The effect of three major co-morbidities on quality of life and outcome of patients with heart failure with reduced ejection fraction
Abstract
Aims
Diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease are prevalent in patients with heart failure with reduced ejection fraction (HFrEF). We have analysed the impact of co-morbidities on quality of life (QoL) and outcome.
Methods and results
A total of 397 patients (58.8 ± 11.0 years, 73.6% with New York Heart Association functional class ≥3) with stable advanced HFrEF were followed for a median of 1106 (inter-quartile range 379–2606) days, and 68% of patients (270 patients) experienced an adverse outcome (death, urgent heart transplantation, and implantation of mechanical circulatory support). Chronic obstructive pulmonary disease was present in 16.4%, diabetes mellitus in 44.3%, and chronic kidney disease in 34.5% of patients; 33.5% of patients had none, 40.0% had one, 21.9% had two, and 3.8% of patient had three co-morbidities. Patients with more co-morbidities reported similar QoL (assessed by Minnesota Living with Heart Failure Questionnaire, 45.46 ± 22.21/49.07 ± 21.69/47.52 ± 23.54/46.77 ± 23.60 in patients with zero to three co-morbidities, P for trend = 0.51). Multivariable regression analysis revealed that furosemide daily dose, systolic blood pressure, New York Heart Association functional class, and body mass index, but not the number of co-morbidities, were significantly (P < 0.05) associated with QoL. Increasing co-morbidity burden was associated with worse survival (P < 0.0001), lower degree of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker treatment (P = 0.001), and increasing levels of BNP (mean of 685, 912, 1053, and 985 ng/L for patients with zero to three co-morbidities, P for trend = 0.008) and cardiac troponin (sm-cTnI, P for trend = 0.0496), which remained significant (P < 0.05) after the adjustment for left ventricular ejection fraction, left ventricular end-diastolic diameter, right ventricular dysfunction grade, body mass index, and estimated glomerular filtration rate.
Conclusions
In stable advanced HFrEF patients, co-morbidities are not associated with impaired QoL, but negatively affect the prognosis both directly and indirectly through lower level of HF pharmacotherapy and increased myocardial stress and injury.
Introduction
Non-cardiac co-morbidities in heart failure (HF) patients are increasingly prevalent.1 Although they are believed to be associated with impaired quality of life (QoL), recently published meta-analysis did not find any study systematically analysing the effect of co-morbidities on QoL.2 Co-morbidities significantly impair the outcome of HF patients,3-6 but the number of studies investigating the combined effect of multiple co-morbidities is still limited.7, 8 The impact of co-morbidities on outcome can vary depending upon both the stage of HF and the degree of HF pharmacotherapy and device therapy. Although co-morbidities negatively influence the outcome of HF patients, it is unclear whether they exert their negative effect directly or indirectly by contributing to cardiac damage or through less optimal HF pharmacotherapy.7 Understanding the mechanisms how co-morbidities affect QoL and outcome of HF patients may help to identify appropriate treatment targets.
Biomarkers reflecting cardiac injury or stress (cardiac troponin and BNP) can be employed to assess the impact of co-morbidities on myocardium. Increased cardiac injury was shown in patients with HF and concomitant diabetes mellitus (DM)9 or moderate chronic obstructive pulmonary disease (COPD)10 as higher level of cardiac troponin was documented in these conditions.
The aim of the study was to assess the impact of co-morbidities on QoL and outcome in advanced HFrEF patients with high degree of guideline-recommended pharmacotherapy and device therapy and to assess the mechanisms how co-morbidities contribute to an adverse outcome. We have employed an ultrasensitive single-molecule counting cardiac troponin assay11 and natriuretic peptide (NP) assessment to determine whether increased co-morbidity burden is associated with increased myocardial stress or injury.
Methods
Study subjects
Patients with stable HF of at least 6 month duration resulting from left ventricular (LV) systolic dysfunction [LV ejection fraction (LVEF) <40%], electively hospitalized at the Institute for Clinical and Experimental Medicine—IKEM in Prague for device implantation (in patients with LVEF ≤ 35%), radiofrequency ablation, coronary angiography/percutaneous coronary intervention, or transplant eligibility evaluation, were screened. Stable patients receiving stable medical therapy for at least 3 months were consecutively enrolled in the study between 2008 and 2011. Patients with reversible LV dysfunction (planned valve surgery, revascularization, or tachycardia-induced cardiomyopathy) were excluded. Patients were followed until March 2018.
Right ventricular dysfunction (RVD) was quantified (0 to 3) in an apical four-chamber view by using tricuspid annular systolic excursion [M-mode tricuspid annular plane systolic excursion (TAPSE)]12 and tissue systolic velocity (Sm)13 with the following cut-offs: RVD0, normal: TAPSE > 20 mm and Sm > 12 cm/s; RVD1, mild impairment: TAPSE 16–20 mm and Sm 9–12 cm/s; RVD2, moderate: TAPSE 10–15 mm and Sm 6–8 cm/s; and RVD3, severe: TAPSE severe: TAPSE < 10 mm and Sm < 6 cm/s. In case of disagreement of criteria, qualitative visual estimation of right ventricular (RV) motion in apical four-chamber was also taken into account.
Patients were prospectively followed for adverse outcome defined as the combined endpoint of death, urgent heart transplantation (HTx), or mechanical circulatory support (MCS) implantation. Because the time to non-urgent HTx reflects donor availability rather than recipient's condition, patients who received a non-urgent HTx were censored as having no adverse outcome event at the day of transplantation.14 At the study enrolment, patients completed a Minnesota Living with Heart Failure Questionnaire (MLHFQ) and had anthropometric tests and echocardiographic study (Vivid-7; General Electric, Milwaukee, WI). LV function and dimensions were measured according to published recommendations.15 The investigation conforms to the principles outlined in the Declaration of Helsinki, the study protocol was approved by the institutional ethics committee, and all subjects signed an informed consent.
Laboratory assessment
Blood was collected into serum separator tubes with clot activator and ethylendiaminetetraacetic acid-anticoagulated tubes upon patient enrolment. Basic biochemical parameters were assessed at the Institute for Clinical and Experimental Medicine. Estimated glomerular filtration rate (eGFR) was calculated by the MDRD equation, based on serum creatinine levels, age, ethnicity, and sex.16 BNP was measured on the ARCHITECT analyzer (Abbott Diagnostics, Abbott Park, IL) using a chemiluminescent immunoassay. Cardiac troponin was assessed at Brigham and Women's Hospital using a highly sensitive assay (sm-cTnI, Singulex, Inc., Alameda, CA) with a lower limit of detection 0.2 ng/L, 99th percentile reference limit 9 ng/L, and total imprecision of 10% at that concentration.
Co-morbidities
The study focused on co-morbidities primarily unrelated to the cardiovascular system—DM, chronic kidney disease (CKD), and COPD. DM was defined according to current recommendation as a patient's history of either known diabetes or fasting glycaemia ≥7.1 mmol/L or Hb1Ac ≥ 48 mmol/mol if diabetes was not known before the study enrolment.17 CKD was defined as estimated glomerular filtration rate below 60 mL/min/1.73 m2. The information about COPD was obtained from patient's history and from medical records.
Statistical analysis
Unpaired t-test and/or Mann–Whitney U test were used to determine differences between parameters in patients with normal renal function and kidney disease. Kolmogorov–Smirnov test was used to evaluate Gaussian distribution. χ2 test was used to compare categorical variables. The effect of biomarker concentration on prognosis was tested using univariate and multivariable Cox model. Event-free survival of patients was analysed by Kaplan–Meier analysis with log-rank test comparison between groups. Calculations were performed using JMP 11 (SAS Institute Inc., Cary, NC) and SPSS Version 19 software (IBM, Chicago, IL).
Results
Patients
A total of 397 patients with HFrEF [LVEF 24.7 ± 5.0% and New York Heart Association (NYHA) 2.8 ± 0.6] were followed for a median of 1106 (inter-quartile range 379–2606) days. During follow-up, 270 patients (68%) experienced an adverse outcome (death, urgent HTx, or MCS implantation). Patient characteristics are summarized in Table 1.
| Total cohort (N = 397) | No. of co-morbidities | P for trend | ||||
|---|---|---|---|---|---|---|
|
0 N = 136 |
1 N = 159 |
2 N = 87 |
3 N = 15 |
|||
| Anthropometry | ||||||
| Age (years) | 58.8 ± 11.0 | 53.61 ± 11.53 | 60.24 ± 10.04 | 63.34 ± 8.85 | 64.26 ± 9.25 | <0.0001 |
| Male gender (%) | 85 | 80.9 | 86.8 | 88.5 | 80.0 | 0.24 |
| BMI (kg/m2) | 27.9 ± 4.7 | 27.23 ± 4.54 | 28.08 ± 4.54 | 27.85 ± 4.99 | 31.15 ± 5.90 | 0.02 |
| Heart failure and co-morbidities | ||||||
| Ischaemic aetiology (%) | 53.6 | 34.6 | 61.6 | 67.8 | 60.0 | <0.0001 |
| HF duration (years) | 8.1 ± 7.0 | 6.96 ± 6.34 | 7.53 ± 6.04 | 10.81 ± 8.64 | 8.75 ± 8.17 | 0.001 |
| NYHA functional class (I/II/III/IV) | 2/103/266/26 (0.5/26.0/67.0/6.5%) | 1/57/70/4 (0.7/41.9/51.5/5.9%) | 0/25/125/9 (0/15.7/78.6/5.7%) | 1/19/60/7 (1.1/21.9/69.0/8.0%) | 0/2/11/2 (0/13.3/73.4/13.3%) | 0.0003 |
| eGFR (mL/min/1.73 m2) | 64.69 ± 22.80 | 83.77 ± 19.75 | 69.13 ± 20.54 | 52.34 ± 16.82 | 47.37 ± 10.66 | <0.0001 |
| COPD (%) | 16.4 | 0 | 15.1 | 29.9 | 100 | <0.0001 |
| Hb1Ac (mmol/mol) | 49.55 ± 16.19 | 41.36 ± 3.70 | 51.40 ± 16.73 | 56.39 ± 19.82 | 62.93 ± 22.30 | <0.0001 |
| Cardiac function | ||||||
| Heart rate (b.p.m.) | 77.2 ± 15.1 | 74.69 ± 14.03 | 78.96 ± 16.0 | 78.07 ± 15.64 | 76.93 ± 9.15 | 0.11 |
| Systolic blood pressure (mmHg) | 114.6 ± 18.8 | 114.46 ± 19.66 | 113.82 ± 17.92 | 116.06 ± 20.04 | 114.80 ± 11.46 | 0.63 |
| LVEF (%) | 24.7 ± 5.0 | 25.20 ± 4.95 | 23.80 ± 4.07 | 25.59 ± 5.97 | 24.20 ± 6.00 | 0.98 |
| LV end-diastolic diameter (mm) | 70.6 ± 8.8 | 70.78 ± 8.65 | 71.26 ± 8.83 | 69.52 ± 9.07 | 69.73 ± 9.79 | 0.34 |
| RV dysfunction grade (0–3, %) | 20.4/27.7/38.3/13.6 | 28.7/36.0/25.7/9.6 | 15.1/22.0/46.5/16.4 | 17.2/26.4/42.6/13.8 | 20.0/20.0/40.0/20.0 | 0.002 |
| Mitral regurgitation (0–2, %) | 38.2/33.3/28.5 | 43.4/27.2/29.4 | 34.0/36.5/29.5 | 36.0/34.9/29.1 | 46.7/46.7/6.6 | 0.94 |
| Tricuspid regurgitation (0–2, %) | 64.6/24.2/11.2 | 75.0/15.4/9.6 | 57.1/29.5/13.4 | 64.0/26.7/9.3 | 53.3/33.3/13.4 | 0.09 |
| IVC (mm) | 20.0 ± 6.0 | 19.10 ± 5.75 | 20.71 ± 5.87 | 19.71 ± 6.06 | 22.67 ± 6.75 | 0.11 |
| Symptoms | ||||||
| MLHFQ total score | 47.43 ± 22.29 | 45.46 ± 22.21 | 49.07 ± 21.69 | 47.52 ± 23.54 | 46.77 ± 23.60 | 0.51 |
| MLHFQ somatic score | 21.49 ± 9.86 | 20.93 ± 9.82 | 22.34 ± 9.77 | 20.48 ± 9.99 | 22.85 ± 10.76 | 0.85 |
| MLHFQ emotional score | 8.29 ± 6.31 | 8.30 ± 6.72 | 8.19 ± 5.88 | 8.81 ± 6.68 | 6.31 ± 5.54 | 0.89 |
| Medication | ||||||
| Furosemide use (daily dose, mg) | 97.9 ± 83.0 | 78.91 ± 67.75 | 96.45 ± 74.39 | 120.15 ± 106.59 | 153.42 ± 86.45 | <0.0001 |
| Beta-blocker use (%) | 92.5 | 94.1 | 93.7 | 88.5 | 86.7 | 0.09 |
| Beta-blocker use (daily dose, 0–3, %) | 7.5/50.7/29.7/12.1 | 5.1/45.6/35.3/14.0 | 6.3/52.8/30.8/10.1 | 11.5/55.2/20.7/12.6 | 13.3/53.3/20.0/13.4 | 0.14 |
| ACEi/ARB use (%) | 85.7 | 91.9 | 84.9 | 80.5 | 66.7 | 0.0014 |
| ACEi/ARB use (daily dose, 0–3, %) | 14.3/47.8/29.7/8.2 | 8.1/51.5/30.9/9.6 | 15.1/49.7/25.8/9.4 | 19.5/41.4/34.5/4.6 | 33.3/33.3/33.4/0 | 0.03 |
| Aldosterone antagonist use (%) | 77.4 | 72.8 | 79.25 | 85.1 | 60.0 | 0.27 |
| Devices | ||||||
| ICD any (%) | 66.6 | 60.3 | 71.1 | 67.8 | 66.7 | 0.22 |
| CRT any (%) | 45.3 | 42.7 | 44.7 | 48.3 | 53.3 | 0.31 |
| Outcome | ||||||
| Death | 173 (43.6%) | 24 (17.6%) | 81 (50.9%) | 57 (65.5) | 11 (73.3) | — |
| Urgent HTx | 63 (15.9%) | 24 (17.6%) | 25 (15.7%) | 12 (13.8) | 2 (13.3) | — |
| Non-urgent HTx | 23 (5.8%) | 10 (7.4%) | 6 (3.8%) | 6 (6.9) | 1 (6.7) | — |
| MCS implantation | 34 (8.6%) | 15 (11.0%) | 15 (9.4%) | 4 (4.6) | 0 (0%) | — |
| Survival without HTx/MCS implantation | 104 (26.2%) | 63 (46.3%) | 32 (20.1%) | 8 (9.2) | 1 (6.7%) | — |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; HTx, heart transplantation; ICD, implantable cardioverter-defibrillator; IVC, inferior vena cava; LV, left ventricular; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; RV, right ventricular.
- Data are presented as mean± SD. Beta-blocker and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker dose was evaluated as follows: 0—no dose, 1—low dose (≤33% of target dose), 2—intermediate dose (>33% and ≤66% of target dose), and 3—high dose (>66% of target dose).
Role of co-morbidities
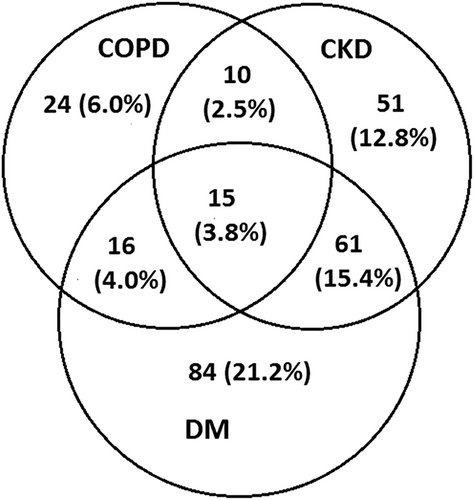
Co-morbidities were prevalent in HF patients—65 patients (16.4%) had COPD, 176 patients (44.3%) had DM, and 137 patients (34.5%) had CKD. The distribution of co-morbidities and co-morbidity combinations is in Figure 1. Patients with more co-morbidities were older and had longer HF history, higher NYHA class, higher degree of RVD, and more often coronary artery disease as an underlying HF aetiology (Table 1).
Therapy of heart failure patients with co-morbidities
Patients with increasing number of co-morbidities were using larger doses of diuretics and were less often treated with angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB). No difference in the use of beta-blockers, mineralocorticoid receptor antagonist, and device therapy was observed (Table 1).
Regression analysis revealed that lower treatment with ACEi/ARB with increasing co-morbidity burden was driven by lower ACEi/ARB treatment in patients with CKD (Supporting Information, Table S1).
Impact of co-morbidities on outcome
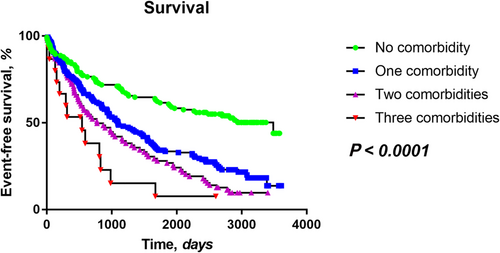
Over a follow-up of 1106 (inter-quartile range 379–2606) days, 270 patients (68%) experienced an adverse outcome. The increasing number of co-morbidities was associated with significantly worse survival (P < 0.0001, Figure 2). Patients with all three co-morbidities had particularly poor survival with majority of events occurring early after the onset of follow-up (i.e. in the first 3 years). Patients with one co-morbidity (COPD, DM, and CKD) had a similar survival irrespective of the type of co-morbidity (P = 0.31, Supporting Information, Figure S2A). Similarly, patients with two co-morbidities showed similar survival irrespective of the co-morbidity combination (P = 0.41, Supporting Information, Figure S2B).
Cox proportional hazard model showed that all co-morbidities were significant independent predictor of adverse outcome in both univariate and multivariable analyses (Table 2).
| Co-morbidity | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| COPD (present vs. absent) | 1.63 | 1.20–2.19 | 0.002 | 1.62 | 1.19–2.17 | 0.003 |
| DM (present vs. absent) | 2.00 | 1.57–2.56 | <0.0001 | 1.87 | 1.46–2.39 | <0.0001 |
| CKD (present vs. absent) | 1.64 | 1.28–2.09 | <0.0001 | 1.49 | 1.16–1.90 | 0.002 |
- CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus.
Nearly identical results were obtained when CKD and DM were replaced with continuous variables (eGFR and Hb1Ac, Supporting Information, Table S2). Additionally, Cox proportional hazard model showed insignificant interaction between all three co-morbidities and their severity (Supporting Information, Figure S3). This suggests that additional co-morbidity negatively impairs outcome regardless of the severity of co-morbidities that are already present.
Patients with COPD had similar degree of eGFR (65.66 ± 19.21 mL/min/1.73 m2) compared with patients without COPD (70.42 ± 23.39, P = 0.12) and Hb1Ac (49.60 ± 16.30 vs. 49.32 ± 15.74, P = 0.90). There was significant but very weak correlation between eGFR and Hb1Ac (r2 = 0.01, P = 0.03). The collinearity between all three co-morbidities was thus very low.
As the number of co-morbidities was associated with lower level of HF pharmacotherapy, higher percentage of HF due to ischaemic aetiology, or higher degree of RVD, we have performed Cox multivariable regression analysis that showed that the number of co-morbidities remained a significant predictor of an adverse outcome even after the adjustment for multiple other potentially confounding variables including BNP, age, HF aetiology, NYHA functional class, ACEi/ARB treatment, and -RV dysfunction grade (Table 3) showing that they indeed deteriorate the prognosis of HF patients. In order to address not only the co-morbidity burden but also their severity, we have repeated the analysis with the inclusion of COPD (as categorical variable) and eGFR and Hb1Ac (continuous variables). The number of co-morbidities (co-morbidity burden) still remained significantly associated with adverse outcome (Supporting Information, Table S3).
| Predictor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Ln (BNP) (ng/L) | 1.77 | 1.55–2.01 | <0.0001 | 1.40 | 1.16–1.70 | 0.0005 |
| Furosemide daily dose (10 mg) | 1.03 | 1.017–1.04 | <0.0001 | 1.01 | 0.99–1.03 | 0.26 |
| Systolic blood pressure (5 mmHg) | 0.90 | 0.87–0.93 | <0.0001 | 0.95 | 0.91–0.99 | 0.02 |
| Sodium (mmol/L) | 0.90 | 0.87–0.93 | <0.0001 | 0.93 | 0.89–0.97 | 0.002 |
| NYHA (III–IV vs. I–II) | 1.72 | 1.30–2.32 | <0.0001 | 1.30 | 0.90–1.90 | 0.17 |
| Total cholesterol (mmol/L) | 0.73 | 0.65–0.84 | <0.0001 | 0.93 | 0.80–1.08 | 0.35 |
| Lymphocyte percentage (%) | 0.97 | 0.96–0.98 | <0.0001 | 0.99 | 0.97–1.01 | 0.19 |
| Sex (male vs. female) | 2.82 | 1.86–4.49 | <0.0001 | 1.32 | 0.80–2.29 | 0.30 |
| Uric acid (100 mmol/L) | 5.39 | 2.46–11.63 | <0.0001 | 1.02 | 0.90–1.16 | 0.74 |
| No. of co-morbidities | 1.51 | 1.34–1.69 | <0.0001 | 1.42 | 1.13–1.77 | 0.002 |
| ACEi/ARB (absent vs. present) | 2.71 | 1.97–3.67 | <0.0001 | 1.83 | 1.19–2.77 | 0.005 |
| BMI (kg/m2) | 0.95 | 0.93–0.98 | 0.0008 | 0.97 | 0.94–1.01 | 0.13 |
| LVEF (5%) | 0.85 | 0.75–0.97 | 0.01 | 1.17 | 0.98–1.41 | 0.09 |
| Heart rate (10 b.p.m.) | 1.07 | 0.99–1.15 | 0.06 | 0.95 | 0.85–1.07 | 0.40 |
| Age (5 years) | 1.01 | 0.95–1.07 | 0.76 | 0.92 | 0.84–1.01 | 0.07 |
| HF duration (years) | 1.02 | 0.997–1.03 | 0.09 | 1.01 | 0.99–1.03 | 0.34 |
| ICD (absent vs. present) | 1.06 | 0.83–1.38 | 0.61 | 1.11 | 0.80–1.52 | 0.54 |
| HF aetiology (CAD vs. non-CAD) | 1.34 | 1.05–1.71 | 0.02 | 1.15 | 0.82–1.66 | 0.42 |
| Beta-blockers (absent vs. present) | 1.60 | 1.04–2.34 | 0.03 | 0.95 | 0.54–1.57 | 0.84 |
| RV dysfunction grade (0–3) | 1.59 | 1.40–1.81 | <0.0001 | 1.10 | 0.91–1.31 | 0.33 |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RV, right ventricular.
- As hazard ratios were very low for 1 mg of furosemide, 1 mmHg of systolic blood pressure, 1 year of age, 1% of LVEF, and 1 μmol/L of uric acid, we have calculated the hazard ratio for 10 mg of furosemide, 5 mmHg of systolic blood pressure, 5 years of age, 5% of LVEF, and 100 μmol/L of uric acid. Please note that calculating hazard ratios for these large units had no impact on P-values.
Impact of co-morbidities on quality of life
Patients with more co-morbidities reported similar QoL assessed by MLHFQ. No difference was found in total MLHFQ score as well as in somatic and emotional scores (Table 1). Similarly, patients with just one co-morbidity (COPD, CKD, and DM) had similar MLHFQ score as co-morbid-free individuals (Supporting Information, Figure S1).
In order to determine the variables with the largest impact on QoL, we have performed multivariable linear regression analysis using the same variables as in outcome analysis. Backward elimination identified furosemide daily dose, systolic blood pressure, NYHA functional class, and body mass index (BMI) (but not the number of co-morbidities) to be significantly associated with QoL. Nevertheless, all these variables were responsible for only 21% of QoL variability (Table 4).
| MLHFQ | MLHFQ (stepwise) | |
|---|---|---|
| Ln (BNP) (ng/L) | 0.16 | — |
| Furosemide daily dose (10 mg) | 0.10 | <0.0001 |
| Systolic blood pressure (5 mmHg) | 0.0004 | <0.0001 |
| Sodium (mmol/L) | 0.02 | — |
| NYHA (III–IV vs. I–II) | <0.0001 | <0.0001 |
| Total cholesterol (mmol/L) | 0.44 | — |
| Lymphocyte percentage (%) | 0.81 | — |
| Sex (male vs. female) | 0.73 | — |
| Uric acid (100 mmol/L) | 0.55 | — |
| No. of co-morbidities | 0.07 | — |
| ACEi/ARB (absent vs. present) | 0.46 | — |
| BMI (kg/m2) | 0.03 | 0.04 |
| LVEF (5%) | 0.75 | — |
| Heart rate (10 b.p.m.) | 0.71 | — |
| Age (5 years) | 0.80 | — |
| HF duration (years) | 0.70 | — |
| ICD (absent vs. present) | 0.81 | — |
| HF aetiology (CAD vs. non-CAD) | 0.67 | — |
| Beta-blockers (absent vs. present) | 0.46 | — |
| RV dysfunction grade (0–3) | 0.72 | — |
| r2 | 0.29 | 0.21 |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; RV, right ventricular.
- The same variables were used as for the Cox proportional hazard analysis (Table 3). First, all parameters were used for analysis, and subsequently, backward selection was performed eliminating all non-significant variables.
Biomarker analysis
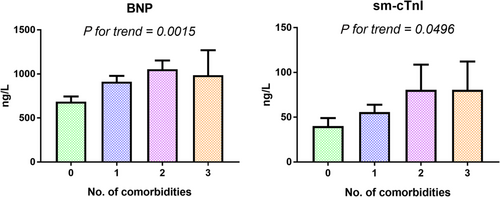
Increasing co-morbidity burden was associated with increasing levels of both BNP and sm-cTnI (Figure 3). Multivariable linear regression showed that the association between co-morbidity burden and BNP and sm-cTnI remained significant even after adjustment for LVEF, LV end-diastolic diameter, RVD grade, BMI, and eGFR (Table 5). This suggests that co-morbidities independently lead to both increased cardiac stress and injury.
| P-value (sm-cTnI) | P-value (BNP) | |
|---|---|---|
| No. of co-morbidities | 0.0498 | 0.0005 |
| LV end-diastolic diameter | 0.44 | 0.04 |
| LV ejection fraction | 0.16 | 0.04 |
| RV dysfunction grade | 0.74 | <0.0001 |
| BMI | 0.58 | <0.0001 |
| eGFR | 0.62 | 0.68 |
- BMI, body mass index; eGFR, estimated glomerular filtration rate; LV, left ventricular; RV, right ventricular.
- Multivariable regression analysis showing that number of co-morbidities was significantly associated with sm-cTnI level and BNP level even after the adjustment for the degree of both LV and RV dysfunction, BMI, and eGFR.
Discussion
In the present study, we have examined the impact of major non-cardiac co-morbidities (DM, COPD, and CKD) on QoL and outcome in a cohort of advanced HFrEF patients with high degree of guideline-recommended pharmacotherapy and device therapy.
Quality of life was associated with parameters reflecting advanced HF (furosemide daily dose, systolic blood pressure, NYHA functional class, and BMI), but not with co-morbidity burden, that additively influenced outcome. Co-morbidities were found to negatively impair prognosis not only directly but also indirectly as they were associated with lower degree of HF pharmacotherapy and higher degree of myocardial stress and injury.
Prevalence
Although the study was carried out in a single centre with younger patients than in previous studies7, 18 (some of them eligible for HTx/MCS implantation), we have observed a comparable prevalence of co-morbidities and overall co-morbidity burden.
Quality of life
t is generally believed that co-morbidities are associated with impaired QoL,7 but recently published meta-analysis did not find a single work systematically analysing QoL of HF patients with co-morbidities.2 To our best knowledge, this is the first study systematically analysing the impact of multiple variables including co-morbidity burden on QoL in HF patients. We have observed that QoL was associated with variables reflecting advanced HF (furosemide daily dose, systolic blood pressure, NYHA functional class, and BMI) but not with co-morbidity burden. This contraintuitive finding suggests that QoL in HF patients is dominantly HF related and will be improved primarily by HF treatment. Conversely, this study raises a question about appropriate treatment targets for HF patients with co-morbidities. As co-morbidities were not associated with lower QoL, it seems unlikely that their treatment would lead to QoL improvement. However, MLHFQ was designed to specifically assess the QoL of HF patients and might be less sensitive to detect the impact of various co-morbid conditions. In order to evaluate the effect of co-morbidities on QoL, different approach might be needed. Additionally, various co-morbidities may have different contribution to impaired QoL. Iron deficiency, for instance, is associated with worse QoL,19 and iron deficiency correction improved QoL in HF patients.20
Adverse effect of co-morbidities
The outcome of HF patients with co-morbidities is driven both by HF itself and by co-morbidities.3-6, 21 The impact of co-morbidities on outcome may vary depending upon both the stage of HF and the degree of HF pharmacotherapy and device therapy. The higher risk of mortality or HF hospitalization was shown for DM in predominantly milder HF patients (72% NYHA I–II).7 We have speculated that in our cohort of advanced HF patients (73.6% NYHA III–IV), the negative effect of co-morbidities on outcome would be less pronounced. However, all three analysed co-morbidities (DM, CKD, and COPD) adversely affected the outcome, and their effect was independent and additive. This might be partly because co-morbidities not only were found to have a direct negative effect but were also associated with less optimal HF pharmacotherapy and increased cardiac stress and injury.
Pharmacotherapy
Similarly to other studies,7 increasing co-morbidity burden was associated with lower degree of ACEi/ARB therapy, which was driven by lower ACEi/ARB treatment in patients with CKD. ACEi/ARB may be feared in patients with HF and CKD as these drugs can be associated with worsening of renal function, but neither maintenance of high doses nor ACEi/ARB nor uptitration was related to long-term worsening of renal function.22 Importantly, patients with HF and CKD derive similar benefit from ACEi/ARB therapy as those with normal renal function.23 ACEi/ARB or mineralocorticoid receptor antagonist treatment in HF patients with CKD is frequently associated with increased risk of hyperkalaemia that can be effectively prevented by patiromer.24 However, this drug was not available in our study.
Cardiac stress and injury
Increasing co-morbidity burden was independently associated with increased cardiac stress (increasing BNP) as well as cardiac injury (increasing sm-cTnI), which corresponds to previously published studies. DM was found to be associated with higher levels of both NT-proBNP and cardiac troponin25; on the other hand, the subanalysis of PARADIGM-HF study reported an increase in cardiac troponin but no difference in NT-proBNP in diabetic patients with HF.9 Moderate COPD was found to be associated with higher level of cardiac troponin, but no difference in BNP,10 and both NPs and cardiac troponins were found elevated in patients with CKD,26 which was likely due to ongoing myocyte damage, LV hypertrophy, or clinically silent microinfarcts27, 28 rather than primarily reduced urinary clearance.29
In comparison with our study, previous studies used both conventional10 and high-sensitivity9, 25 (but not ultrasensitive) cardiac troponin assays, analysed milder HF patients (24% in NYHA III9 and 25% in NYHA III25), and focused on one particular co-morbidity.9, 10, 25 Advanced imaging studies demonstrated both subclinical RVD and LV dysfunction in patients with COPD,30 subclinical LV dysfunction in patients with diabetes,31 and abnormal LV mechanics in patients with CKD.32 Using biomarker approach (ultrasensitive cardiac troponin assay and BNP), our data support the concept in HF patients (even if they achieve high degree of guideline-recommended pharmacotherapy) co-morbidities are progressively and independently associated with increased cardiac stress and injury.
Clinical implication
The improvement of QoL and prognosis are two interrelated goals of HF treatment. NP level and cardiac troponin33 are strong predictors of adverse outcome in HF patients. If HF therapy is associated with NP lowering, it will likely result in outcome improvement,34 but significant NP lowering is not a prerequisite of effective HF therapy as other mechanisms may play significant role. SGLT2 inhibitors (dapagliflozin and empagliflozin) were shown to significantly improve outcome of HF patients despite only modest NP lowering.35, 36 However, based upon our results, we hypothesize that co-morbidity treatment leading to NP and troponin lowering might have the potential to improve the outcome of HF patients but would unlikely lead to the improvement of QoL. Co-morbidity treatment leading to NP and troponin lowering might be promising candidates that deserve further investigation.
Study limitations
Our study was performed in a heart centre offering a complex cardiovascular programme including MCS implantation and HTx. Because this could introduce bias related to the analysis of prognostic value, urgent HTx and MCS implantation were considered adverse outcomes,14 while the patients receiving non-urgent HTx were censored as having no adverse outcome on the day of transplantation. In addition, it was a single-centre study with a substantial predominance of men. Our study cohort included patients with rather advanced HF. Consequently, the results might not be fully applicable to patients with milder HF or to older patients. Data about HF rehospitalizations were not available in all patients, so this endpoint could have not been included in the analysis. Data about COPD were obtained from anamnestic records; the diagnosis was not validated by spirometry. Consequently, it was not possible to quantify COPD severity. As a significant number of patients were referred for transplant eligibility evaluation, those with most severe co-morbid conditions are missing. Data about iron status of patients were not available. The study focused on the most prevalent co-morbidities, and the effect of other co-morbidities (e.g. depression, sleep-disordered breathing, thyroid dysfunction, stroke, periphery artery disease, and cancer) was not analysed.
Conclusion
In stable advanced HFrEF patients, co-morbidities are not associated with impaired QoL but negatively affect the prognosis both directly and indirectly through lower level of HF pharmacotherapy and increased myocardial stress and injury.
Conflict of interest
J.K. is a member of advisory boards for Bayer, Boehringer Ingelheim, Daiichi Sankyo, Biosense Webster, Boston Scientific, Medtronic, LivaNova, and St. Jude Medical. He has received speaker honoraria from the previously mentioned companies and from Biotronik. P.J. received research support from Abbott Laboratories, Amgen Inc., AstraZeneca LP, Beckman Coulter, Daiichi Sankyo, Inc., GlaxoSmithKline, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation and speaker honoraria from Roche Diagnostics Corporation.
Funding
This work was supported by the Ministry of Health of the Czech Republic (Ministerstvo Zdravotnictví Ceské Republiky)—conceptual development of research organization (Institute for Clinical and Experimental Medicine—IKEM-IN 00023001), by grants 17-28784A, and NV19-02-00130 and by fund of the Biomarker Research and Clinical Trials Laboratory at Brigham and Women's Hospital (103517).




