Use of angiotensin receptor blocker is associated with improved 1 year mortality in heart failure with mid-range ejection fraction
Abstract
Aims
Current evidence about the effect of angiotensin receptor blocker (ARB) on the outcome of heart failure with mid-range ejection fraction (HFmrEF) is lacking. We aim to assess the association between use of ARB and 1 year all-cause mortality after hospitalization for HFmrEF.
Methods and results
We analysed the data of patients with ejection fraction of 40–49% in China Patient-centred Evaluative Assessment of Cardiac Events Prospective Heart Failure Study; 4907 patients hospitalized for heart failure from 52 Chinese hospitals were enrolled from August 2016 to May 2018. Use of ARB was determined by prescriptions at discharge. Patients who died during hospitalization or were using angiotensin-converting enzyme inhibitors at discharge were excluded. The association between the use of ARB and outcome was assessed using stabilized inverse probability of treatment weighting-adjusted Kaplan–Meier and Cox regression analyses. A total of 701 patients with HFmrEF were included for analysis. The mean age was 66.4 ± 12.8 years, and 267 (38.1%) were female. Of them, 244 were treated (34.8%) with ARB. During the 1 year follow-up period, patients treated with ARB had lower all-cause mortality compared with untreated patients (11.5% vs. 21.9%, P = 0.0005). Inverse probability of treatment weighting-adjusted Cox regression analysis showed that use of ARB was associated with significantly reduced all-cause mortality (adjusted hazard ratio 0.44, 95% confidence interval 0.28–0.69, P = 0.0004).
Conclusions
Among patients hospitalized for HFmrEF, the use of ARB was associated with lower 1 year mortality after discharge.
Introduction
Heart failure (HF) with ejection fraction in 40–49% accounts for nearly one-fifth of the population with HF, and its prognosis is comparable with HF with reduced ejection fraction (HFrEF).1-6 Until recently, as international clinical guidelines recognized this subtype of HF as an intermediate phenotype, called HF with mid-range ejection fraction (HFmrEF)7 or HF with borderline ejection fraction,8 this ‘grey’ area in HF is gaining increasing attention in recent studies.3 The current guidelines recommend HFmrEF to be managed in the same way as HF with preserved ejection fraction (HFpEF).7, 8 However, different from HFrEF, evidence with regard to the effect of medication treatment on the outcome of HFmrEF is sparse.9
It was suggested that angiotensin receptor blocker (ARB), one of the cornerstone treatments of HFrEF, was likely to improve the outcome of HFmrEF, but the conclusion has yet to be corroborated. Post hoc analysis of CHARM trial including 1322 HFmrEF patients indicated that candesartan reduced cardiovascular death and hospitalization of patients with HFmrEF.10 However, the robustness of the result was weakened by multiple comparisons of outcomes and the post hoc nature of the analysis, leading to the increased risk of a chance finding.10 Additionally, the patients were selected through strict criteria of the trial; whether the results can be generalized to a broader population in clinical practice is unclear.11-14 Further study regarding the effect of ARB on the outcome of HFmrEF in a real-world setting is required to provide insights for future recommendations on the management of HFmrEF.
Accordingly, we aim to explore the association of ARB use with 1 year all-cause mortality among the patients with HFmrEF, using data of a multicentre prospective cohort of patients hospitalized primarily for HF.
Methods
Study design and population
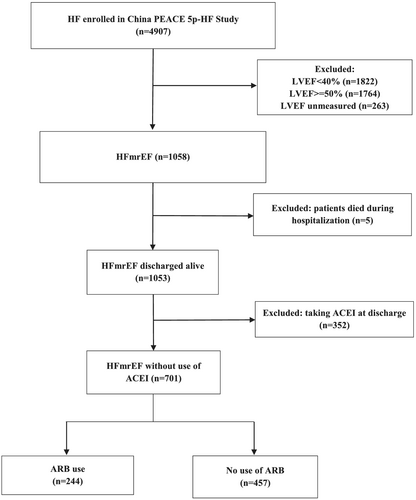
In China Patient-centred Evaluative Assessment of Cardiac Events Prospective Heart Failure Study (China PEACE 5p-HF Study), we consecutively enrolled patients hospitalized primarily for HF from 52 Chinese hospitals located in 20 provinces between August 2016 and May 2018. The protocol of China PEACE 5p-HF Study has been published.15 Patients aged 18 years or older, and hospitalized with a primary diagnosis of new-onset HF or decompensation of chronic HF, as assessed by the local physician, were enrolled in the China PEACE 5p-HF Study. In this study, we only included the patients with HFmrEF, which was defined as left ventricular ejection fraction (LVEF) in 40–49% according to the measurement through echocardiography during hospitalization (n = 1058). We excluded those who died during the index hospitalization (n = 5). In order to eliminate the confounding effect of angiotensin-converting enzyme inhibitor (ACEI), we further excluded patients with prescription of ACEI at discharge (n = 352). In total, 701 eligible patients were included in our analysis (Figure 1). Enrolled patients completed a baseline interview during hospitalization and were followed up at 1, 6, and 12 months after discharge. All the enrolled patients signed an informed consent, and their blood and urine samples were taken within 48 h after admission for central laboratory analysis.
The investigation conforms to the principles outlined in the Declaration of Helsinki. The central ethics committee at Fuwai Hospital and local ethics committees at study hospitals approved the China PEACE 5p-HF Study. The study was registered at http://www.clinicaltrials.gov (NCT02878811).
Data collection
For all enrolled patients, we obtained demographic (age and sex), socio-economic (marital status and education level), and clinical characteristics through abstraction of medical charts of the index hospitalization and in-person interviews during index hospitalization. Clinical variables included medical history, symptoms and signs, laboratory test, and medications prescribed at discharge. The abstraction was performed centrally according to standardized procedures of abstraction and data dictionaries, and accuracy of abstraction exceeded 98%. LVEF was measured during index hospitalization by trained clinicians according to the standard echocardiogram protocol. The use of ARB was determined by the prescriptions with medications recorded at discharge.
Outcome
The outcome of the study was 1 year all-cause mortality. Information regarding patients' survival status during the 12 months of follow-up was collected from follow-up interview, medical documents, and death registry. All the events were centrally adjudicated at the national coordinating centre by trained clinicians.
Statistical analysis
We applied the approach of stabilized inverse probability of treatment weighting (IPTW) to obtain treatment effects of ARB in patients with HFmrEF.16 We first fitted a logistic regression model to predict the probability of receiving the ARB treatment with following baseline covariates according to clinical relevance: age, sex, smoking status, marital status, education level, New York Heart Association functional class, hypertension, diabetes mellitus (DM), atrial fibrillation, stroke, previous myocardial infarction, dilated cardiomyopathy, valvular heart disease, peripheral artery disease, chronic obstructive pulmonary disease, anaemia, history of percutaneous coronary intervention or coronary artery bypass grafting, systolic blood pressure at discharge, estimated glomerular filtration rate (eGFR) at discharge, blood urea nitrogen at discharge, and prescription of beta-blocker and spironolactone at discharge. Detailed definitions of medical history in the study were listed in Supporting Information, Table S1. We calculated the Harrell C statistic to assess the overall predictive accuracy17 and the Hosmer–Lemeshow goodness-of-fit test to assess calibration.18 Then we calculated stabilized IPTW based on the propensity score.19 We used standardized differences to evaluate and re-evaluate difference between treated and untreated patients of all the covariates in the original population and IPTW-weighted population, with difference less than 10% accepted. We used boxplots to describe the distribution of propensity scores in the unweighted population and IPTW-weighted population. IPTW-adjusted Kaplan–Meier analysis was performed to compare overall survival between the treated and untreated patients.20 We further fitted an IPTW-weighted Cox regression model21 adjusting for unbalanced characteristics (age, education level, and co-morbidity of valvular heart disease). For sensitivity analyses, given the potential confounding effect of concomitant medications, we further adjusted the use of beta-blocker and spironolactone in the IPTW-weighted Cox model. We also fitted a Cox regression model with propensity score as a covariate to test the robustness of our findings. To test heterogeneous effect of ARB in patients with renal dysfunction, we added interaction term of ARB and eGFR level (<45 and ≥45 mL/min/1.73 m2) in the IPTW-weighted Cox regression model.
We used percentages to describe categorical variables and means with standard deviation to describe continuous variables; 17 (2.4%) of serum creatinine and 17 (2.4%) of serum urea nitrogen were missing and were imputed with mean value of the overall population, respectively. A value of P < 0.05 (two-sided test) was considered statistically significant. All analyses were performed in SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patient characteristics
Our study sample included 701 patients of HFmrEF. The mean age was 66.4 ± 12.8 years, and 267 (38.1%) were female; 244 patients (34.8%) were prescribed with ARB at discharge. Unweighted and weighted baseline characteristics of patients with HFmrEF according to use of ARB were reported in Table 1. Standardized differences of unweighted population showed that clinical characteristics differed significantly between treated and untreated patients. After IPTW adjustment, standardized differences were considerably smaller and less than 10% for most characteristics except for age, education level, and co-morbidity of valvular heart disease. Propensity score distribution was similar in treated and untreated patients after IPTW adjustment (Supporting Information, Figure S1). Results of multivariable logistic regression analysis determining the use of ARB were reported in Supporting Information, Table S2, with C statistics 0.81 and Hosmer–Lemeshow goodness of fit 0.10, indicating the adequate predictive accuracy and goodness of fit of the model.
| Patient characteristics | Unweighted population | Standardized difference (%) | Weighted population | Standardized difference (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 701) | ARB use (n = 244) | No ARB use (n = 457) | Overall | ARB use | No ARB use | |||
| Age, year, mean (SD) | 66.4 (12.8) | 66.5 (12.6) | 66.4 (12.9) | 1.3 | 67.0 (12.8) | 68.0 (11.8) | 66.5 (13.3) | 12.3 |
| Female, n (%) | 267 (38.1) | 96 (39.3) | 171 (37.4) | 4.0 | 267 (36.6) | 96 (33.8) | 171 (38.0) | −8.7 |
| Current smoker, n (%) | 200 (28.5) | 61 (25.0) | 139 (30.4) | −12.1 | 200 (28.3) | 61 (28.1) | 139 (28.4) | −0.7 |
| Unmarried, n (%) | 143 (20.4) | 55 (22.5) | 88 (19.3) | 8.1 | 143 (21.2) | 55 (19.5) | 88 (22.1) | −6.5 |
| Education level below high school, n (%) | 495 (70.6) | 170 (69.7) | 325 (71.1) | −3.2 | 495 (72.2) | 170 (75.3) | 325 (70.5) | 10.7 |
| NYHA Class III or IV, n (%) | 555 (79.2) | 192 (78.7) | 363 (79.4) | −1.8 | 555 (78.3) | 192 (77.0) | 363 (79.0) | −4.9 |
| Medical history, n (%) | ||||||||
| Hypertension | 434 (61.9) | 194 (79.5) | 240 (52.5) | 59.5 | 434 (61.9) | 194 (59.9) | 240 (62.9) | −6.2 |
| Diabetes mellitus | 252 (35.9) | 100 (41.0) | 152 (33.3) | 16.0 | 252 (35.8) | 100 (34.5) | 152 (36.5) | −4.2 |
| Atrial fibrillation | 277 (39.5) | 84 (34.4) | 193 (42.2) | −16.1 | 277 (39.5) | 84 (39.5) | 193 (39.5) | −0.2 |
| Stroke | 155 (22.1) | 65 (26.6) | 90 (19.7) | 16.5 | 155 (19.4) | 65 (17.3) | 90 (20.5) | −8.2 |
| Previous MI | 192 (27.4) | 76 (31.1) | 116 (25.4) | 12.8 | 192 (28.9) | 76 (30.9) | 116 (27.9) | 6.4 |
| DCM | 89 (12.7) | 32 (13.1) | 57 (12.5) | 1.9 | 89 (13.7) | 32 (14.5) | 57 (13.3) | 3.6 |
| VHD | 108 (15.4) | 26 (10.7) | 82 (17.9) | −20.9 | 108 (17.3) | 26 (21.8) | 82 (15.0) | 17.8 |
| PAD | 85 (12.1) | 33 (13.5) | 52 (11.4) | 6.5 | 85 (11.6) | 33 (10.0) | 52 (12.4) | −7.5 |
| COPD | 120 (17.1) | 47 (19.3) | 73 (16.0) | 8.6 | 120 (18.9) | 47 (19.9) | 73 (18.4) | 3.7 |
| Anaemia | 196 (28.0) | 49 (20.1) | 147 (32.2) | −27.8 | 196 (26.8) | 49 (27.0) | 147 (26.7) | 0.6 |
| History of PCI or CABG, n (%) | 153 (21.8) | 60 (24.6) | 93 (20.4) | 10.2 | 153 (21.7) | 60 (20.9) | 93 (22.1) | −2.9 |
| Systolic BP, mmHg, mean (SD) | 123 (15.1) | 124 (13.5) | 123 (15.9) | 11.0 | 123 (15.4) | 123 (13.8) | 123 (16.1) | 1.4 |
| Serum creatinine, mg/dL, mean (SD) | 1.2 (0.8) | 1.1 (0.5) | 1.3 (1.0) | −23.8 | 1.2 (0.8) | 1.2 (0.6) | 1.2 (0.9) | −7.7 |
| eGFR, mL/min/1.73 m2, mean (SD) | 80.4 (35.3) | 82.4 (33.4) | 79.3 (36.3) | 8.8 | 80.2 (36.4) | 78.6 (33.7) | 81.0 (37.8) | −6.8 |
| Blood urea nitrogen, mg/dL, mean (SD) | 8.2 (4.9) | 7.5 (3.3) | 8.6 (5.5) | −25.1 | 8.2 (4.7) | 8.3 (4.0) | 8.2 (5.0) | 3.9 |
| Medication, n (%) | ||||||||
| Beta-blocker | 371 (52.9) | 188 (77.0) | 183 (40.0) | 81.1 | 371 (55.2) | 188 (56.1) | 183 (54.8) | 2.6 |
| Spironolactone | 400 (57.1) | 192 (78.7) | 208 (45.5) | 72.8 | 400 (57.7) | 192 (55.9) | 208 (58.6) | −5.5 |
- ARB, angiotensin receptor blocker; BP, blood pressure; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SD, standard deviation; VHD, valvular heart disease.
Treatment effect of angiotensin receptor blocker
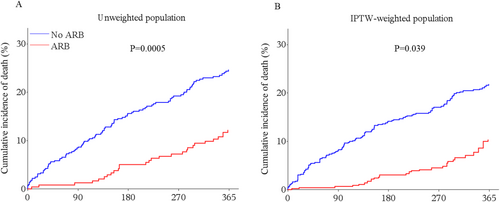
In the overall study population, 128 (18.3%) patients died during the 1 year follow-up. Among them, 28 (11.5%) patients treated with ARB and 100 (21.9%) patients not treated with ARB died (P = 0.0005). Cumulative incidences of 1 year all-cause mortality with Kaplan–Meier method by use of ARB before and after IPTW adjustment were shown in Figure 2. After IPTW adjustment, all-cause mortality was significantly lower in treated patients than untreated patients (P = 0.039). In IPTW-weighted Cox regression analysis, use of ARB was associated with a significant reduced all-cause mortality [hazard ratio (HR) 0.44, 95% confidence interval (CI) 0.28–0.69, P = 0.0004]. In the sensitivity analyses, IPTW-weighted Cox regression analysis adjusting for concomitant medications or adding propensity score as a covariate in the Cox regression model showed similar results (Table 2).
| Analysis | All-cause death | P value |
|---|---|---|
| No. of events/no. of patients at risk | 128/701 (18.3%) | — |
| Angiotensin receptor blocker | 28/244 (11.5%) | — |
| No angiotensin receptor blocker | 100/457 (21.9%) | — |
| Crude analysis—hazard ratio (95% CI) | 0.48 (0.32–0.73) | 0.0006 |
| Propensity score analyses—hazard ratio (95% CI) | ||
| IPTW-weighted analysis | 0.44 (0.28–0.69) | 0.0004 |
| IPTW-weighted adjusting for concomitant medicationsa | 0.43 (0.28–0.68) | 0.0003 |
| Adjust for propensity score | 0.46 (0.30–0.71) | 0.0004 |
- CI, confidence interval; IPTW, inverse probability of treatment weighting.
- a Beta-blocker and spironolactone.
With regard to the effect of ARB in patients with renal dysfunction, 107 (15.3%) patients had eGFR <45 mL/min/1.73 m2. ARB was prescribed in 28 (26.2%) and 216 (36.4%) patients with eGFR <45 and ≥45 mL/min/1.73 m2, respectively. Similar effect of ARB on the outcome was found between two groups (eGFR <45 mL/min/1.73 m2, HR 0.39, 95% CI 0.16–0.96; eGFR ≥45 mL/min/1.73 m2, HR 0.45; 95% CI 0.27–0.76, P for interaction = 0.80).
Discussion
In this prospective study of the patients with HFmrEF, we found that use of ARB at discharge was significantly associated with lower 1 year all-cause mortality. To date, this is the first study to show the beneficial effect of ARB on 1 year outcome of HFmrEF using the data from the real-world setting.
The baseline profiles of the patients with HFmrEF in this study are similar with the prior large registry studies. Compared with the Swedish Heart Failure Registry22 and Get With The Guidelines—Heart Failure,1 patients with HFmrEF in this study were similar except being younger (Supporting Information, Table S3). In contrast to these real-world studies, there were fewer female patients (29.9%) and lower prevalence of hypertension (56.2%), DM (28.6%), and atrial fibrillation (25.6%) in the population of HFmrEF in the CHARM trial. Notably, consistent with previous registry studies or trial,10, 22, 23 our studies demonstrated substantially high proportion of ischaemic heart disease among patients with HFmrEF.
Our study corroborated the beneficial effect of ARB on the outcome of HFmrEF using the data from a real-world setting. Previous studies suggest that ARB may exert beneficial effect on the outcome of HFmrEF, but the evidence is inconclusive because they did not investigate the effect of ARB separately. A study of the Swedish Heart Failure Registry including HF with ejection fraction >40% demonstrated a stronger association of renin–angiotensin receptor inhibitor treatment with reduced all-cause mortality in subgroup of HFmrEF than HFpEF.24 Another pooled analysis from two Korean registry studies found that therapies of renin–angiotensin receptor inhibitor may reduce the risk of all-cause mortality of HFmrEF.25 Interestingly, subgroup analysis of PARAGON-HF trial26 also indicated the potential benefit of sacubitril–valsartan in the lower spectrum of LVEF (<57%) on the composite outcome of cardiovascular death and HF hospitalization. Based on these studies, our study provided important therapeutic evidence for the management of HFmrEF.
Our study demonstrated the homogenous effect of ARB in HFmrEF in different eGFR levels. This is particularly of interest given the high prevalence of renal dysfunction in HF27 and concern of ARB use in patients with renal dysfunction in clinical practice.28 Our finding was consistent with previous studies on use of renin–angiotensin receptor inhibitor in patients with HFrEF and chronic kidney disease, even in severe renal dysfunction (eGFR <30 mL/min/1.73 m2).29, 30 Given the limited sample size, our study could not further analyse the effect in patients with severe renal dysfunction.
The underlying mechanism of favourable effect of ARB on the outcome of HFmrEF remains unclear, because the pathophysiology of HFmrEF is still poorly understood. Despite controversial, accumulating evidence suggests that HFmrEF is a not distinct phenotype of HF but a transition group from HFrEF to HFpEF. The characteristics that HFmrEF shares with HFrEF may partially explain the favourable effect of ARB in HFmrEF, including higher prevalence of ischaemic heart disease and similar biomarker patterns.31 Thus, it has been proposed to rename HFmrEF as HF with mildly reduced ejection fraction.32 In addition, given the substantial co-morbidities observed in the patients with HFmrEF, ARB may improve their outcomes by treating hypertension, DM, ischaemic heart disease, and chronic kidney disease. More investigations are needed to elucidate the pathophysiology of HFmrEF and mechanism of ARB on HFmrEF.
Our findings will inform decision making in the clinical management of HFmrEF and may help guide future recommendations for treatment of HFmrEF. ARB and other guideline- recommended medical therapies of HFrEF, including beta-blocker and mineralocorticoid antagonist, are widely used in vast majority of patients with HFmrEF to control blood pressure or treat other co-morbidities, and evidence is urgently needed to fill these gaps of the indications for these treatments. Moreover, the results of our study may facilitate the design and implementation of future clinical trial of HFmrEF.
The main limitation of our study was its nature of observational design. Of note, the present analyses may be subject to confounding, which we attempted to address by using an IPTW-adjusted approach. Second, multi-pharmacy is highly prevalent in the management of HF from the real-world scenario,33 and our study was unable to evaluate the association between use of ARB added to beta-blocker or/and spironolactone and outcome of patients with HFmrEF given the limited sample size. However, by using propensity score approach, we balanced the baseline difference of treatment beta-blocker and spironolactone. We believed that the improved mortality of HFmrEF was largely attributed to the ARB use, rather than other medications. Third, we did not account for the adherence to the ARB and crossover during the follow-up period, but if any, our study would have underestimated the potential benefit of ARB. Fourth, our study did not collect information of LVEF during the follow-up and were thus unable to explore the effect of ARB on dynamic transition from HFmrEF into HFpEF or HFrEF.
Conclusions
Use of ARB was associated with lower 1 year all-cause mortality among patients with HFmrEF. Our study will provide valuable insights for decision making and future recommendations for the management of HFmrEF.
Conflict of interest
J.L. discloses that she is a recipient of research grants from the government of China, through Fuwai Hospital, for research to improve the management of hypertension and blood lipids and to improve care quality and patient outcomes of cardiovascular disease; is a recipient of research agreements with Amgen, through the National Center for Cardiovascular Diseases (NCCD) and Fuwai Hospital, for a multicentre trial to assess the efficacy and safety of omecamtiv mecarbil and for dyslipidaemic patient registration; is a recipient of a research agreement with Sanofi, through Fuwai Hospital, for a multicentre trial on the effects of sotagliflozin; is a recipient of a research agreement with the University of Oxford, through Fuwai Hospital, for a multicentre trial of empagliflozin; and was a recipient of a research agreement, through NCCD, from AstraZeneca for clinical research methods training. No other disclosure is reported.
Funding
This project was supported by the National Key Research and Development Program (2018YFC1312400 and 2018YFC1312401) from the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (2020-I2M-2-007, 2016-I2M-2-004 and 2017-I2M-2-002), the National Key Technology R&D Program (2015BAI12B02) from the Ministry of Science and Technology of China, the 111 Project from the Ministry of Education of the People's Republic of China (B16005), and the Peking Union Medical College Innovation Fund for Postgraduates (2018-1002-01-12).




