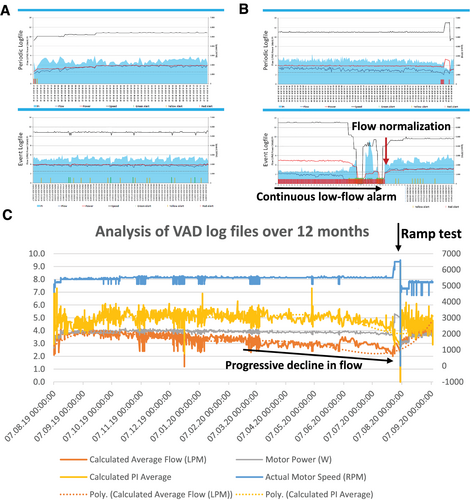
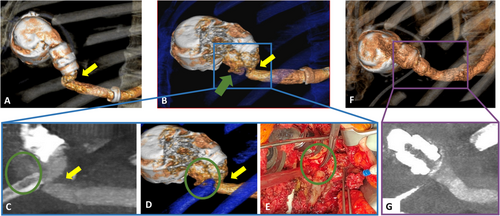
Four months after pump exchange, the patient presented to the emergency room with continuous low-flow alarm (Figure 3). Log files showed a flow of 1.2 L over approximately 20 minutes. Patient history and clinical demonstration revealed posture dependency of the alarm, with the alarm going off when lying on his left side and subsiding when lying straight or standing up. We proceeded to perform computed tomography angiography (CTA) both in a supine and in a left lateral decubitus position demonstrating posture-dependent, dynamic outflow graft compression with a minimal luminal diameter measuring 5 × 7 mm in left lateral position and 11 × 13 mm in supine position, respectively. (Figure 4; published previously, adapted with permission from the publisher).3
As the symptoms were minor, low-flow alarms manageable when avoiding sleeping in a left lateral position, and a repeat intervention in a hostile environment not desirable, a wait-and-see approach was agreed upon.
Over the next 6 months, the patient was regularly seen in the outpatient VAD clinic. He recognized a slow decline in functional capacity. In line with progressive outflow graft obstruction, LVAD flow decreased from 4.0 to 2.7 L/min (Figure 3). Serial echocardiograms showed an increase in left ventricular end-diastolic diameter from 58 mm after VAD implantation to 67 mm. Under echocardiographic guidance, the revolutions per minute were increased from 5500 to 6500 without a significant increase in LVAD flow (from 2.7 to 3.0 L/min). The patient was hospitalized for a repeat CTA and interdisciplinary discussion of interventional and surgical options.
Computed tomography angiography showed progression of the outflow graft obstruction in the left lateral decubitus position (3.5 × 4 mm). The obstruction of the graft was thought to be associated with a progressive kink due to a costal hypomochlion at the surgical site and graft size incompatibility. Stenting was considered,4 yet the thought was abandoned because of the proximity of the obstruction to the origin of the outflow graft and the associated risk of getting a wire caught in the VAD rotor, before we proceeded with a surgical revision of the outflow graft.
During surgery, a pseudoaneurysm aggravating the pre-existing kink in the form of an external graft compression was found as the underlying cause of the progressive low-flow situation. While dissecting the outflow graft, major bleeding occurred, necessitating circulatory arrest in deep hypothermia. Upon inspection, the Dacron graft had been damaged by the remnants of the resected ribs, causing a 2-mm-sized hole (Figure 4). The damaged segment of the HeartMate 3 outflow graft—Dacron was resected; after a new end-to-end anastomosis with the HeartWare outflow graft, no residual kinking was observed. LVAD flow normalized immediately. No further alarms were registered during follow-up. A repeat computed tomography angiogram confirmed a straightened outflow tract without signs of obstruction (Figure 4).
Discussion
According to the International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support Registry, infections and bleeding remain the most common complications in mechanical circulatory support.5
Balancing bleeding risk and thromboembolic complications presents a major challenge in the management of VAD patients. While much rarer, the consequences of VAD pump thrombosis are typically more severe, as they include life-threatening device malfunction and thromboembolic stroke.
Anticoagulation management and time in the therapeutic international normalized ratio (INR) range play a key role in preventing late-onset pump thrombosis (>3 months after implantation) and bleeding complications, as an INR between 2.4 and 2.6 was associated with both low thromboembolic and bleeding risk.6 The factors determining early VAD thrombosis remain less clear, as no apparent association with adequate target INR (2–2.5) was seen in the first three post-operative months.6
Post-operative bridging with heparin, timely initiation of post-operative anticoagulation, and antithrombotic therapy play a key role (see Table 1 for management at Inselspital Bern), but likely so do the choice of VAD system, operative technique, and optimization of pump speed and haemodynamics during the intraoperative and early post-operative period.7
| Time after surgery | ||||
|---|---|---|---|---|
| 0 | VAD implantation | |||
| 6 | If no significant post-operative bleeding | → | Start heparin 10 000/24 h i.v. | |
| 24 | 1 | Drains <50 mL/h | → | Heparin up-titration, target: anti-Xa 0.2–0.3 IU/mL |
| 48 | 2 | Drains <50 mL/h | → |
Heparin target: anti-Xa 0.2–0.3 IU/mL Start aspirin 100 mg/day |
| 3–5 | After removal of drains | → |
Start anticoagulation with vitamin K antagonist (target INR 2–2.5) Continue heparin (anti-Xa 0.2–0.3 IU/mL) until target INR is reached Continue aspirin 100 mg/day |
|
| h | Post-operative day | |||
- INR, international normalized ratio; VAD, ventricular assist device.
In our patient, haematuria and VAD alarms pointed towards a VAD pump thrombosis, and laboratory signs of haemolysis were instrumental in confirming the diagnosis8 (Figure 1). Depending on clinical presentation, treatment strategies for VAD pump thrombosis range from optimizing antithrombotic therapy and anticoagulation to thrombolysis or surgical therapy (e.g. pump exchange or transplantation).
During each thrombosis, our patients remained haemodynamically stable and symptom-free. Thus, we decided to proceed with systemic thrombolysis. The mechanism of the initial pump thrombosis remains unclear, as routine haematological work-up prior to VAD implantation excluded coagulopathies in our patient. The recurring thromboses were likely associated with the damaged surface area of the VAD, leading to increased thrombogenicity. The decision to wait with a VAD pump exchange was driven by the lack of patient symptoms, rapid and adequate response without complications to systemic haemolysis, and the challenging operating site after redo cardiac surgery.
Outflow graft obstruction
Continuous low-flow alarms were the first sign of outflow tract obstruction in our patient. Alarms and symptoms of progressive heart failure are the most common presentation (in 92% of patients) leading to hospital admission in these cases.9
After exclusion of hypovolaemia, arrhythmias, or hypertension, posture dependency of the low-flow alarm pointed towards an outflow graft obstruction, and the diagnosis was confirmed using CTA.3 In our increasingly symptomatic patient, a steady decline in LVAD flow (Figure 3) without an increase in VAD power and accompanying progressive left ventricular dilatation hinted towards progressive outflow graft obstruction. Other echocardiographic signs may include increased mitral regurgitation or aortic valve opening,10 but were not present in our patient. The blunted change in LVAD flow seen during the echocardiographic ramp study is consistent with outflow tract obstruction and may be further supported by an invasive ramp test without translation of increasing pump speed into increased cardiac output or reduced pulmonary capillary wedge pressure.
Computed tomography angiography is an important tool when planning therapeutic intervention for outflow tract obstruction and may point towards the underlying mechanism. Interventional therapy and stenting of the outflow tract obstruction may be an option11 to prevent a surgical redo procedure. The heart team's decision to proceed with a surgical revision was based on the technical difficulties encountered during the pump exchange surgery and the substantial outflow graft kinking seen in CTA. The mobility of the replacement VAD pump likely caused the initial outflow graft kink, allowing displacement towards the resected rib stump, subsequent puncture of the outflow graft, and pseudoaneurysm formation. As substantial bleeding was encountered after incision of the pseudoaneurysm, the decision to proceed with surgery appears, retrospectively, to have been the correct one.
Conflict of interest
None declared.




