Development and implementation of a remote patient monitoring program for heart failure: a single-centre experience
Abstract
Aims
Remote patient monitoring (RPM) in the management of heart failure (HF), including telemonitoring, thoracic impedance, implantable pulmonary artery pressure (PAP) monitors, and cardiac implantable electronic device (CIED)-based sensors, has had varying outcomes in single platform studies. Uncertainty remains regarding the development of single-centre RPM programs; additionally, no studies examine the effectiveness of dual platform RPM programs for HF. This study describes the implementation and outcomes of a dual platform RPM program for HF at a single centre.
Methods and results
An RPM program was developed to include two platforms (e.g. CardioMEMS™ HF System and HeartLogic™ HF Diagnostic). To examine changes within each participant over time, study-related outcomes including total hospitalizations (TH), total length of stay (TLOS), cardiac hospitalizations (CH), cardiac LOS (CLOS), and cardiac-related emergency department (ED) visits were compared in two timeframes: 12 months pre-enrolment and post-enrolment into RPM. For 141 participants enrolled, there was a significant reduction in the likelihood of experiencing a CH by 19% (0.77 vs. 0.61 events/patient-year; HR: 0.81, 95% CI: 0.67–0.97, P = 0.03) and a cardiac-related ED visit by 28% (0.48 vs. 0.34 events/patient-year; HR: 0.72, 95% CI: 0.55–0.93, P = 0.01). There was also a 51% decrease (SE = 1.41, 95% CI: 2.79–8.38 days, P < 0.001) and 62% decrease (SE = 1.24, 95% CI: 3.35–8.22 days, P < 0.001) in TLOS and CLOS, respectively.
Conclusions
A dual platform RPM program for HF using structured education, RPM-capable devices, and alert-specific medication titration reduces the likelihood of experiencing a cardiac hospitalization and cardiac-related ED visit in this single-centre study.
Introduction
Heart failure (HF) affects approximately 26 million people worldwide with more than one million hospitalizations annually in both the USA and Europe, accounting for 1–2% of all hospital admissions.1 Every hospitalization for acute decompensated HF (ADHF) is a predictor of readmission with a 1 month readmission rate of 30% and a 6 month readmission rate of 50%.2 Hospitalization for HF is the leading cause of readmission within 60 days and is a strong predictor of mortality.3-5 This highlights the importance of care coordination, patient monitoring, and early intervention to reduce HF hospitalizations and readmissions.
After several neutral remote patient monitoring (RPM) trials, some recent studies have yielded positive results.6 The success of the CardioMEMS™ HF System in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA [New York Heart Association] Functional Class III HF Patients) trial demonstrated reduced HF hospitalization risk in outpatients with advanced HF, regardless of ejection fraction.7 The CardioMEMS Post-Approval Study demonstrated a 57% reduction in risk of HF hospitalization and a 27% reduction in all-cause hospitalizations during the year after implantation.8 The newer Cordella™ HF System was also shown to provide safe and accurate pulmonary artery pressure (PAP) monitoring for the proactive management of HF.9 The international, multicentre, nonrandomized MultiSENSE (Multisensor Chronic Evaluation in Ambulatory HF Patients) study showed that the HeartLogic index from cardiac implantable electronic device (CIED)-based sensors is a predictor of impending HF decompensation and is able to alert clinicians prior to HF events10, 11; however, the HeartLogic index has not been tested for efficacy to decrease HF hospitalizations. More recent studies have also demonstrated positive results for RPM using CardioMEMS in select countries in Europe12 and during the SARS-CoV-2 pandemic.13 However, such encouraging results have only been evaluated in a single platform RPM program.
These recent successes with implantable PAP monitors and CIED-based multisensor indexes have led to renewed interest in RPM programs to reduce HF hospitalizations and readmissions. However, there are no studies that evaluate patient outcomes in a dual platform setting. In October 2017, we started a dual platform RPM program using two RPM-capable devices. We believed this approach would allow us to enrol a broader patient population and determine the effectiveness of a more patient-oriented RPM program for HF. This article details the development and implementation of a dual platform RPM program for HF and discusses the 1 year outcomes for hospitalizations, hospital length of stay, and emergency department (ED) visits.
Methods
Study population
In this report, we describe the development and implementation of a dual platform RPM program to improve the quality of care and clinical outcomes for HF patients at a facility that previously provided no RPM. In October 2017, an RPM program to include two available platforms (e.g. CardioMEMS™ HF System and HeartLogic™ HF Diagnostic) was started under the supervision of a physician and HF nurse manager who provided individualized monitoring and alert-specific medication titration. These providers also offered patient and caregiver support and education, specifically with environmental and behavioural factors such as dietary changes and/or medication compliance. Participants were compared in two timeframes: 12 months pre-enrolment and post-enrolment into RPM. The local institutional review board approved the study, and informed consent was not required due to the retrospective study design.
Recruitment, enrolment, and intervention
Recruitment and enrolment
The algorithm for patient recruitment into RPM is detailed in Figure 1. HF clinic patients with new or existing devices with RPM capability were offered enrolment. After eligible participants were identified, informed, and agreed, they were enrolled in RPM. HF clinic patients underwent device selection that was determined on an individual basis depending on their history of any previously implanted devices that were eligible for RPM without the implantation of a new device. If patients did not have any implantable device (i.e. ICD or CRT) and met CHAMPION trial inclusion criteria (18 years and older, NYHA Class III for at least 3 months, and one or more HF hospitalization in the past 12 months),7 they were informed of the CardioMEMS HF System. CardioMEMS recipients were required to transmit daily readings after appropriate education, and trainings were provided to patients and their caregivers. Each CardioMEMS recipient had an individualized pulmonary artery diastolic (PAD) pressure goal, usually 15 ± 3 (based on the initial degree in elevation of the pulmonary capillary wedge pressure) plus the pulmonary artery diastolic pressure gradient (DPG), determined at the time of implant and calibration via right heart catheterization. Those patients that had a previously implanted ICD or CRT device with HeartLogic HF Diagnostic capability were offered enrolment into RPM. The inclusion criteria included a currently implanted HeartLogic HF Diagnostic capable device, LVEF ≤35%, NYHA II/III, and without exclusion criteria from the MultiSENSE study.10 These patients, under the HeartLogic index monitoring guidelines, provide a weekly report utilizing a proprietary algorithm.10, 11
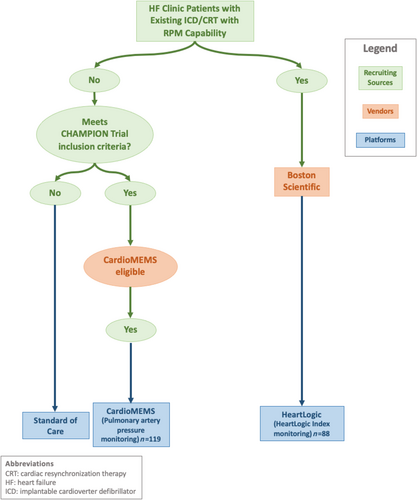
Intervention
Our RPM program is individualized, algorithm-based, and utilizes a physician and HF nurse manager for comprehensive HF management. Educational material was used to train participants in platform use, including the remote reporting of alerts from their corresponding device. The care team ensured that the participant understood that their device provides alerts prior to becoming symptomatic, but to always contact the care team should they ever develop HF symptoms. Alerts were reviewed within 24 h of being sent to the care team, excluding weekends and holidays, with daily readings from CardioMEMS and weekly readings from HeartLogic. The care team responded to additional alerts when they were outside of the patient's individualized goal, as provided by the Merlin.net™ system or the Latitude NXT™ Remote Patient Management System.
In response to these alerts, the HF nurse manager first performed telephone calls to address potential dietary changes and/or medication non-adherence prior to any alterations in medical therapy. Participants preferentially had guideline-directed medical therapy (GDMT) titration, as appropriate, in response to changes in PAD pressure or HeartLogic index outside of the set goal. Special consideration was given to starting or increasing angiotensin receptor-neprilysin inhibitors (ARNi), mineralocorticoid receptor antagonists (MRA), and sodium-glucose cotransporter 2 inhibitors (SGLT2i), when appropriate. In the event of an inadequate response to titration of GDMT, patients received changes to their oral diuretic or were given ambulatory intravenous diuretic therapy if required. After receiving an alert that was outside of goal, the following measures were taken: (i) Participants with CardioMEMS with an alert less than or greater than 6 mmHg from set PAD pressure goal who were asymptomatic received a corresponding decrease or increase in loop diuretic by 25–50%. Participants who were symptomatic with PAD >6 mmHg above goal were started on a thiazide diuretic for 1–3 days. For participants who were symptomatic with PAD <6 mmHg below goal, all diuretics were discontinued temporarily, and the PAD pressure was reassessed. In the case that PAD was between 4 and 6 mmHg below or above goal, there was a corresponding decrease or increase in loop diuretic dosage by 10–25%. If PAD pressure remained above goal, then a thiazide diuretic was initiated for 1–3 days. After initiating any change in medical therapy, a basic metabolic panel was checked in 3–5 days and the PAD pressure was reassessed. (ii) For participants with a HeartLogic index between 16 and 32 the loop diuretic dosage was increased by 10–25% if the participant was symptomatic. If the HeartLogic index was greater than 32, the loop diuretic dosage was increased by 10–25% regardless of symptoms.
Data collection, outcomes, and statistical analysis
To examine changes within each participant over time, study-related outcomes in two timeframes—12 months pre-enrolment and post-enrolment into RPM—were compared. Primary outcomes included the number of total hospitalizations (TH), cardiac hospitalizations (CH), and cardiac-related emergency department (ED) visits. CH and cardiac-related ED visits included any presentation that was cardiac in nature, including acute coronary syndrome, HF, electrolyte abnormalities secondary to HF treatment, and arrhythmias. For each primary outcome, the Andersen–Gill extension of the Cox proportional hazards model was used to compare the rate of events during the 12 month pre-enrolment to the rate during 12 month post-enrolment period. Because a significantly greater proportion of patients were taking an MRA in the post-enrolment period compared with the pre-enrolment period (P < 0.001) (Table 2), all models were adjusted for MRA use. Likewise, as death prevents later cardiac events, inclusion of death as a covariate in the models controlled for rate and risk in these and future time points.14-16 For all primary outcomes, MRA use as a covariate was highly significant (all Ps < 0.001), while death was always non-significant (all Ps > 0.10). Our primary reports concern effects of the time-dependent factor of RPM (pre-enrolment vs. post-enrolment). As such, significant findings indicate that RPM contributes to rate change above and beyond that of MRA use or death.
The secondary outcome measures were CH length of stay (CLOS) and TH length of stay (TLOS). Every event was individually adjudicated to determine the discharge diagnosis for the presenting problem. A linear mixed-effects model was utilized to assess differences in CLOS and TLOS, with pre-enrolment and post-enrolment time period as a categorical within-subjects factor, adjusting for the between-subjects factors of MRA use and participant deceased status. P-values <0.05 were considered indicative of statistical significance.
Results
From October 2017 to May 2020, 238 participants were enrolled in RPM for HF (CardioMEMS 119 and HeartLogic 88). Of these, 141 participants (CardioMEMS 89 and HeartLogic 52) had complete 12 month pre-enrolment and post-enrolment RPM data and were included in the analyses.
Demographics and baseline characteristics
Participant demographics and baseline characteristics, as well as haemodynamic parameters via right heart catheterization at the time of CardioMEMS implantation are outlined in Table 1. Prior to RPM enrolment, 92% were on a beta-blocker, 77% were on an ACEi/ARB/ARNi, and 41% were on an MRA. Table 2 shows the difference in medication rates from the pre-enrolment to post-enrolment period; only MRA use showed a significant difference between pre-enrolment and post-enrolment periods. There were 11 deaths (7.80%) in the 1 year post-enrolment period.
| Demographics and characteristics | Study population (N = 141) |
|---|---|
| Average age (years) | 68.4 ± 13.8 |
| Males (%) | 97 (68.8%) |
| Race (%) | |
| Caucasian | 118 (83.7%) |
| African American | 21 (14.9%) |
| Hispanic | 2 (1.4%) |
| CardioMEMS (%) | 89 (63.1%) |
| HeartLogic (%) | 52 (36.9%) |
| HFrEF (%) | 118 (83.7%) |
| HFpEF (%) | 23 (16.3%) |
| Average LVEF | 33.8 ± 13.3 |
| Ischaemic (%) | 57 (40.4%) |
| Non-ischaemic (%) | 84 (59.6%) |
| NYHA class II (%) | 22 (15.6%) |
| NYHA class III (%) | 75 (53.2%) |
| NYHA class not well-defined (%) | 44 (31.2%) |
| Stage C (%) | 129 (91.5%) |
| Stage D (%) | 9 (6.4%) |
| Average BMI, kg/m2 | 30.8 ± 7.8 |
| Average SBP, mm Hg | 120 ± 16.4 |
| Average DBP, mm Hg | 71 ± 11.4 |
| Average HR, b.p.m. | 76 ± 12.8 |
| Average Creatinine, mg/dL | 1.54 ± .77 |
| Average BUN, mg/dL | 32 ± 19.4 |
| Average GFR, mL/min/1.73 m2 | 49 ± 21.7 |
| Average Hgb A1c, % | 6.7 ± 1.4 |
| Hypertension (%) | 117 (83.0%) |
| COPD (%) | 26 (18.4%) |
| Diabetes mellitus (%) | 69 (48.9%) |
| Dyslipidaemia (%) | 98 (69.5%) |
| Atrial fibrillation (%) | 72 (51.1%) |
| CAD (%) | 92 (65.2%) |
| Previous MI (%) | 46 (32.6%) |
| Valvular disease (%) | 34 (24.1%) |
| Ventricular arrhythmia (%) | 25 (17.7%) |
| Tobacco use (%) | 80 (56.7%) |
| Alcohol use (%) | 19 (13.5%) |
| Average PASP, mm Hg | 47.0 ± 15.6 |
| Average PADP, mm Hg | 21.2 ± 9.3 |
| Average PAMP, mm Hg | 30.4 ± 10.3 |
| Average PCWP, mm Hg | 16.8 ± 7.9 |
| Average TD CO, L/min | 4.7 ± 1.4 |
| Average TD CI, L/min/m2 | 2.2 ± 0.6 |
| Average Fick CI, L/min/m2 | 2.3 ± 1.7 |
| Average PA O2 saturation, % | 59.8 ± 7.3 |
- This table demonstrates the average age, percent (%) male, race, % with CardioMEMS, % with HeartLogic, % with HF with reduced ejection fraction (HFrEF), % with HF with preserved ejection fraction (HFpEF), and the mean ejection fraction (EF). The table is further broken down into ischaemic versus non-ischaemic aetiology, NYHA class, and stage. NYHA class not well-defined includes those that were documented as multiple classes (NYHA Class I/II, II/III, or III/IV). The mean body mass index (BMI), systolic blood pressure (SBP) [mmHg], diastolic blood pressure (DBP), heart rate (HR), creatinine, blood urea nitrogen (BUN), glomerular filtration rate (GFR), and haemoglobin A1c at time of enrolment. The table also demonstrates patient co-morbidities including hypertension, COPD, diabetes, dyslipidaemia, atrial fibrillation, coronary artery disease (CAD), previous myocardial infarction (MI), valvular disease, and ventricular arrhythmia. Social history is included as tobacco and alcohol use %. The average haemodynamics for the CardioMEMS implants are also included. Values are mean ± standard deviation or n (%).
| Measure | Pre-RPM | Post-RPM | P-value |
|---|---|---|---|
| Loop diuretic | 122 (86.5%) | 125 (88.7%) | 0.469 |
| Thiazide diuretic | 35 (24.8%) | 44 (31.2%) | 0.060 |
| Beta-blocker | 130 (92.2%) | 133 (94.3%) | 0.407 |
| ACEi/ARB/ARNi | 108 (76.6%) | 107 (75.9%) | 0.828 |
| Nitrate | 60 (42.6%) | 62 (44.0%) | 0.595 |
| Hydrazaline | 14 (9.9%) | 16 (11.3%) | 0.566 |
| MRA | 58 (41.1%) | 94 (66.7%) | <0.001 |
- This table shows the percentage (%) of patients on guideline-directed medical therapy (GDMT) pre-enrolment and post-enrolment into the RPM program. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor-neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist. Values are shown as n (%).
Primary outcomes
Hospitalizations
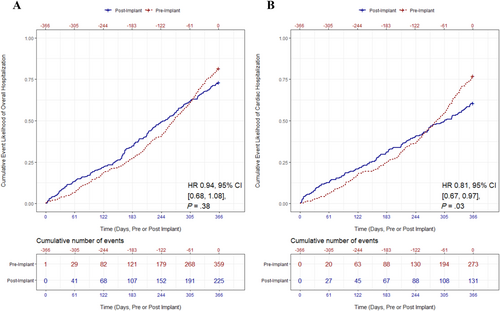
In the year preceding enrolment, 87% of participants were hospitalized for any reason compared with 65% of patients in year after enrolment. For CH, 80% of participants were hospitalized during the pre-enrolment period compared with 48% in the post-enrolment period. Across all participants, TH decreased from 359 in the pre-enrolment period to 225 in the post-enrolment period; however, the cumulative incidence rate was not statistically significant between the pre-enrolment and post-enrolment time periods (P = 0.38, Figure 2). In contrast, the cumulative rate of CH significantly decreased by 19%, resulting in a reduction from 273 pre-enrolment to 131 in the 12 month period after enrolment (0.77 vs. 0.61 events/patient-year; hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.67–0.97, P = 0.03, Figure 2). Statistically, Kaplan–Meier curves crossed between months 6 and 10, with pre-enrolment rate of risk accelerating and post-enrolment rate diminishing during this period. By 11 months, the rates were significantly different, with post-enrolment patients having a lower rate of CH. Because the curves crossed, this indicated a violation of the proportional hazards assumption, utilized in traditional survival analysis. Previous research has discussed that a primary issue of the log-rank test in these circumstances is that it may lose statistical power.17 Namely, this would likely make the actual CH difference more robust, relative to non-significant. To address this limitation, alternatives to the robust log-rank test were utilized. A likelihood ratio (LR) test—statistically more powerful—and a Wald test, which does not assume independence of observations within a cluster, were conducted. Results indicated that, like the log-rank test, both the LR test and the Wald test were significant, suggesting a reduction in CH.
Cardiac-related emergency department visits
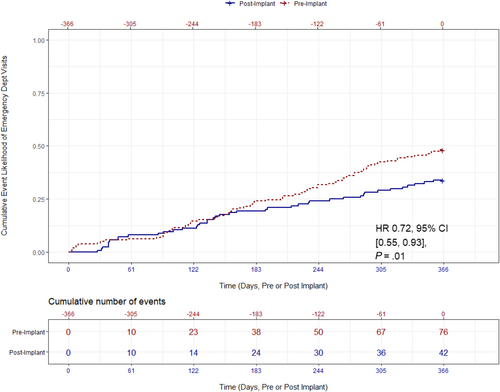
A total of 35% of participants had a cardiac-related ED visit at least once in the 12 month period before enrolment, compared with 23% of participants in the 12 months after enrolment. The cumulative number of cardiac-related ED visits decreased from 76 in the pre-enrolment time period to 42 in the post-enrolment time period. Relative to the year before enrolment, the rate of cardiac-related ED visits significantly decreased by 28% (0.48 vs. 0.34 events/patient-year; HR: 0.72, 95% CI: 0.55–0.93, P = 0.01, Figure 3).
Secondary outcomes
Length of stay
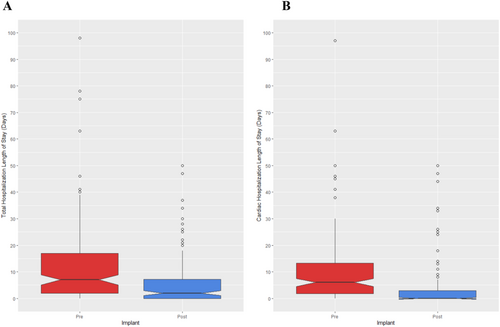
There was an 51% decrease in TLOS, reduced from 12.00 days pre-enrolment to 5.92 post-enrolment on average (SE = 1.41, 95% CI: 2.79–8.38 days, P < 0.001, Figure 4). CLOS had a 62% reduction from 9.70 to 3.70 days (SE = 1.24, 95% CI: 3.35–8.22 days, P < 0.001, Figure 4). Out of 141 participants, 11 deaths occurred within the post-enrolment observation period, detracting from the maximum possible number of days they could be admitted. To address this limitation, we asymmetrically decreased the maximum number of days a patient could be admitted. Those that died early would have their maximum LOS of 365 reduced to the number of days they survived; to illustrate, for participants living to day 365, 20 days in the hospital would represent LOS of 5.75% (20 days out of 365). For a participant who died on day 100, they would have LOS of 20% (20 days out of 100). These supplemental analyses reflected our initial findings as there was a 57% reduction of TLOS from 4.05% pre-period to 1.74% post-period (SE = 0.83, 95% CI: 0.44–3.71%, P = 0.01). Likewise, there was a 66% reduction of CLOS from 3.20% pre-period to 1.07% post-period (SE = 0.60, 95% CI: 0.83%–3.18%, P < 0.01).
Discussion
To our knowledge, this is the first study to show positive clinical outcomes using a dual platform RPM program for a large cohort of HF patients at a single centre. This study translates the effectiveness of RPM in the multicentre clinical trial setting into the individual clinical practice setting, which is important to support the widespread use of RPM at other medical centres. Not only does this study demonstrate the feasibility of RPM at a single centre, but also demonstrates that a dual platform RPM program for HF decreases the likelihood of CH as well as decreases CLOS and cardiac-related ED visits at 1 year post-enrolment compared with the year prior.
The list of failed therapies for ADHF is extensive and includes nesiritide, levosimendan, seralaxin, ularitide, tolvaptan, low-dose dopamine, and so forth.18 Therefore, a pivot from treating ADHF to preventing may be in order. Worsening congestion drives most hospitalizations for ADHF. In a recent retrospective analysis of 496 patients enrolled in two previous ADHF trials (DOSE-AHF and CARRESS-HF), only 52% were discharged free of congestion.19 Thus, the limitations of current therapies and management strategies mean that many hospitalized patients are discharged with clinically significant congestion. Importantly, patients who are still congested at hospital discharge are at increased risk for readmission or death.20, 21 Beyond empiric treatment with loop diuretics, what else may be useful in achieving the goal of euvolaemia at hospital discharge and at outpatient follow-up with normalized cardiac filling pressures? The long-awaited answer to this question may be RPM. Indeed, our data provides evidence that a solution to the problem of ADHF and HF hospitalization is early recognition and prevention of congestion via timely responses to RPM indices with appropriate medication changes.
Studies of the CardioMEMS HF System in patients with chronic HF have been shown to improve quality of life and reduce HF hospitalizations, mortality, and cost in the setting of a single platform program compared with standard-of-care.7, 22, 23 Studies of the HeartLogic HF Diagnostic have shown it to be useful in detecting worsening HF and allow better risk stratification.10, 11, 24 However, there is currently no data evaluating a dual platform RPM program. To bridge this gap, this study demonstrates for the first time that a dual platform RPM program, primarily including CardioMEMS and HeartLogic, is effective in reducing the incidence rate of CH and cardiac-related ED visits. It is known that the longer duration of a HF hospitalization increases the risk of death,25 and in our dual platform RPM experience, we observed that there was a reduction in CLOS from the pre-enrolment to post-enrolment 12 month period. If a participant is unfortunately rehospitalized after RPM enrolment we routinely interrogate RPM devices in the hospital to guide inpatient decongestion and provide reassurance for continued outpatient monitoring, all of which allows for earlier hospital discharge. Thus, this is the first study to show the benefit of RPM in both the outpatient and inpatient settings.
Overall, RPM has had efficacy in improving HF outcomes in prior studies and holds promise to overcome various barriers to care.6, 26, 27 A main limitation to implementing RPM for HF has been the cost and technical barriers to replicating clinical trial outcomes in a single centre. Our single-centre experience has demonstrated effectiveness in managing HF patients, and our results suggest that a properly implemented local RPM program can provide similar outcomes to those found in clinical trials. Key components appear to be institutional commitment, a multidisciplinary HF care team, organized enrolment, and algorithm-based patient management. We believe the success of RPM in the ‘real-world’ depends on individualized medication titration in response to changes in PAP and other monitoring indices. Although an algorithm cannot anticipate every patient change and outcome, this is an excellent place to begin when managing RPM patients. When adjusting medications, we favour optimizing GDMT—especially with an ARNi, MRA, or SGLT2i when appropriate—as the initial therapeutic change before starting or adjusting diuretics. Notably, a high percentage of participants were on GDMT prior to RPM enrolment with only a significant difference in MRA use after RPM enrolment; however, all outcomes were adjusted for MRA use and demonstrated an incremental benefit in RPM for HF beyond the benefit of an MRA.
We look forward to the upcoming results of the GUIDE-HF (Haemodynamic-GUIDEd Management of HF) trial (ClinicalTrials.gov Identifier: NCT03387813), as the randomized arm was recently closed due to full enrolment in December 2019, which will examine an expanded HF patient population outside of the present indication for the CardioMEMS HF System. Additionally, the PREEMPT-HF (Precision Event Monitoring for Patients With Heart Failure Using HeartLogic) trial (ClinicalTrials.gov Identifier: NCT03579641) to evaluate extended applications of the HeartLogic HF Diagnostic in a broad spectrum of HF patients and the PROACTIVE-HF (PROACTIVE-HF IDE Trial Heart Failure NYHA Class III) trial (ClinicalTrials.gov Identifier: NCT04089059) to evaluate the safety and effectiveness of the Cordella™ HF System in NYHA Class III HF patients are actively enrolling. Future studies should evaluate multiplatform RPM in a broader patient population and evaluate the cost-savings and cost-effectiveness at an individual hospital system level to assess the financial impact of single-centre RPM programs.
Study limitations
As this was a retrospective cohort study comparing the outcomes before and after RPM enrolment, not a prospective randomized study, it is possible that selection bias or changes in HF management in the period after enrolment may have confounded the results. Moreover, adjusting for death statistically may be a limitation as this variable affects the post-enrolment period outcomes, but not the pre-enrolment period outcomes. Additionally, patient and caregiver support and education could have impacted the outcomes independent of RPM. However, as HF is a progressive disease, it is also possible that these results may reflect a conservative estimate of the reductions in our studied outcomes. Both the CardioMEMS HF System and HeartLogic HF Diagnostic were included in this dual platform RPM program, but future studies directly comparing the efficacy of the various available RPM platforms are needed.
The conclusions from this study show changes within an individual over time, but comparisons between HF patients with and without RPM are not feasible given the current design. Thus, while a randomized design would strengthen the conclusions from study results, an advantage of the present study is that it evaluates RPM in a clinical practice, which demonstrates both the feasibility and effectiveness of implementing a dual platform RPM program outside of a research context.
Conclusions
Our single-centre, dual platform experience with the use of RPM for HF patients via appropriate medication changes in response to alerts demonstrates significant reductions in the likelihood of experiencing a CH and cardiac-related ED visit. Participants also showed reduced CLOS from the pre-enrolment to post-enrolment period. Future studies should evaluate multiplatform RPM in a broader patient population and evaluate the cost-savings and cost-effectiveness at an individual hospital system level to assess the financial impact of single-centre RPM programs.
Acknowledgements
The authors are grateful to Sarah S. Matthews, NP, Katherine A. Sorrel, PA-C, and Veronica Smith, in their triage and medical management of our HF patients within our RPM program.
Conflict of interest
None declared.
Funding
None.
Author contributions
B. N. B. conceived and designed the analysis, collected the data, and wrote the paper. K. A. B. collected the data, contributed data or analysis tools, and wrote the paper. D. G. V. conceived and designed the analysis and wrote the paper. R. B. collected the data and contributed data or analysis tools. D. B. conceived and designed the analysis, contributed data or analysis tools, and performed the analysis. T. P. T. conceived and designed the analysis, contributed data or analysis tools, performed the analysis, and wrote the paper. J. L. G. conceived and designed the analysis and wrote the paper.




