Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock
Abstract
Aim
The management of cardiogenic shock remains a clinical challenge even in well-developed healthcare systems, best illustrated by its high mortality despite numerous innovative proposals for management. The aim of this study was to describe temporal trends in incidence, causes, use of mechanical circulatory support, and mortality in cardiogenic shock in Germany.
Methods and results
Data on all cardiogenic shock patients treated in German hospitals between 2005 and 2017 were obtained from the Federal Bureau of Statistics. The data set comprised 441 696 patients with cardiogenic shock, mean age 71 (±13.8) years, 171 383 (39%) female patients. Incidence rates increased from 33.1/100 000 population in 2005 (27 246 cases) to 51.7/100 000 population in 2017 (42 779 cases). Acute myocardial infarction was the most common cause of cardiogenic shock in 2005–07 (43 422 of 82 037 cases, 52.9%), but the proportion of cases caused by it decreased until 2014–17 (73 274 of 165 873 cases, 44.2%). Over time, intra-aortic balloon pump (2005: 5104; 2017: 973 cases) was used less frequently, whereas use of extracorporeal-membrane-oxygenation (2007: 35; 2017: 2414 cases) and percutaneous left ventricular assist devices (2005: 27; 2017: 1323 cases) increased. Mortality remained high at around 60% without relevant temporal trends in patients without acute myocardial infarction and slightly decreased in patients with acute myocardial infarction.
Conclusions
In this large, nation-wide study, annual incidence of cardiogenic shock was growing, its causes were changing, and mortality was high despite a shift towards use of novel mechanical circulatory support devices. This highlights the need to address the evidence gap in this field, in particular for cardiogenic shock caused by diseases other than acute myocardial infarction.
Introduction
Cardiogenic shock is defined as the acute inability of the heart to provide sufficient blood flow, which leads to hypoperfusion of peripheral tissue and organs.1 Although cardiogenic shock can be a complication of several diseases, for example, end-stage heart failure or pulmonary embolism, it has mainly been described as a complication of acute myocardial infarction.2 In patients admitted with acute myocardial infarction, cardiogenic shock occurs in up to 6% and is associated with high mortality.3 Importantly, the overall mortality risk of patients with acute myocardial infarction complicated by cardiogenic shock has apparently plateaued on a high level in the past years.4
Traditionally, inotropes are used to treat cardiogenic shock irrespective of the underlying cause. However, these agents increase myocardial oxygen demand and might therefore further aggravate the clinical situation of affected patients.5 Mechanical circulatory support has been suggested to increase cardiac output without the negative consequences of inotropes.1, 6 These devices support cardiac output and improve haemodynamics and potential outcome. The first type of device, intra-aortic balloon pump (IABP), showed a neutral effect in cardiogenic shock complicating acute myocardial infarction.7, 8 Other types of mechanical support such as veno-arterial extracorporeal membrane oxygenation (ECMO) (VA-ECMO) or percutaneous left ventricular assist devices (pLVADs) are currently evaluated in three large randomized controlled trials.6, 9-11 However, almost all clinical research so far focused on cardiogenic shock complicating acute myocardial infarction, traditionally the most common cause of cardiogenic shock.6-11 Little is known about the incidence and outcome of cardiogenic shock in patients with conditions other than acute myocardial infarction. Furthermore, there is hardly any information on the current clinical use of mechanical circulatory support in the broad cardiogenic shock population.
The aim of this study was to characterize temporal trends in incidence, causes, use of mechanical circulatory support, and mortality in a nation-wide sample of German patients with cardiogenic shock between 2005 and 2017.
Methods
Setting
The Federal Bureau of Statistics in Germany (http://www.destatis.de) routinely collects administrative data on characteristics and outcomes of patients treated in German hospitals. The data submission is tied to reimbursement, ensuring complete reporting and auditing. Diagnoses are coded using the German modification of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10-GM); procedures are coded using the German Operational and Procedural codes (OPS).
These patient-level data are completely anonymized and centrally stored at the Research Data Centre of the Federal Bureau of Statistics (Wiesbaden, Germany). The study was performed in accordance to the Declaration of Helsinki. As the investigators did not have direct access to any patient-level data but only to anonymized statistical reports, approval by an ethics committee or informed consent was not required by German law.
For this study, all patients treated at a German hospital with the diagnosis of cardiogenic shock (ICD-10-GM code R57.0; either present at hospital admission or developed during the hospital stay) between 2005 and 2017 were included. Patients younger than 18 years were excluded. For included patients, data on coexisting diseases and used treatments were obtained via ICD-10-GM codes or OPS codes (variable definitions are shown in Table S1).
Statistics
A detailed analysis plan was written, reviewed, and submitted to the Federal Bureau of Statistics (B. S., A. G., and D. W.). Aggregate data were checked for consistency and prepared for publication. Patient-level data remained at the Federal Bureau at all times. Categorical variables are shown as frequencies and percentages and compared using the χ2 test; all continuous variables were normally distributed (assessed via histograms) and shown as mean and standard deviation and compared using the one-way analysis of variance or student's t test. Patients were categorized in four treatment groups in the main analysis: treated with IABP, pLVAD, VA-ECMO, or no mechanical circulatory support device. If multiple devices were used, the patient was categorized as treated with the device providing the highest cardiac output support (e.g. IABP < pLVAD < VA-ECMO). Annual incidence of cardiogenic shock as well as use of mechanical circulatory support per year was calculated based on cases being reported between 1 January and 31 December of that year. To evaluate associations between patient characteristics and use of mechanical circulatory support, logistic regression models with the respective treatment as the dependent and the variable of interest as the independent variable were fitted. Cardiogenic shock-related mortality was estimated as in-hospital mortality and patients were censored at discharge from hospital.
Similarly, in these patients with cardiogenic shock, relative frequency, use of mechanical circulatory support, and mortality were investigated in regard to presence vs. absence of acute myocardial infarction as well as in regard to sex, presence vs. absence of intra-hospital cardiopulmonary resuscitation, and presence vs. absence of mechanical ventilation.
All statistical analyses were programmed in R statistical software (version 3.5.2)12 by A. G. and performed at the Federal Bureau of Statistics in Wiesbaden.
Results
Between 2005 and 2017, 441 696 patients with cardiogenic shock were treated in German hospitals. The mean age of the study population was 70.97 (±13.75) years, and 171 383 (38.8%) were female patients. Cardiovascular comorbidities such as hypertension and diabetes and multi-organ failure as illustrated by mechanical ventilation and dialysis were common, irrespective of the absence/presence of acute myocardial infarction (Tables 1 and 2). However, patients were younger and more likely to have multi-organ failure if selected for mechanical circulatory support therapy (Table 1). Additional baseline characteristics on the study cohort stratified by sex, presence/absence of mechanical ventilation, and presence/absence of intra-hospital cardiopulmonary resuscitation are presented in Tables S2–S4.
| IABP treated (N = 42 636; 10%) | pLVAD treated (N = 4063; 1%) | VA-ECMO treated (N = 9825; 2%) | No device (N = 385 172; 87%) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 67.08 ± 11.96 | 66.7 ± 12.5 | 59.37 ± 15.58 | 71.74 ± 13.69 |
| Sex, female | 12 438 (29.18%) | 1085 (26.7%) | 2662 (27.09%) | 155 198 (40.3%) |
| Comorbidities | ||||
| Arterial hypertension | 16 595 (38.92%) | 1474 (36.28%) | 3316 (33.75%) | 138 048 (35.84%) |
| Hypercholesterinemia | 12 436 (29.17%) | 968 (23.82%) | 2153 (21.91%) | 71 027 (18.44%) |
| Diabetes mellitus | 13 029 (30.56%) | 1172 (28.85%) | 2370 (24.12%) | 112 348 (29.17%) |
| Peripheral vascular disease | 2732 (6.41%) | 303 (7.46%) | 747 (7.6%) | 26 661 (6.92%) |
| Prior stroke | 1313 (3.08%) | 130 (3.2%) | 672 (6.84%) | 8857 (2.3%) |
| Atrial fibrillation | 12 704 (29.8%) | 1203 (29.61%) | 3482 (35.44%) | 124 413 (32.3%) |
| COPD | 3163 (7.42%) | 266 (6.55%) | 684 (6.96%) | 40 006 (10.39%) |
| Pulmonary hypertension | 2869 (6.73%) | 302 (7.43%) | 1255 (12.77%) | 22 761 (5.91%) |
| Hyperthyroidism | 905 (2.12%) | 89 (2.19%) | 195 (1.98%) | 8113 (2.11%) |
| Chronic kidney disease | 10 368 (24.32%) | 928 (22.84%) | 2013 (20.49%) | 110 971 (28.81%) |
| History of coronary bypass graft | 2834 (6.65%) | 199 (4.9%) | 780 (7.94%) | 23 203 (6.02%) |
| Presentation | ||||
| Acute myocardial infarction | 33 017 (77.44%) | 2999 (73.81%) | 4903 (49.9%) | 171 073 (44.41%) |
| Severe pulmonary embolism | 173 (0.41%) | 30 (0.74%) | 363 (3.69%) | 17 314 (4.5%) |
| Acute myocarditis | 174 (0.41%) | 48 (1.18%) | 186 (1.89%) | 1062 (0.28%) |
| Post cardiothoracic surgery | 4254 (9.98%) | 151 (3.72%) | 2256 (22.96%) | 9060 (2.35%) |
| Pre-hospital cardiac arrest | 8432 (19.78%) | 1120 (27.57%) | 2296 (23.37%) | 54 065 (14.04%) |
| Intra-hospital cardiac arrest | 17 113 (40.14%) | 2114 (52.03%) | 4964 (50.52%) | 143 260 (37.19%) |
| Treatment | ||||
| Invasive ventilation | 24 314 (57.03%) | 2443 (60.13%) | 6536 (66.52%) | 163 879 (42.55%) |
| Non-invasive ventilation | 4923 (11.55%) | 659 (16.22%) | 1149 (11.69%) | 47 170 (12.25%) |
| Pulmonary artery catheter | 7667 (17.98%) | 555 (13.66%) | 1477 (15.03%) | 13 690 (3.55%) |
| Dialysis | 12 487 (29.29%) | 1419 (34.92%) | 5327 (54.22%) | 54 543 (14.16%) |
| IABP | 42 636 (100%) | 298 (7.33%) | 1651 (16.80%) | 0 (0%) |
| pLVAD | 0 (0%) | 4063 (100%) | 869 (8.84%) | 0 (0%) |
| VA-ECMO | 0 (0%) | 0 (0%) | 9825 (100%) | 0 (0%) |
- COPD, chronic obstructive pulmonary disease; IABP, intra-aortic balloon counter-pulsation pump; pLVAD, percutaneous left ventricular assist device; VA-ECMO, veno-arterial extracorporeal membrane oxygenation therapy.
| Cardiogenic shock without acute myocardial infarction (N = 229 704; 52%) | Cardiogenic shock with acute myocardial infarction (N = 211 992; 48%) | |
|---|---|---|
| Demographics | ||
| Age (years) | 70.76 ± 14.97 | 71.19 ± 12.3 |
| Sex, female | 94 929 (41.33%) | 76 454 (36.07%) |
| Comorbidities | ||
| Arterial hypertension | 80 111 (34.88%) | 79 322 (37.42%) |
| Hypercholesterinemia | 37 068 (16.14%) | 49 516 (23.36%) |
| Diabetes mellitus | 64 109 (27.91%) | 64 810 (30.57%) |
| Peripheral vascular disease | 14 921 (6.5%) | 15 522 (7.32%) |
| Prior stroke | 5359 (2.33%) | 5613 (2.65%) |
| Atrial fibrillation | 86 893 (37.83%) | 54 909 (25.9%) |
| COPD | 27 461 (11.95%) | 16 658 (7.86%) |
| Pulmonary hypertension | 19 336 (8.42%) | 7851 (3.7%) |
| Hyperthyroidism | 5251 (2.29%) | 4051 (1.91%) |
| Chronic kidney disease | 71 231 (31.01%) | 53 049 (25.02%) |
| History of coronary bypass graft | 15 301 (6.66%) | 11 715 (5.53%) |
| Presentation | ||
| Severe pulmonary embolism | 16 419 (7.15%) | 1461 (0.69%) |
| Acute myocarditis | 1341 (0.58%) | 129 (0.06%) |
| Post cardiothoracic surgery | 9001 (3.92%) | 6720 (3.17%) |
| Pre-hospital cardiac arrest | 27 350 (11.91%) | 38 563 (18.19%) |
| Intra-hospital cardiac arrest | 82 489 (35.91%) | 84 962 (40.08%) |
| Treatment | ||
| Coronary angiography | — | 129 553 (61.11%) |
| Coronary intervention | — | 109 998 (51.89%) |
| Coronary bypass graft | — | 15 441 (7.28%) |
| Invasive ventilation | 100 034 (43.55%) | 97 138 (45.82%) |
| Non-invasive ventilation | 29 400 (12.8%) | 24 501 (11.56%) |
| Pulmonary artery catheter | 11 063 (4.82%) | 12 326 (5.81%) |
| Dialysis | 41 622 (18.12%) | 32 154 (15.17%) |
| IABP | 10 264 (4.47%) | 34 321 (16.19%) |
| pLVAD | 1437 (0.63%) | 3495 (1.65%) |
| VA-ECMO | 4922 (2.14%) | 4903 (2.31%) |
- COPD, chronic obstructive pulmonary disease; IABP: Intra-aortic balloon counter-pulsation pump; pLVAD: Percutaneous left ventricular assist device; VA-ECMO: Veno-arterial extracorporeal membrane oxygenation therapy.
Incidence, cause, and mortality of cardiogenic shock
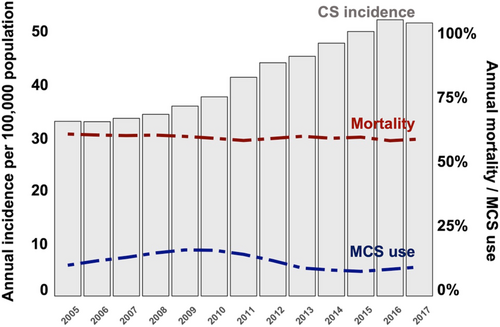
Between 2005 and 2009, the annual incidence of cardiogenic shock was relatively stable (33.1/100 000 population, 27 246 cases in 2005 and 35.9/100 000 population, 29 390 cases in 2009). However, there was a relevant increase in the annual incidence of cardiogenic shock from 2010 to 2017, with 45/100 000 population (30 808 cases) in 2010 and 51.7/100 000 population (42 779 cases) in 2017 (Figure 1).
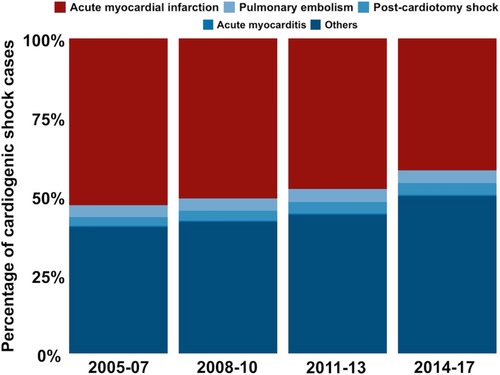
Acute myocardial infarction was the most common single cause of cardiogenic shock. Although the absolute number of patients with cardiogenic shock caused by acute myocardial infarction increased over time, the proportion of cardiogenic shock cases caused by it decreased (43 422 cases, 52.9% in 2005–07; 44 917 cases, 50.8% in 2008–10; 50 379 cases, 47.8% in 2011–13; 73 274 cases, 44.2% in 2014–17; Figure 2). The proportion of patients with shock due to pulmonary embolism (3.7% in 2005–07, 3.8% in 2008–10, 4.1% in 2011–13, and 4.3% in 2014–17), acute myocarditis (0.3% in 2005–07, 0.3% in 2008–10, 0.4% in 2011–13, and 0.4% in 2014–17) and shock after cardiothoracic surgery (2.8% in 2005–07, 3.2% in 2008–10, 3.6% in 2011–13, and 4.0% in 2014–17) remained relatively stable over time. Ultimately, the absolute number and proportion of cardiogenic shock cases due to other diseases (diseases without a specific ICD-10-GM code, e.g. cardiogenic shock caused by decompensated heart failure) increased (32 992 cases, 40% in 2005–07; 36 969 cases, 42% in 2008–10; 46 468 cases, 44% in 2011–13; 87 669 cases, 53% in 2014–17; Figure 2).
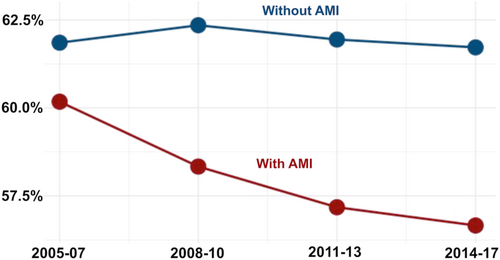
During a mean follow-up of 13 (±18) days, 60% of all patients with shock (264 869) died. Annual in-hospital mortality remained relatively constant over time (61% in 2005 and 59% in 2017, Figure 1) in the overall patient cohort, although there was a trend towards lower mortality over time in patients with acute myocardial infarction, which was not observed in patients without acute myocardial infarction (Figure 3).
Use of mechanical circulatory support
Overall, mechanical circulatory support devices were used in 56 524 (13%) of the cardiogenic shock patients treated in Germany between 2005 and 2017. The use of mechanical circulatory support devices increased from 12% in 2005 to 18% in 2009, decreased from 2010 onwards, and plateaued at a level comparable with that in 2005 at the end of this study (17% in 2010 and 11% in 2017; Figure 1).
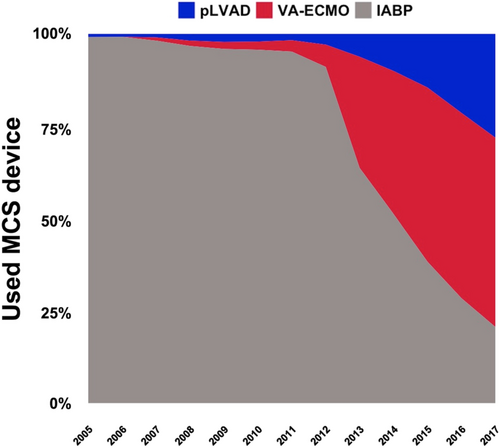
Intra-aortic balloon pump was the most frequently used device (used in 42 636 patients, 75%). IABP use increased between 2005 and 2010 (from 3152 patients in 2005 to 5104 in 2010) and decreased drastically in the following years (down to 973 in 2017). The use of pLVAD (from 27 patients in 2005 to 1323 in 2017) and VA-ECMO (from 35 patients in 2007 to 2414 in 2017; Figure 4) increased constantly throughout the observation period. This trend was consistent even if the study cohort was stratified by presence/absence of acute myocardial infarction (Figure S1).
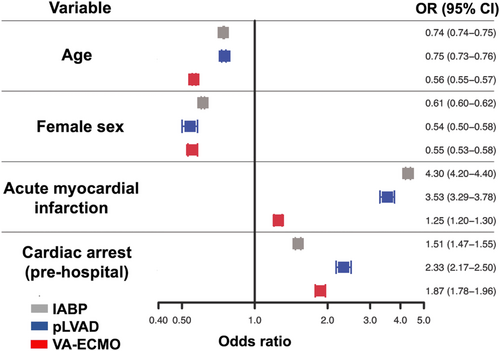
Overall, factors associated with a higher likelihood of mechanical circulatory support use were acute myocardial infarction and pre-hospital cardiac arrest, whereas higher age and female sex were associated with a lower likelihood of mechanical circulatory support use (Figure 5).
Discussion
- Cardiogenic shock is common, and its incidence is increasing from 33.1/100 000 population to 51.7/100 000 population in Germany.
- The increase in cardiogenic shock cases is mainly due to cardiogenic shock outside of an acute myocardial infarction.
- In-hospital mortality of cardiogenic shock remains unacceptably high at 60% without clear improvements in patients without acute myocardial infarction, although we observed a trend towards lower mortality in patients with acute myocardial infarction
- Mechanical circulatory support shows clear temporal patterns, with high usage of IAPB until 2010 and a growing use of ECMO and pLVAD thereafter, although these devices were overall more likely to be used in patients with acute myocardial infarction.
Incidence and causes of cardiogenic shock
In the past decades, new therapies have led to an overall improvement in care and outcomes of patients with cardiovascular diseases. As an example, early revascularization and adequate medical therapy have significantly reduced the mortality rate of acute myocardial infarction.4 However, this has led to an increase in chronic cardiovascular conditions, for example, heart failure.13 In the present study, we did not only observe a growing incidence of cardiogenic shock over the past years but also observed that more than 50% of patients developed shock outside of acute myocardial infarction (and also outside of severe pulmonary embolism, acute myocarditis, or post-cardiothoracic surgery). Although a certain percentage of these observations might be explained by differences in case reporting, it is more likely that these changes are caused by a shift towards more non-ischemic cardiogenic shock cases. Similarly, the incidence of acute myocardial infarction has decreased in the past decades, whereas the prevalence of heart failure caused by ischemic cardiomyopathy has increased.14 The rapidly growing group of non-ischemic cardiogenic shock cases will include patients with severe heart failure due to distant myocardial infarction, due to cardiomyopathies, and due to other causes such as hypertension. A similar pattern was reported in a small study from the USA and in a small study from our own institution, where the majority of the patients presented with non-ischemic cardiogenic shock and less than a third of the cases could be attributed to acute myocardial infarction.15, 16 Additionally, formation of cardiac arrest centres and shock teams might have contributed to the observed increase in case numbers, as both might have facilitated early diagnosis (e.g. identifying cardiogenic shock as the leading cause of admission) and correct triage of cardiogenic shock patients, thereby raising awareness for and correct labelling of these cases.17, 18 Another interesting observation was that among patients with ischemic cardiogenic shock, the use of coronary angiography and revascularization was relatively low (~60% each). To a certain proportion, this could be explained by patients presenting with severe cardiogenic shock and dying before/during transportation to the catheterization laboratory or operation room. A recent study showed that the annual rate of these procedures in patients with ischemic cardiogenic shock has increased over the past years, indicating improvements in early diagnosis, correct triage, and transfer.19
Mortality and use of mechanical circulatory support in cardiogenic shock
Another important observation of this study was that the annual in-hospital mortality rate of patients with cardiogenic shock has plateaued at about 60% over the past years in the overall study cohort. This might stem from the paucity of effective treatments in this setting: Inotropes, although frequently used, increase myocardial oxygen demand, which hampers myocardial recovery and seems to increase mortality risk.5 In cardiogenic shock due to acute myocardial infarction, early revascularization of the culprit artery is an effective approach to improve outcomes and might explain the trend towards lower mortality in these patients observed in our study.20 Nevertheless, the latest trial on this topic still reported an unacceptable high mortality rate of more than 40%, despite enrolling a selected study population (exclusion of patients with prolonged cardiac arrest).20 Of note, mortality in patients with cardiogenic shock outside of acute myocardial infarction was constantly high throughout the study duration, which highlights the need to develop specific treatments for this patient population,16, 21 especially as our analysis indicated that mechanical circulatory support devices were less frequently used in these patients.
Mechanical circulatory support devices have been suggested as a potential treatment for cardiogenic shock.21 In this study, we observed that mechanical circulatory support devices were used only in about 10% of the cardiogenic shock patients between 2005 and 2017 and that annual use of these devices has plateaued in this range in the past years. The IABP was the most frequently used device in this study, but its annual use decreased to a relevant degree after 2010. This coincides with the publication of the IABP-SHOCK II trial, which showed that the IABP does not reduce mortality in cardiogenic shock due to acute myocardial infarction.8 A similar observation has been made in the USA, although the decrease in annual IABP use in the USA was more modest as compared with our findings.22 Interestingly, our data indicate that other mechanical circulatory support devices (pLVAD and VA-ECMO) have been increasingly used, illustrating a perceived clinical need for mechanical circulatory support devices. Currently, there is no randomized controlled trial showing that these therapies improve outcomes in cardiogenic shock; and retrospective data showed mixed results in regard to mortality, but a clear trend towards a higher risk of complications.23-26 Fortunately, randomized controlled trials evaluating pLVAD or VA-ECMO in this setting are currently ongoing.9-11 Although these trials are limited to patients with cardiogenic shock caused by acute myocardial infarction, their results will be of utmost importance to guide further use of these devices.
Furthermore, this study indicated that pLVAD and VA-ECMO were more likely to be used in patients with acute myocardial infarction or cardiac arrest and less likely to be used in female and elderly patients.1, 6 This reflects general trends to use heart failure devices less often in female/elderly patients.27 As there is no general recommendation on selection criteria for mechanical circulatory support in Germany, the presented data indeed reflect the most commonly used selection criteria, and the results are therefore valid to identify overall patterns in use of these devices. In addition to the important ongoing randomized trials, our analysis illustrates a clear need for future research projects testing novel therapeutic concepts, including mechanical circulatory support, in patients with non-ischemic cardiogenic shock. Understanding the mechanisms leading to cardiogenic shock outside of acute myocardial infarction will be critical to develop new prognostic therapies for this growing group of patients with a grim prognosis.
Limitations
Strengths of the study include the nation-wide, near-complete data collection and capture of outcomes in a highly developed healthcare system over a long period. This allowed us to derive reliable numbers on annual incidence, causes, use of mechanical circulatory support, and mortality.
Limitations are linked with the non-randomized nature of this study and therefore with residual/unmeasured confounding and selection bias. Furthermore, follow-up was limited to the hospital stay, so that we could not capture events occurring after discharge, and the case definition was based on ICD-10-GM codes with a potential for simplification. Clinical variables such as laboratory values or physiological markers were not available, so that patients could not be classified based on commonly used cardiogenic shock scores.28 Similarly, data points in this administrative database are not reported with a time stamp, so that we could not assess important prognostic markers such as development of non-cardiac organ failure29 or distinguish between concomitant use of vs. escalation from one to another mechanical circulatory support device.26 While misclassification or upcoding of administrative data (e.g. to obtain financial incentives linked to mechanical circulatory support devices) might have possibly enhanced the reported case numbers, this is unlikely to have a larger impact on the results in view of the robust rules for reporting and auditing by hospitals and payors. Additionally, the same patient might have had multiple registrations from different hospitalization in the database, which could have influenced the results. While the data are extremely valid for Germany, other countries with different healthcare systems may see different trends and outcomes. Lastly, the data for this study originate from an administrative database, and not from database created and maintained by clinician scientists, which limits its clinical depth and accuracy.
Conclusions
In this large, nation-wide study of cardiogenic shock patients treated between 2005 and 2017, there was an increase in the annual incidence of cardiogenic shock and a growing sub-population of patients without acute myocardial infarction. The decreasing use of IABP after 2010 led to a shift towards increasing pLVAD and VA-ECMO use. We observed a trend towards lower mortality in patients with acute myocardial infarction, but annual mortality rate has plateaued at about 60% in patients with cardiogenic shock outside of acute myocardial infarction.
Overall, these findings highlight the need to address the lack of effective treatments in this field, in particular for cardiogenic shock caused by diseases other than acute myocardial infarction.
Conflict of interest
B.S. has received speakers fee from AstraZeneca (unrelated to the submitted work).
P.K. receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years. He is also listed as an inventor on two patents held by the University of Birmingham (Atrial Fibrillation Therapy WO 2015140571 and Markers for Atrial Fibrillation WO 2016012783).
S.K. received research support by Ambu, E.T.View Ltd, Fisher & Paykel, Pfizer, and Xenios. He also received lecture honorarium from ArjoHuntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll. He received consultant honorarium from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios, all unrelated to the submitted work.
M.S. reports personal fees from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, and Medtronic (unrelated to the submitted work).
S.B. reports grants and personal fees from Abbott Diagnostics, Bayer, SIEMENS, and Thermo Fisher, grants from Singulex, and personal fees from Abbott, Astra Zeneca, AMGEN, Medtronic, Pfizer, Roche, SIEMENS Diagnostics, and Novartis (unrelated to the submitted work).
D.W. reports speakers fee from AstraZeneca, Bayer, and Novartis (unrelated to the submitted work).
Funding
The study was funded by the University Heart and Vascular Centre Hamburg. B.S. is currently funded by the German Research Foundation and the Else Kröner-Fresenius-Stiftung.




