Low-fat hypocaloric diet reduces neprilysin in overweight and obese human subjects
Abstract
Aims
Neprilysin (NEP), a zinc metallopeptidase, degrades a variety of bioactive peptides including natriuretic peptides terminating their biological action on arterial blood pressure and natriuresis. Pharmacological inhibition of NEP reduces mortality in patients with heart failure with reduced ejection fraction. Physiological interventions reducing NEP levels are unknown in humans. Because obesity leads to increased NEP levels and increases the risk for heart failure, we hypothesized that weight loss reduces NEP concentrations in plasma and tissue.
Methods and results
We randomized overweight to obese human subjects to a low-fat or low-carbohydrate hypocaloric 6 month weight loss intervention. Soluble NEP was determined in plasma, and NEP mRNA was analysed from subcutaneous adipose tissue before and after diet. Low-fat diet-induced weight loss reduced soluble NEP levels from 0.83 ± 0.18 to 0.72 ± 0.18 μg/L (P = 0.038), while subcutaneous adipose tissue NEP mRNA expression was reduced by both dietary interventions [21% (P = 0.0057) by low-fat diet and 16% (P = 0.048) by low-carbohydrate diet]. We also analysed the polymorphisms of the gene coding for NEP, rs9827586 and rs701109, known to be associated with plasma NEP levels. For both single-nucleotide polymorphisms, minor allele carriers (A/A) had higher baseline plasma NEP levels (rs9827586: β = 0.53 ± 0.23, P < 0.0001; rs701109: β = 0.43 ± 0.22, P = 0.0016), and minor allele carriers of rs9827586 responded to weight loss with a larger NEP reduction (rs9827586: P = 0.0048).
Conclusions
Our study identifies weight loss via a hypocaloric low-fat diet as the first physiological intervention in humans to reduce NEP in plasma and adipose tissue. Specific single-nucleotide polymorphisms further contribute to the decrease. Our findings may help to explain the beneficial effect of weight loss on cardiac function in patients with heart failure.
Background
Numbers of patients with heart failure (HF) are dramatically increasing with 26 million people affected worldwide. Once patients are diagnosed, the mortality rate is high with 50% dying within 5 years.1 Research on proteins and mechanisms involved in HF are therefore of big interest. One interesting enzyme in the field is neprilysin (NEP), a zinc-dependent membrane metallopeptidase located on the cell surface, which inactivates several bioactive peptides, such as natriuretic peptides (NPs).2-4 Atrial NP and brain NP are synthesized as pre-prohormones in the heart, regulating arterial blood pressure in an endocrine fashion by inhibiting renal sodium reabsorption, suppressing aldosterone secretion from the adrenals, and a direct vasodilatory effect.5-7 Therefore, NP clearance by NEP is of high clinical relevance.
Neprilysin is mainly synthesized in adipose tissue and can be separated from the cell surface to circulate in a soluble, catalytic active form in the blood stream.8 Pharmacological inhibition of NEP leads to enhanced diuresis and natriuresis,5 likely because the degradation of NPs is inhibited. Accordingly, the combined renin–angiotensin system and NEP inhibitor LCZ693 (sacubitril and valsartan)9 conveys important clinical benefit in patients with HF with reduced ejection fraction.
The development and progression of HF is highly linked to obesity as HF is slowed down by maintaining low body fat and high insulin sensitivity.10 Interestingly, combined renin–angiotensin system and NEP inhibitors also improve insulin sensitivity in obese individuals and glucose control in patients with diabetes.11, 12 Together, these data highlight the role of NEP inhibition in cardiovascular—but also metabolic disease. So far, clinical data on the interaction between dietary interventions and NEP concentrations or activity are missing in humans. Our study aimed at determining how low-calorie–low-carbohydrate and low-calorie–low-fat diets affect NEP plasma levels.
Aims
Primarily, we assessed for the first time soluble plasma NEP levels and adipose tissue NEP RNA expression from obese patients before and after weight reduction due to two hypocaloric diet regimens. Secondarily, we also tested if the two single-nucleotide polymorphisms (SNPs) rs9827586 and rs701109 in the NEP locus, which have been reported to be associated with changed NEP levels,13 affect NEP regulation in these subjects.
Material and methods
We included overweight and obese but otherwise healthy individuals (52 women and 10 men) of the B-SMART study (ClinicalTrials.gov identifier: NCT00956566). Data were generated in a prospective, randomized manner comparing weight reduction by either a hypocaloric low-carbohydrate or low-fat diet over a 6 month intervention period.14 Details can be found in Haufe et al.14 All anthropometric and cardio-metabolic outcomes presented here were measured as previously described. Body fat content was assessed by nuclear magnetic resonance.14, 15 Additionally, circulating soluble NEP as well as other cardiovascular and metabolic measurements were obtained at baseline and after 6 month dietary weight loss. Soluble NEP in plasma samples was determined with a Solid Phase Sandwich ELISA (R&D Systems, Minneapolis, MN, USA), and NEP gene expression in subcutaneous adipose tissue (SAT) samples was obtained by needle biopsies as described.16 NEP mRNA expression was measured with SYBR Green (Thermo Fisher Scientific, Wilmington, DE, USA) and was normalized to relative expression of HPRT.
DNA from 62 patients was isolated from whole blood using a commercial DNA isolation kit, and SNP genotyping was performed based on Ilumina Human610-Quadv1. Data were analysed with the GenomeStudio Genotyping from Ilumina. For both SNPs, the minor allele showed a frequency of 15.6% [rs9827586: 15.6% (AA), 54.4% (AG), and 30% (GG); rs701109: 15.6% (AA), 51.1% (AG), and 33.3% (GG)].
All statistical analyses were performed with SPSS 18 (SPSS, Inc., Chicago, IL, USA) or JMP 13.0 (SAS, Institute Cary, NC, USA). Univariate associations between ∆NEP and ∆body weight were tested using Pearson correlation. To test differences of clinical characteristics between NEP tertiles at baseline or ∆NEP, we used one-way ANOVA with Bonferroni's post hoc test without and with Bonferroni–Holm correction for multiple testing. To test for interactions between diet groups over 6 month period (diet × time), we used a two-way ANOVA for repeated measurements. Within-group differences in response to 6 month diet were analysed using paired two-tailed t-tests. The association of genetic variation in the NEP locus with circulating NEP concentrations was tested by multiple linear regression analyses under an additive inheritance model. NEP concentrations and age were log-transformed prior to analysis to approximate normal distribution. Significance was assumed when P ≤ 0.05.
Results
Participants were divided into low (n = 21), middle (n = 21), and high tertiles (n = 20) of baseline NEP plasma concentrations. High NEP levels were associated with high intramyocellular fat, end-diastolic volume index, VO2 max, pericardial fat, and trend with higher fat mass (P = 0.07) (Table 1). After correction for multiple testing, only VO2 max remained associated with NEP levels at baseline (Table 1). Other baseline data and clinical outcomes for both diet interventions were previously reported in detail.14, 15
| Clinical characteristics | Baseline | P value | |||
|---|---|---|---|---|---|
| Low tertile (NEP) | Middle tertile (NEP) | High tertile (NEP) | Unadjusted | Adjusted for multiple testing | |
| NEP (ng/mL) | 0.21 ± 0.14 | 0.60 ± 0.12 | 2.26 ± 1.94 | 0.103 | Ns |
| N (men/women) | 4/17 | 2/19 | 4/16 | 0.62 | Ns |
| Age (years) | 48 ± 8 | 45 ± 8 | 42 ± 10 | 0.15 | Ns |
| Body weight (kg) | 94.1 ± 17.1 | 90.2 ± 16.7 | 86.7 ± 13.2 | 0.34 | Ns |
| BMI (kg/m2) | 33 ± 3 | 32.7 ± 4.4 | 31.5 ± 3.8 | 0.26 | Ns |
| FM (%) | 37.4 ± 3.8 | 35.1 ± 6.0 | 33.5 ± 5.8a | 0.07 | Ns |
| Abdominal subcutaneous fat (kg) | 10.5 ± 2.7 | 9.3 ± 2.6 | 8.6 ± 2.8 | 0.11 | Ns |
| Abdominal visceral fat (kg) | 2.1 ± 0.8 | 1.6 ± 0.6 | 1.7 ± 1.4 | 0.20 | Ns |
| Intrahepatic fat content (%) | 10.6 ± 11.9 | 7.2 ± 6.4 | 8.8 ± 12.2 | 0.59 | Ns |
| Intramyocellular lipid content [IMCL (creatine)] | 3.1 ± 1.1 | 3.8 ± 2.2 | 5.4 ± 2.4a | 0.005* | Ns |
| Glucose metabolism | Ns | ||||
| Fasting glucose (mg/dL) | 96.1 ± 8.3 | 99.8 ± 8.5 | 93.7 ± 8.2b | 0.08 | Ns |
| Fasting insulin (U/mL) | 6.7 ± 3.8 | 8.5 ± 5.9 | 6.0 ± 3.4 | 0.18 | Ns |
| Glucose at 2 h OGTT (mg/dL) | 129.7 ± 33.2 | 147.4 ± 37.8 | 140.1 ± 38.7 | 0.31 | Ns |
| HOMA-IR (arbitrary unit) | 1.6 ± 1.0 | 2.0 ± 1.3 | 1.4 ± 0.9 | 0.19 | Ns |
| Cardiovascular parameter | Ns | ||||
| RR 24 h sys V0 (mm/Hg) | 120.1 ± 11.5 | 120.2 ± 10.2 | 117.3 ± 6.8 | 0.59 | Ns |
| RR 24 h dia V0 (mm/Hg) | 72.4 ± 9.1 | 73.5 ± 6.8 | 72.6 ± 5.6 | 0.88 | Ns |
| LV mass index (g/m) | 41.7 ± 5.9 | 45.8 ± 7.8 | 46.7 ± 7.1a | 0.06 | Ns |
| Ejection fraction (%) | 62.0 ± 4.7 | 61.9 ± 5.6 | 59.1 ± 6.0 | 0.17 | Ns |
| EDV index (mL/m2) | 74.4 ± 11.7 | 75.5 ± 10.2 | 80.5 ± 8.0 | 0.13 | Ns |
| ESV index (mL/m2) | 28.5 ± 6.7 | 28.8 ± 6.0 | 33.0 ± 6.3a | 0.05* | Ns |
| Cardiac output (L/min) | 6248.0 ± 1079.4 | 6326.8 ± 1013.4 | 6925.5 ± 1387.6 | 0.15 | Ns |
| Stroke volume (mL) | 95.5 ± 21.1 | 98.0 ± 27.0 | 94.7 ± 16.4 | 0.88 | Ns |
| Myocardial fat (mm) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.89 | Ns |
| Epicardial fat (mm) | 6.4 ± 2.7 | 6.3 ± 2.7 | 5.3 ± 3.2 | 0.56 | Ns |
| Pericardial fat (mm) | 29.2 ± 14.1 | 18.7 ± 5.4a | 21.6 ± 12.6 | 0.03* | Ns |
| PFRE/PFRA ratio | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.5 ± 0.5 | 0.68 | Ns |
| Cardiorespiratory fitness | |||||
| VO2 max (mL/min/kg) | 18.8 ± 3.5 | 24.1 ± 4.6a | 23.2 ± 5.0 | <0.001* | 0.017 |
| Free fatty acids (mmol/L) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.49 | Ns |
| Triglyceride (mmol/L) | 1.0 ± 0.4 | 1.2 ± 0.5 | 1.2 ± 0.7 | 0.48 | Ns |
| HDL (mmol/L) | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.5 ± 0.8 | 0.71 | Ns |
| LDL (mmol/L) | 3.2 ± 0.8 | 2.9 ± 0.8 | 2.9 ± 0.8 | 0.44 | Ns |
| Total adiponectin (μg/mL) | 6.2 ± 1.7 | 6.0 ± 2.4 | 5.7 ± 1.9 | 0.78 | Ns |
| CRP (μg/mL) | 1.9 ± 1.0 | 1.3 ± 0.8 | 1.2 ± 0.9 | 0.11 | Ns |
| FABP4 (ng/mL) | 34.6 ± 10.3 | 31.9 ± 10.1 | 32.0 ± 14.8 | 0.71 | Ns |
| Leptin (ng/mL) | 32.8 ± 10.6 | 34.7 ± 13.2 | 27.4 ± 14.7 | 0.27 | Ns |
| mr-proANP (pmol/L) | 59.8 ± 31.8 | 56.9 ± 22.6 | 46.5 ± 20.0 | 0.46 | Ns |
- BMI, body mass index; CRP, C-reactive protein; dia, diastolic; EDV, end-diastolic volume; ESV, end-systolic volume; FABP4, fatty acid-binding protein 4; FM, fat mass; HDL, high-density lipoprotein; HOMA-IR, Homeostasis Model Assessment for Insulin Resistance; LDL, low-density lipoprotein; LV, left ventricular; mr-proANP, mid-regional pro-atrial natriuretic protein; NEP, neprilysin; Ns, not significant; OGTT, oral glucose tolerance test; PFRE/PFRA, early peak filling rate/atrial peak filling rate; RR, Riva-Roci; sys, systolic; VO2 max, maximum oxygen uptake during exercise testing.
- Differences between groups were analysed by one-way ANOVA.
- * P ≤ 0.05.
- a P < 0.1 compared with low tertile.
- b P < 0.1 compared with middle tertile with Bonferroni's post hoc test without and with Bonferroni–Holm correction for multiple testing. Data are mean ± SD.
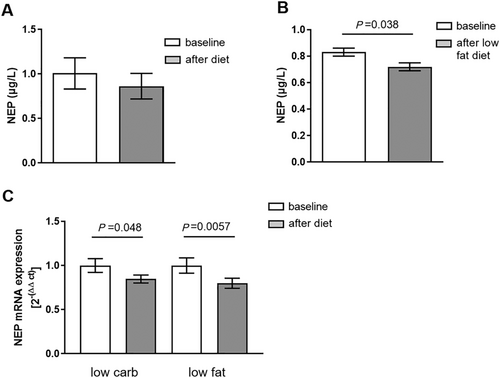
Overall, after 6 months of the hypocaloric diet intervention, patients on a low-carbohydrate diet (n = 26; 2 male and 24 female, age: 42.5 ± 9.1 years) lost 7.02 ± 4.21 kg, and patients on a low-fat diet (n = 36; 8 male and 28 female, age: 47.5 ± 8.7 years) lost 6.66 ± 4.4 kg of body weight. Weight loss was similar in tertiles according to NEP baseline concentration (low: 6.2 ± 4.1 kg; middle: 7.6 ± 4.2 kg; and high: 9.5 ± 4.6 kg; between tertiles P = 0.472). When combining both dietary inventions, NEP levels before and after diet did not change (Figure 1). Yet, when analysing the low-fat-diet intervention separately, we observed a decrease of plasma NEP levels from 0.83 ± 0.18 to 0.72 ± 0.18 μg/L (n = 36; P = 0.038, Figure 1) during the study, whereas in the low-carbohydrate diet group, the reduction in NEP levels was not significantly reduced (1.23 ± 0.34 to 1.04 ± 0.25 μg/L, n = 26; P = 0.373). Correlation analysis between decrease in NEP with the decrease in body weight resulted in a positive association between both parameters (r = 0.239; P = 0.062). SAT mRNA expression of NEP was markedly reduced by 21% by the low-fat diet (1 ± 0.086 to 0.799 ± 0.057, n = 34; P = 0.0057) and by 16% by the low-carbohydrate diet (1 ± 0.079 to 0.847 ± 0.045, n = 29; P = 0.048) (Figure 1). Changes in NEP plasma levels and mRNA levels between the two diet groups over time did not differ significantly (time × diet: NEPplasma, P = 0.671; NEPmRNAexpression, P = 0.336). We next analysed the contribution of changes in soluble NEP to improvement of clinical characteristics and the contribution of clinical characteristics at baseline for changes in soluble NEP. Dividing NEP changes in tertiles (high reduction, no reduction, and increase), we observed associations between ∆NEP and food habits at baseline. Larger reductions in NEP were observed in subjects ingesting less fat (P = 0.052) and more carbohydrates (P = 0.005) at baseline, when correlating food consumption before the study and NEP reduction after 6 month independent of dietary group (data not shown). The data also suggest a role of food fat content in the regulation of plasma NEP levels.
In 51 participants of in which genetic analyses were available, the contribution of the SNPs rs9827586 and rs701109 located in the NEP locus to soluble NEP levels at baseline was analysed. The SNPs were previously reported to affect soluble NEP levels.13 In our cohort, minor allele carriers of both rs9827586 (β = 0.53 ± 0.23, P < 0.0001) and rs701109 (β = 0.43 ± 0.22, P = 0.0016) showed higher soluble NEP concentrations at baseline. This association remained valid after adjustment for sex and age (rs9827586: P = 0.0002; rs701109: P = 0.0017). Minor allele carriers of rs9827586 responded with a larger reduction in NEP (P = 0.0048), while minor allele carriers of rs701109 showed only a tendency (P = 0.059).
Conclusion
We conclude that weight loss with a low-calorie low-fat dietary intervention reduces soluble NEP concentrations and SAT NEP mRNA expression in overweight to obese humans. Our findings are in line with the notion that soluble NEP levels are controlled by the nutritional state. In mice, weight reduction reduced NEP levels, whereas a high-fat diet increased NEP concentrations in visceral adipose tissue as well as plasma.17 In parallel, human subjects with obesity have been reported to have higher NEP levels correlating positively with their body mass index.17, 18 Previous data have shown that NEP is expressed on mature adipocytes and also released by them in a soluble form.17, 19 Furthermore NEP promotes adipogenesis, leading to a positive feedback loop, promoting obesity.17 Yet, in our study, we did not observe a correlation between body mass index, fat mass, and soluble NEP or NEP mRNA. However, we did observe a positive correlation between soluble NEP and intramyocellular lipid content in skeletal muscle of our obese patients at baseline, which is in line with our previously shown data that NPs, which are inactivated by NEP, increase mitochondrial metabolism in skeletal muscle and induce lipid oxidation.20, 21 Our study has limitations. It includes only overweight to obese participants, and the dietary intervention of 6 months is relatively short. Furthermore, we cannot exclude that our sample size, especially in the dietary subgroups, might have hindered detection of smaller effects. Therefore, larger studies in additional patient populations are needed to test transferability of the results to the general population.
Nevertheless, given the recent results of the PARADIGM trial showing that inhibiting NEP in patients with HF with reduced ejection fraction improves overall survival,9 nutritional strategies to reduce NEP may also contribute to a better outcome in these patients and contribute to the beneficial effect of weight loss on glucose control and cardiac function.
Acknowledgement
The author would like to thank Kathrin Saar from the Max Delbrück Center for contributing to this work.
Conflict of interest
None declared.
Funding
The work was supported by an unrestricted grant from Novartis Pharma GmbH. The funder was not involved in analysis or interpretation of the study.




