Nurse-led ambulatory care supported by non-invasive haemodynamic assessment after acute heart failure decompensation
Abstract
Heart failure (HF) is characterized by frequent decompensation and an unpredictable trajectory. To prevent early hospital readmission, coordinated discharge planning and individual therapeutic approach are recommended.
Aims
We aimed to assess the effect of 1 month of ambulatory care, led by nurses and supported by non-invasive haemodynamic assessment, on the functional status, well-being, and haemodynamic status of patients post-acute HF decompensation.
Methods and results
This study had a multicentre, prospective, and observational design and included patients with at least one hospitalization due to acute HF decompensation within 6 months prior to enrolment. The 1 month ambulatory care included three visits led by a nurse when the haemodynamic state of each patient was assessed non-invasively by impedance cardiography, including thoracic fluid content assessment. The pharmacotherapy was modified basing on haemodynamic assessment. Sixty eight of 73 recruited patients (median age = 67 years; median left ventricular ejection fraction = 30%) finished 1 month follow-up. A significant improvement was observed in both the patients' functional status as defined by New York Heart Association class (P = 0.013) and sense of well-being as evaluated by a visual analogue score (P = 0.002). The detailed patients' assessment on subsequent visits resulted in changes of pharmacotherapy in a significant percentage of patients (Visit 2 = 39% and Visit 3 = 44%).
Conclusions
The proposed model of nurse-led ambulatory care for patients after acute HF decompensation, with consequent assessment of the haemodynamic profile, resulted in: (i) improvement in the functional status, (ii) improvement in the well-being, and (iii) high rate of pharmacotherapy modifications.
Introduction
Heart failure (HF) is a complex clinical syndrome characterized by frequent decompensation and an unpredictable trajectory.1 It has been proven that chest fluid retention starts even weeks before clinical symptoms occur.2, 3 On the other hand, even HF patients who have been previously stable for a long time can suddenly deteriorate within days and even hours.1 Each hospitalization due to HF deterioration negatively influences prognosis.3, 4 Discharge is recommended to be arranged when the patient is clinically stable and euvolemic.1 However, it was shown that in real-world conditions, over 50% of patients leave the hospital while being insufficiently decongested.5, 6 Moreover, comprehensive assessments are not frequently performed, and patients often receive a suboptimal titration of life-saving medications.3 The early post-discharge period, also called the ‘vulnerable phase’, is a crucial time in which many clinical haemodynamic and therapeutic changes may occur.3
To prevent early hospital readmission, coordinated discharge planning is recommended, including early outpatient follow-up appointments.1, 7 Such a strategy provides an opportunity to ensure the safe and optimal dosing of medicines, to deliver adequate education, and to detect disease progression that may require a change in management.1 Unfortunately, limited access to ambulatory cardiologists restricts the application of these guidelines.8
An effective healthcare programme should consider the potential of telemedicine to better manage medical resources. In the AMULET project (‘A new Model of medical care with Use of modern methods of non-invasive cLinical assEssment and Telemedicine in patients with heart failure’),9 we piloted the creation of ambulatory care points to reduce the involvement of cardiologists in outpatient care to only activities that were absolutely necessary. In the first work package of the AMULET project, we arranged for ambulatory care points, which were operated by the trained HF nurse. To improve patient assessment, we implemented non-invasive haemodynamic profiling via impedance cardiography (ICG)10, 11 to measure parameters such as heart rate and blood pressure, as well as thoracic fluid load. Together with oxygen saturation and body mass, we obtained a set of vital signs that were essential for assessing the clinical state of patients and making therapeutic decisions.
In this pilot study, we aimed to assess the effect of 1 month of patient care involving regular visits to ambulatory care points on the functional state, well-being, and haemodynamic status of patients post-acute HF decompensation. This was led by nurses and supported by non-invasive haemodynamic assessment.
Methods
Study group
This study had a multicentre (three centres), prospective, and observational design. We recruited chronic HF patients of both sexes and age over 18 years with at least one hospitalization due to acute HF decompensation within 6 months prior to enrolment, clinical presentation of New York Heart Association (NYHA) Classes III and IV and a left ventricular ejection fraction (LVEF) ≤49%. The exclusion criteria were as follows: (i) cardiogenic shock; (ii) acute coronary syndrome (ST-segment elevation acute coronary syndrome, non-ST-segment elevation acute coronary syndrome, or unstable angina) as the main cause of hospitalization within the last 40 days prior to recruitment; (iii) stroke within 40 days prior to recruitment; (iv) cardiac surgery within 90 days prior to recruitment; (v) pulmonary embolism within 40 days prior to recruitment; (vi) chronic obstructive pulmonary disease (Stage C/D), uncontrolled asthma, or pulmonary hypertension (World Health Organization Classes III and IV); (vii) chronic kidney disease (Stage 5 and/or requiring dialysis); (viii) severe inflammatory disease, including pneumonia, as the main cause of hospitalization and sepsis; (ix) severe mental and physical disorders; (x) life expectancy of less than 12 months in the opinion of the physician due to reasons other than HF; and (xi) patients' refusal to participate.
The study was approved by the local ethics committee and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki (1996). Each study participant provided written informed consent to participate in the study.
Study schedule
The ambulatory care plan was for a duration of 1 month for each patient and included three visits in an ambulatory care point led by a nurse as follows: an initial recruitment visit (Visit 1), a second visit after 7–10 days (Visit 2), and a third visit after 30 ± 7 days (Visit 3). The recruitment visit included data collection regarding current patient basic characteristics (i.e. medical history and physical examination), comorbidities, and details of the last hospitalization for worsening HF (i.e. clinical state at admission and diagnostics). The patient's functional state was assessed using the NYHA classification and the self-assessment of well-being using a visual analogue scale (VAS) ranging from 1 (I feel extremely bad) to 10 (I feel great) points. LVEFs determined using echocardiography, radionuclide angiography, or cardiac magnetic resonance imaging and documented up to 6 months before enrolment were noted.
Impedance cardiography
The haemodynamic state of each patient was assessed by ICG (Niccomo, Medis, Illmenau, Germany). This non-invasive method is based on measuring thoracic impedance and its change with each heart cycle due to blood volume variations as blood conducts electricity better than other tissues.11 The haemodynamic data were collected after 10 min of resting in a sitting position. The following parameters were analysed: heart rate (HR), systolic and diastolic blood pressure (SBP and DBP), stroke volume (SV), cardiac output (CO), systemic vascular resistance index (SVRI), and thoracic fluid content (TFC).
Recommendation support module and decision making
The prescribed pharmacotherapy was modified according to each patient's clinical state and haemodynamic assessment. The following vital signs were selected as the treatment targets: HR, SBP, DBP, and TFC (to detect pulmonary congestion). Whole data sets were pictured on a recommendation support module (RSM), which presented predefined target values and alarms (Figure 1, Table 1). The physician cross-referenced RSM indications with the patient's clinical data and gave the final recommendations, including those related to pharmacotherapy.
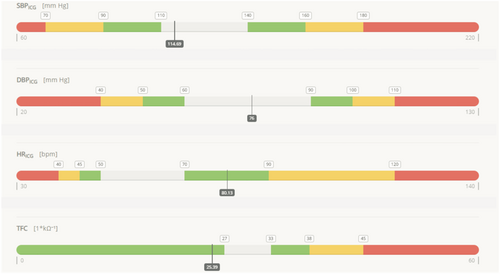
| Alarm grading | Recommendations |
|---|---|
| White | No changes in drug dosage are recommended, and direct physician consultation is not needed. |
| Green | There are slight deviations in haemodynamic parameters, and eventual treatment modification might be considered. |
| Yellow | There are significant deviations in haemodynamic parameters, and treatment modification should be considered. |
| Red | There are critical deviations in haemodynamic parameters, the patient's health is in danger, and urgent admission to hospital should be considered. |
At every visit, the patients were also educated by the nurse about HF causes, symptoms and trajectories, advice on maintaining a healthy lifestyle (fluid and salt intake, physical activity counselling, and abstinence from alcohol), and general principles of self-assessment (self-monitoring of signs and symptoms, especially those related to clinical stability).
Statistical analysis
Statistical analyses of the obtained data set were performed using Stata 16.1 (StataCorp LLC, USA). Data distribution and normality were assessed by visual inspection of histograms and the Shapiro–Wilk test. Descriptive statistics included means and standard deviations and medians and interquartile ranges for continuous variables, as well as frequencies and percentages for categorical variables. Generalized estimating equations with identity as a link function and exchangeable structures of within-subject correlation matrices were used to assess the influence of selected parameters on continuous outcome variables (VAS scoring, SBP, DBP, HR, CI, SVRI, and TFC), and random-effect ordered logistic models were used to assess influence of selected parameters on the ordinal outcome variable (NYHA class).
Results
Basic characteristics
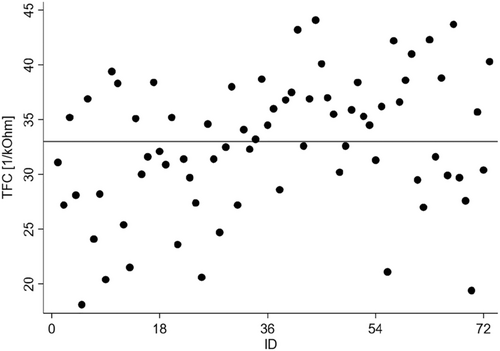
Of 73 recruited patients, three withdrew from participation after Visit 1, one withdrew after Visit 3, and one died in the period between Visits 2 and 3. The withdrawals were due to reasons unrelated to HF. The study group was predominantly composed of patients recruited on their last day of hospitalization due to HF decompensation (44%). Another 19% of patients were enrolled before the 30th day post-discharge, and only one-fourth were recruited more than 90 days post-discharge. Detailed basic characteristics of the study group are shown in Table 2. The patients presented with low LVEFs (median 30%), and most of them were in NYHA functional Classes II and III (63% and 23%, respectively). Ischaemic heart disease was the predominant HF aetiology, and the next most common comorbidities were hypertension (67%) and atrial fibrillation (59%). Most patients reported exercise intolerance (dyspnoea, 55%). Pulmonary crepitations and peripheral oedema were detected in 21% and 22% of subjects, respectively. In general, the patients were treated according to current guidelines as follows: 83% of patients were taking angiotensin-converting-enzyme inhibitors (ACEIs), 7% were taking angiotensin-receptor blockers, 1% were taking angiotensin receptor-neprilysin inhibitors, 97% were taking beta-blockers, and 69% were taking mineralocorticoid receptor antagonists. Almost 90% of subjects needed diuretics. Assessments of thoracic fluid content dispersion suggested that almost half of the subjects (49%) may have been congested at the recruitment visit (Figure 2).
| Variable | Value |
|---|---|
| Male/female, n (%) | 55/18 (75/25) |
| Age (years), mean ± SD; median (IQR) | 66 ± 13; 67 (19) |
| BMI (kg/m2), mean ± SD; median (IQR) | 29.4 ± 5.5; 29.9 (7.4) |
| LVEF (%), mean ± SD; median (IQR) | 31 ± 10; 30 (17) |
| haemoglobin (g/dL), mean ± SD; median (IQR) | 13.5 ± 2.7; 14.3 (2.8) |
| eGFR (mL/min), mean ± SD; median (IQR) | 66 ± 20; 67 (28) |
| SpO2 (%), mean ± SD; median (IQR) | 97 ± 2; 97 (3) |
| VAS (points), mean ± SD; median (IQR) | 6 ± 2; 6 (3) |
| NYHA (points), mean ± SD; median (IQR) | 2.1 ± 0.6; 2.0 (0.0) |
| Ischaemic aetiology of HF, n (%) | 48 (66) |
| History of MI, n (%) | 31 (42) |
| Hypertension, n (%) | 49 (67) |
| Atrial fibrillation, n (%) | 43 (59) |
| Diabetes, n (%) | 34 (47) |
| COPD, n (%) | 6 (8) |
| CKD (Stage 3 or higher), n (%) | 14 (19) |
| Implanted ICD/CRT, n (%) | 16/5 (22/7) |
| Haemodynamics (impedance cardiography) | |
| HR (bpm), mean ± SD; median (IQR) | 74 ± 13; 74 (14) |
| SBP (mmHg), mean ± SD; median (IQR) | 110 ± 22; 107 (30) |
| DBP (mm Hg), mean ± SD; median (IQR) | 69 ± 13; 69 (14) |
| CI (L/min/m2), mean ± SD; median (IQR) | 2.9 ± 0.6; 2.9 (0.8) |
| SVRI (dyn * s * m2/cm5), mean ± SD; median (IQR) | 2,140 ± 644; 1,997 (894) |
| TFC (1/kOhm), mean ± SD; median (IQR) | 32.7 ± 6.1; 32.6 (7.4) |
| Signs and symptoms, n (%) | |
| Dyspnoea at rest, n (%) | 3 (4) |
| Dyspnoea at exercise, n (%) | 40 (55) |
| Orthopnoea, n (%) | 6 (8) |
| Paroxysmal nocturnal dyspnoea, n (%) | 6 (8) |
| Palpitations, n (%) | 10 (14) |
| Dizziness, n (%) | 11 (15) |
| Tachypnoea, n (%) | 0 (0) |
| Peripheral oedema, n (%) | 16 (22) |
| Ascites, n (%) | 0 (0) |
| Pulmonary crepitations, n (%) | 15 (21) |
| Tachycardia, n (%) | 4 (6) |
- BMI, body mass index; CI, cardiac index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, hear failure; HR, heart rate; ICD, implanted cardioverter defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation; SVRI, systemic vascular resistance; TFC, thoracic fluid content.
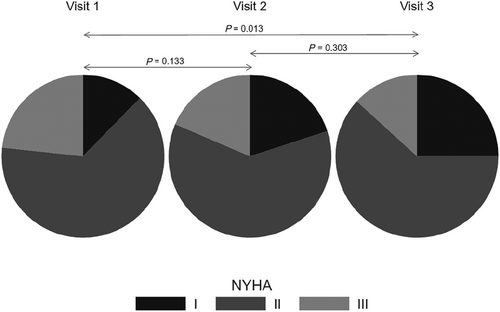
Visit-to-visit change in functional state and well-being
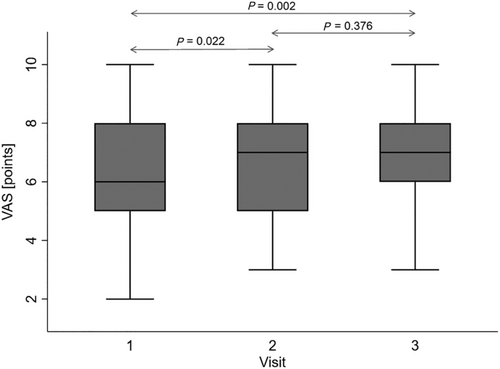
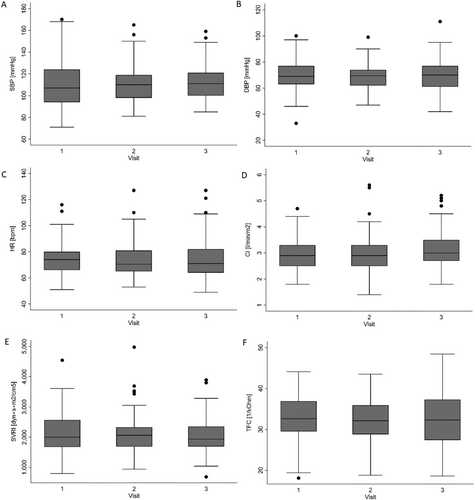
A significant improvement was observed in both the patients' functional status as defined by NYHA class and sense of well-being as assessed by the VAS. The likelihood of having a lower NYHA class category was significantly increased at Visit 3 compared with Visit 1, as presented in Figure 3 (13.2% vs. 23.3% of patients achieving NYHA Class III and 25.0% vs. 12.3% for NYHA Class I at Visits 3 and 1, respectively). Likewise, VAS scores significantly increased from a mean of 6.35 points at baseline (Visit 1) to 6.76 points at Visit 2 and 7.33 points at Visit 3 (Figure 4). There were no significant visit-to-visit group changes observed in haemodynamic parameters (SBP, DBP, HR, CI, SVRI, and TFC) and body weight (87 kg vs. 87 kg vs. 88 kg for Visits 1 to 3, respectively).
Interventions
The assessment of patients' clinical data with reference to RSM on subsequent visits resulted in changes in pharmacotherapy in a significant percentage of patients. At Visit 1, we focused mostly on education and self-care recommendations. Accordingly, the prescribed dose of ACEI was only reduced in one patient. Conversely, during Visits 2 and 3, we modified pharmacological interventions for larger proportions of patients (39% and 44%, respectively). The most frequently modified medications were diuretics, and the modifications mostly comprised dosage increases. In parallel, the dosages of ACEIs and beta-blockers were also increased quite frequently (Table 3).
| Medicines | ||||
|---|---|---|---|---|
| Taken before visit | Changes in pharmacotherapy on visit | |||
| n (%) | Number of changes, n (%) | Started/increased (●) | Stopped/decreased (○) | |
| Visit 2 | ||||
| ACEI, n (%) | 58 (83) | 10 (14) | ●●●●●●● | ○○○ |
| ARB, n (%) | 5 (7) | 0 (0) | — | — |
| Beta-blockers, n (%) | 68 (97) | 5 (7) | ●●●● | ○ |
| MRA, n (%) | 48 (69) | 4 (6) | ●●● | ○ |
| ARNI, n (%) | 1 (1) | 0 (0) | — | — |
| Diuretic, n (%) | 61 (87) | 20 (29) | ●●●●●●●●●●●●●● | ○○○○○○ |
| Visit 3 | ||||
| ACEI, n (%) | 56 (82) | 7 (10) | ●●●●● | ○○ |
| ARB, n (%) | 5 (7) | 0 (0) | — | — |
| Beta-blockers, n (%) | 65 (96) | 7 (10) | ●●●●●●● | — |
| MRA, n (%) | 47 (69) | 4 (6) | ● | ○○○ |
| ARNI, n (%) | 1 (1) | 0 (0) | — | — |
| Diuretic, n (%) | 57 (84) | 22 (32) | ●●●●●●●●●●●●●●● | ○○○○○○○ |
- ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin-receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Discussion
The results of this pilot study demonstrated that 1 month of ambulatory care supported by non-invasive haemodynamic assessment positively influenced the functional state and well-being of patients after acute HF decompensation. ICG was revealed to be a useful tool in optimizing pharmacotherapy. The assessment of lung impedance revealed that a clinically significant proportion of HF patients may have been sub-optimally decongested.
Readmissions following HF exacerbation are a significant burden for healthcare systems.1, 12 Up to 10% of patients in Europe and 25% in the US who are discharged from the hospital after HF decompensation will die or be readmitted within 30 days post-discharge.13, 14 The cause of this phenomenon is complex and is related not only to patient factors but also to limitations in access to post-discharge care programmes.12 Given that nearly half of all HF readmissions are preventable,15 the transition from inpatient to outpatient care is a crucial phase in the prevention of HF progression and mortality.1, 12, 16
The AMULET project aims to meet the need for a comprehensive protocolized approach to the management of HF patients in the post-discharge period.17 Being aware that the availability of cardiologists is limited, we have based our concept on ambulatory care points led by qualified HF nurses, thus limiting the role of physicians to data interpretation and clinical inference. We assumed that detailed assessment of vital signs, including congestion status, would provide additional diagnostic value, allowing for easier, faster, and individualized therapeutic decisions. To gauge patient stability and bridge gaps in the treatment, we provided education and pharmacotherapy adjusted to the haemodynamic goals of care. The verification of this concept was a crucial step in paving the way to introduce telemedicine solutions which are planned to be implemented in an ongoing randomized controlled trial (ClinicalTrials.gov Identifier: NCT03476590).
The main benefit for the subjects participating in the study was realized in the functional status and the self-assessed well-being of the patients (Figures 3 and 4), who improved from visit to visit in terms of both NYHA class and VAS scoring. In our opinion, the effect from Visits 1 to 2 should be attributed mostly to non-pharmacological interventions, such as education and providing a sense of security. The later effects seem to be more related to pharmacological interventions.
Nursing interventions, such as scheduled outpatient follow-up appointments, self-monitoring education, and remote telemonitoring, have been shown to improve the prognosis of patients and reduce rehospitalization rates in HF patients.18-21 In a prospective, multicentre randomized controlled WHICH trial,18 it was shown that even home-based nurse intervention provided benefits for HF patients, such as reductions in the duration of hospitalization and lower healthcare costs. Additionally, You et al.19 demonstrated the positive effects of a telephone-based, nurse-led post-discharge programme, which improved the adherence of patients to guideline-directed medication therapy for 12 weeks, their quality of life, and rate of rehospitalization. The holistic perception of the clinical state of patients by experienced HF nurses and personal relationships with patients and their caregivers may provide even better opportunities to deliver education tailored to the needs of individual patients to a greater extent than general practitioners and specialists.22
The ICG assessment and recruitment revealed increases in TFC in 49% of our patients, which is in line with previous reports showing that even clinically improved and stable patients can still be congested.17, 23, 24 Accordingly, the uptitration of diuretics was the most common change in pharmacotherapy (Table 3). However, some patients were advised to reduce their dosage of diuretics due to their low TFCs and the absence of signs of congestion. Additionally, other medications were changed quite frequently, resulting in 39% of patients experiencing modifications in pharmacological interventions at Visit 2 and 44% at Visit 3. The different directions in therapy modifications may account for the overall lack of significant changes in haemodynamic in the summary trends for the whole group (Figure 5).
Limitations
The small sample size and the lack of control group is an obvious limitation of this pilot study and prevented the possibility of sub-analyses. A more detailed evaluation of the AMULET strategy will be performed in an ongoing randomized, controlled trial. The differences in the duration between hospital discharge and recruitment to the study could also influence the results, as patients who are more stable usually benefit less from such a short-term intervention than those recruited in the vulnerable post-discharge period. We also did not evaluate mortality and cardiovascular endpoints, mostly because of non-random sample selection and short follow-up. However, only one patient died within this period, and none were readmitted. Additionally, the possibility of a placebo effect related to patients cannot be excluded.
Conclusions
A 1 month period of care based on regular visits to an ambulatory point led by a nurse and supported by non-invasive haemodynamic assessment resulted in improvements in the functional state and well-being of patients after acute HF decompensation. The consequent assessment of the individually differentiated haemodynamic profiles, especially with regard to congestion status, resulted in a high rate of pharmacotherapy modifications.
Acknowledgements
The authors would like to thank the medical staff of their centres for the nursing care and data collection.
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Funding
This work was supported by the National Centre for Research and Development under the programme ‘Prevention and treatment of lifestyle diseases’—STRATEGMED III (STRATEGMED3/305274/8/NCBR/2017).
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




