An aetiology-based subanalysis of the Telerehabilitation in Heart Failure Patients (TELEREH-HF) trial
Abstract
Aims
The aim of our study was to analyse the benefits of a 9 week hybrid comprehensive telerehabilitation (HCTR) programme in heart failure (HF) patients according to aetiology, as a subanalysis of the Telerehabilitation in Heart Failure Patients (TELEREH-HF) trial.
Methods and results
Overall, 555 (65.3%) patients with ischaemic (IS) and 295 (34.7%) patients with non-ischaemic (NIS) HF aetiology were randomized. There were no differences between the effect of HCTR and usual care (UC) on the primary outcome of number of days alive and out of the hospital in 26 months from the time of randomization in either aetiology (Wilcoxon–Mann–Whitney test), and no heterogeneity of effect between the aetiologies was noted (van Elteren test, P = 0.746). In Cox proportional hazards regression analysis, treatment was not independently associated with the secondary outcomes. For all-cause mortality, the adjusted hazard ratio for HCTR vs. UC was 0.90 (95% confidence interval, 0.54–1.51) in IS and 1.42 (95% confidence interval, 0.69–2.94) in NIS (P interaction = 0.316). Differences between HCTR and UC in terms of change in the 6 min walk test distance and cardiopulmonary exercise test time after 9 weeks reached statistical significance in the IS arm (P = 0.015 and P < 0.001, respectively), but not in the NIS arm; however, tests of heterogeneity indicated no statistically significant differences.
Conclusions
The trial showed no difference between HCTR and UC in the primary outcome of percentage of days alive and out of the hospital for either IS or NIS aetiology. Moreover, the magnitude of changes in the clinical and functional statuses of the HF patients did not differ by aetiology. HCTR might have had beneficial effects on the 6 min walk test distance and cardiopulmonary exercise test time after 9 weeks in the IS patients; however, the effect was not statistically significantly different from that observed in the NIS patients.
Introduction
Exercise intolerance is the hallmark of heart failure (HF), and it is of critical relevance in HF patients because it is associated with low quality of life and increased mortality. Although a reduced cardiac reserve is considered the principal cause of symptoms in HF patients,1 impaired exercise and functional capacity are the products of multisystem dysfunction, including aging, decreased pulmonary reserve, and peripheral and respiratory skeletal muscle dysfunction, which can variably and meaningfully contribute to the syndrome.2 Loss of skeletal muscle, the so-called sarcopenia, is frequently observed in patients with advanced HF, and it results in reduced exercise capacity, which is independently associated with increased mortality.3 Moreover, wasting and weakness can occur in the respiratory musculature.4 Some systematic reviews and meta-analyses that were conducted within small studies have shown the benefits of exercise training in terms of exercise tolerance, health-related quality of life, and hospitalization due to worsening HF.5, 6 The rationale for proposing cardiac rehabilitation in HF patients is based on broadly confirmed evidence, which shows that abnormalities of the peripheral vasculature and skeletal muscles play a major role in impaired exercise capacity and functional status.7, 8 Moreover, it has been shown that an ischaemic (IS) HF aetiology is a significant predictor of cardiac-related events in comparison with a non-ischaemic (NIS) aetiology.1, 9-11 Likoff et al. showed that an IS HF aetiology independently predicts an increased risk of mortality in comparison with idiopathic HF.12 In addition, patients with IS disease have lower survival rates and exercise capacity than NIS patients.13
A few previous reports have proposed that HF aetiology might influence functional capacity, regardless of left ventricular function, as functional capacity is related to different levels of peripheral vascular disease, skeletal muscle myopathy, and cardiac adaptation to excessive wall stress.14 Therefore, the objective of the present study was to examine the impact of HF aetiology on the outcomes of the hybrid comprehensive telerehabilitation (HCTR) programme assessed by functional and clinical parameters in patients enrolled in the Telerehabilitation in Heart Failure Patients (TELEREH-HF) trial.
Methods
Type of study
The design and main results of the TELEREH-HF trial have been reported previously.15, 16 The TELEREH-HF study is a randomized, multicentre, prospective, open-label, parallel-group, controlled trial performed at five locations in Poland. In brief, the study enrolled clinically stable patients (New York Heart Association class I, II, or III) who had left ventricular ejection fraction (LVEF) ≤ 40% after a hospitalization, within 6 months prior to the randomization. The study was designed to determine whether potential improvements in functional and quality of life outcomes after a 9 week training period translated into improvement in clinical outcomes during an extended follow-up period.15, 16 The research protocol was registered in a clinical database (ClinicalTrials.gov NCT02523560), and the study procedure was in conformity with the ethical guidelines of the Declaration of Helsinki and its later amendments.17 The ethics committees in all centres where the study had been conducted approved the research protocol. Informed consent was obtained from all patients.
Study population
Between 8 June 2015 and 28 June 2017, 850 eligible patients were randomized in a 1:1 ratio to either a HCTR + usual care (UC) group (HCTR group) or a UC-only group. A total of 425 HF patients with reduced ejection fraction, as defined by the European Society of Cardiology guidelines,18 for whom exercise training was not contraindicated and who could undergo HCTR, were enrolled in the TELEREH-HF trial. The patients were randomized to the HCTR and UC arms. The inclusion and exclusion criteria are shown in Table 1.
| IS aetiology group (n = 555) | NIS aetiology group (n = 295) | |||||
|---|---|---|---|---|---|---|
| IS-HCTR n = 281 | IS-UC n = 274 | P1 | NIS-HCTR n = 144 | NIS-UC n = 151 | P2 | |
| Men, n (%) | 261 (92.9) | 252 (92.0) | 0.685 | 116 (80.6) | 124 (82.1) | 0.730 |
| Age (years), mean ± SD | 65.5 ± 8.6 | 64.1 ± 8.8 | 0.076 | 57.0 ± 12.4 | 58.5 ± 11.6 | 0.257 |
| LVEF (%), mean ± SD | 31.0 ± 6.9 | 29.7 ± 6.9 | 0.033 | 30.9 ± 7.0 | 31.3 ± 7.4 | 0.619 |
| Atrial fibrillation or atrial flutter, n (%) | 44 (15.7) | 39 (14.2) | 0.638 | 35 (24.3) | 41 (27.1) | 0.576 |
| BMI (kg/m2), mean ± SD | 28.5 ± 4.7 | 28.9 ± 4.4 | 0.329 | 29.1 ± 5.7 | 29.4 ± 5.3 | 0.620 |
| NT-proBNP [pg/mL] | 870 [401–1924] | 962 [434–2128] | 0.359 | 969 [266–2462] | 751 [317–1801] | 0.334 |
| 6MWTD (m) | 4.75 ± 1.44 | 4.56 ± 1.56 | 0.147 | 5.02 ± 1.86 | 5.18 ± 2.00 | 0.462 |
| CPET time (s) | 371.6 ± 168.5 | 356.8 ± 174.5 | 0.311 | 404.7 ± 206.2 | 403.9 ± 198.2 | 0.974 |
| Peak VO2 (mL/kg/min) | 16.57 ± 5.04 | 15.96 ± 5.34 | 0.170 | 17.5 ± 6.5 | 17.9 ± 6.8 | 0.588 |
| RER | 0.96 ± 0.15 | 0.97 ± 0.12 | 0.803 | 0.96 ± 0.12 | 0.98 ± 0.14 | 0.361 |
| Past medical history, n (%) | ||||||
| Coronary artery disease (MI; PCI; CABG) | 275 (97.9) | 264 (96.3) | 0.286 | 5 (3.5) | 7 (4.6) | 0.613 |
| Myocardial infarction | 246 (87.5) | 240 (87.6) | 0.987 | 4 (2.8) | 3 (2.0) | 0.717 |
| Angioplasty | 196 (69.7) | 191 (69.7) | 0.991 | 4 (2.8) | 5 (3.3) | 1.000 |
| Coronary artery bypass grafting | 70 (24.9) | 70 (25.5) | 0.863 | 0 (0) | 0 (0) | NA |
| Hypertension | 196 (69.7) | 204 (74.5) | 0.217 | 61 (42.4) | 73 (48.3) | 0.302 |
| Stroke | 20 (7.1) | 27 (9.8) | 0.247 | 8 (5.6) | 6 (4.0) | 0.523 |
| DM | 103 (36.6) | 106 (38.7) | 0.621 | 36 (25) | 46 (30.5) | 0.295 |
| Chronic kidney disease | 53 (18.9) | 53 (19.3) | 0.885 | 25 (17.4) | 18 (11.9) | 0.186 |
| Hyperlipidaemia | 159 (56.6) | 133 (48.5) | 0.058 | 51 (35.4) | 53 (35.1) | 0.954 |
| Depressiona | 58 (24.9) | 62 (26.5) | 0.692 | 24 (19.7) | 41 (32.5) | 0.021 |
| Functional status by NYHA level | ||||||
| NYHA I | 32 (11.4) | 23 (8.4) | 0.072 | 22 (15.3) | 27 (17.9) | 0.510 |
| NYHA II | 201 (71.5) | 184 (67.2) | 92 (63.9) | 100 (66.2) | ||
| NYHA III | 48 (17.1) | 67 (24.4) | 30 (20.8) | 24 (15.9) | ||
| Treatment, n (%) | ||||||
| Beta-blocker | 268 (95.4) | 268 (97.8) | 0.115 | 141 (97.9) | 148 (98.0) | 1.000 |
| ACEI/ARB | 260 (92.5) | 257 (93.8) | 0.554 | 135 (93.7) | 141 (93.4) | 0.896 |
| Digoxin | 22 (7.8) | 21 (7.7) | 0.942 | 30 (20.8) | 31 (20.5) | 0.949 |
| Loop diuretics | 200 (71.2) | 208 (75.9) | 0.206 | 116 (80.6) | 126 (83.4) | 0.518 |
| Spironolactone/eplerenone | 227 (80.8) | 220 (80.3) | 0.884 | 124 (86.1) | 128 (84.8) | 0.744 |
| Aspirin/clopidogrel | 206 (73.3) | 205 (74.8) | 0.685 | 37 (26.7) | 37 (24.5) | 0.813 |
| Anticoagulants | 65 (23.1) | 73 (26.6) | 0.339 | 60 (41.7) | 55 (36.4) | 0.356 |
| NOAC | 51 (18.1) | 34 (12.4) | 0.060 | 20 (13.9) | 28 (18.5) | 0.279 |
| Statins | 258 (91.8) | 257 (93.8) | 0.367 | 88 (61.1) | 93 (61.6) | 0.933 |
| CIEDs | 217 (77.2) | 222 (81.0) | 0.271 | 118 (81.9) | 125 (82.8) | 0.850 |
| Implantable cardioverter–defibrillator | 153 (70.5) | 158 (71.2) | 0.973 | 53 (44.9) | 67 (53.6) | 0.609 |
| CRT-P | 1 (0.5) | 1 (0.4) | 3 (2.5) | 3 (2.4) | ||
| CRT-D | 61 (28.1) | 60 (27.0) | 61 (51.7) | 54 (43.2) | ||
| Remote monitoring CIEDs | 138 (49.1) | 35 (12.8) | <0.0001 | 75 (52.1) | 27 (17.2) | <0.0001 |
- 6MWTD, 6 min walk test distance; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CIEDs, cardiovascular implantable electronic devices; CPET, cardiopulmonary exercise test; CRT-D, cardiac resynchronization therapy–defibrillator; CRT-P, cardiac resynchronization therapy–pacemaker; DM, diabetes mellitus; IS, ischaemic; IS-HCTR, patients with ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; IS-UC, patients with ischaemic aetiology of heart failure randomized to the usual care group; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; NIS, non-ischaemic; NIS-HCTR, patients with non-ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; NIS-UC, patients with non-ischaemic aetiology of heart failure randomized to the usual care group; NOAC, non-vitamin K antagonist oral anticoagulant; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; Peak VO2, peak oxygen uptake; RER, respiratory exchange ratio; SD, standard deviation.
- a Defined by a Beck Depression Inventory-II score of more than 13 points.
Intervention
Patients with both aetiologies (IS and NIS) underwent a 9 week HCTR programme, which comprised two stages: an initial stage (1 week) conducted in a hospital and a basic, home-based stage (8 weeks) in which HCTR was performed five times weekly. The goals of the initial stage were a baseline clinical examination, treatment optimization, patient education, planning of exercise training, and offering five monitored educational training sessions. The basic stage, which was conducted in the patients' homes, consisted of two parts: consent to access each training session and a training session. The monitoring system included a special remote device for supervised exercise training controlled by tele-electrocardiogram (ECG) (also called a telerehabilitation set; Pro Plus Company, Poland), which consisted of an EHO mini device, a blood-pressure-measuring device, and a weighing scale; a data transmission set via a mobile telephone; and a monitoring centre capable of receiving and storing patients' medical data. The EHO mini device can record ECG data from three precordial leads and transmit the data via a mobile telephone network to the monitoring centre. Additionally, the device has pre-programmed individualized training sessions (with a defined exercise duration, breaks, and timing of ECG recordings).15, 16
Parameters
Functional capacity parameters were evaluated using the symptom-limited cardiopulmonary exercise test (CPET), which was performed on a treadmill (Schiller MTM-1500 MED, Baar, Switzerland), with an incremental workload, according to the ramp protocol and the 6 min walk test (6MWT). CPET was performed twice: on admission (within 5 days of hospitalization in the HCTR group and within 3 days of hospitalization in the UC group) and after completing the 9 week programme (within 3 days of hospitalization in both groups). Maximal exercise was defined as a respiratory exchange ratio ≥1 or as maximum fatigue, according to the Borg scale (fatigue level of 14–16 on a 20-point scale). Oxygen uptake was analysed on a continuous breath-by-breath basis. The obtained peak oxygen uptake (VO2 peak) values were presented as per kilogram of body mass per minute (mL/kg/min) and as a percentage of normal values (pVO2% N) for each patient with considerations of gender, age, weight, and height, according to the criteria developed by Wasserman. CPET was continued until symptoms indicating the need to discontinue the test, in accordance with the European Society of Cardiology recommendations, were noted.19 The 6MWT was performed according to published guidelines.20
Endpoints
The primary study hypothesis was that the benefits of HCTR would be maintained during an extended follow-up period (minimum, 14 months; maximum, 26 months after randomization) in patients randomized according to IS and NIS HF aetiology, resulting in an increased probability of a higher number of days alive and out of the hospital. The secondary outcomes were all-cause mortality, cardiovascular (CV) mortality, CV hospitalizations, HF hospitalizations, composite of all-cause mortality or all-cause hospitalization, composite of all-cause mortality or CV hospitalization, composite of all-cause mortality or HF hospitalization, and composite of CV mortality or HF hospitalization. Tertiary outcomes were defined as changes in functional status assessed using the New York Heart Association class, CPET duration, and 6MWT distance.15, 16
Statistical analysis
A total of 850 clinically stable, eligible patients in five centres in Poland were randomized in a 1:1 ratio (block size of 2, stratified by site) to either the HCTR + UC group or the UC-only group. All patients were followed up for a minimum of 12 months and a maximum of 24 months after the 9 week period (not later than 31 March 2019), with up to two check-up visits. The primary outcome, which was the number of days alive and out of the hospital, was compared between treatment arms (HCTR and UC) within each aetiology-based (IS and NIS) group using the Wilcoxon–Mann–Whitney rank sum test, and the P-value of the heterogeneity was obtained based on the independence of patients within each aetiology-based group (van Elteren test). The secondary time-to-event outcomes were compared using the log-rank test. Additionally, using Cox proportional hazards regression, with age, LVEF baseline, and depression as covariates, the heterogeneity of treatment effect was assessed using an interaction term between the aetiology-based groups and the treatment arms. The proportional hazards assumption was checked using the weighted Schoenfeld residuals. The probabilities of event-free survival were estimated by means of Kaplan–Meier methods. Continuous tertiary outcomes were compared using analysis of covariance, with adjustments for age, baseline LVEF, and severity of depression traits. All analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC, USA), and a P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Aetiological screening was performed to classify patients into the IS (n = 555) or NIS (n = 295) aetiology groups. Some patients with IS HF aetiology had a history of myocardial infarction (486/555), coronary percutaneous angioplasty (387/555), or coronary artery bypass surgery (140/555).
Within the aetiologies, there were no statistically significant differences between patients in the HCTR and UC arms in all variables, with the exception of LVEF, which was higher among IS patients randomized to the HCTR group (IS-HCTR) vs. those randomized to the UC group (IS-UC) (31.0 ± 6.9% vs. 29.7 ± 6.9%, P = 0.033).
The mean body mass index did not differ between the groups. It was 28.5 ± 4.7 kg/m2 in the IS-HCTR group, 29.1 ± 5.7 kg/m2 in the NIS patients randomized to the HCTR group (NIS-HCTR), 28.9 ± 4.4 kg/m2 in the IS-UC group, and 29.4 ± 5.3 kg/m2 in the NIS patients randomized to the UC group (NIS-UC). The baseline characteristics of the cohort, stratified by HF aetiology at inclusion, are presented in Table 1.
Primary and secondary outcome measures
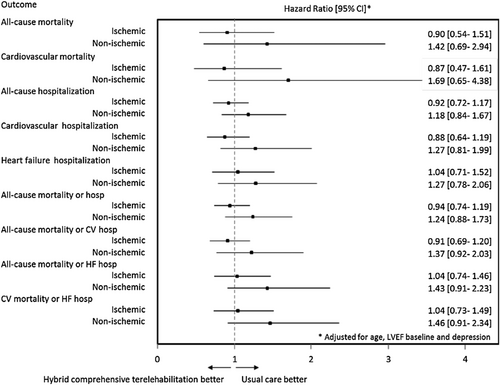
One hundred eight out of the IS-HCTR group (38.4%) and 120 out of the IS-UC group (43.8%) were hospitalized or died of CV causes. Fifty-eight out of the NIS-HCTR group (40.2%) and 59 out of the NIS-UC group (41.1%) were hospitalized or died of CV causes. During the course of the 26 months of follow-up, 37, 36, 17, and 16 patients in the IS-HCTR, IS-UC, NIS-HCTR, and NIS-UC groups, respectively, died. Further, hazard ratios comparing HCTR with UC for all-cause and CV mortalities, as well as for all-cause, CV, and HF hospitalizations, were not statistically significant for either aetiology and were not different between the aetiologies (Figure 1; Supporting Information, Table S2).
In the IS-HCTR, IS-UC, NIS-HCTR, and NIS-UC groups, the mean % of days out of the hospital (%DAOH was calculated as the ratio of the DAOH divided by the total possible days of follow-up for each patient) were 99.6, 99.6, 99.3, and 99.6 days, respectively, with no statistically significant differences (IS-HCTR vs. NIS-HCTR, P = 0.429; IS-UC vs. NIS-UC, P = 0.669). There were no differences between the treatment arms in either aetiology in the number of days alive and out of the hospital 26 months after the randomization, and there was no heterogeneity of effect between the aetiologies (Table 2).
| IS group | NIS group | P-value IS vs. NIS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IS-HCTR n = 281 | IS-UC n = 274 | P | P (DAOHHCTR > DAOHUC) | NIS-HCTR n = 144 | NIS-UC n = 151 | P | P (DAOHHCTR > DAOHUC) | HCTR | UC | P (DAOHHCTR > DAOHUC) | |
| %DAOH | 99.6 [97.0–100] | 99.6 [96.7–100] | 0.801 | 0.506 [0.549–0.552] | 99.3 [94.8–100] | 99.6 [96.3–100] | 0.369 | 0.471 [0.407–0.535] | 0.429 | 0.669 | 0.746 |
- %DAOH, % of days alive and out of hospital; IS, ischaemic; IS-HCTR, patients with ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; IS-UC, patients with ischaemic aetiology of heart failure randomized to the usual care group; NIS, non-ischaemic; NIS-HCTR, patients with non-ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; NIS-UC, patients with non-ischaemic aetiology of heart failure randomized to the usual care group; P (DAOHHCTR > DAOHUC), probability that hybrid comprehensive telerehabilitation extends %DAOH vs. usual care.
- The results are expressed as follows: %DAOH: median [Q1–Q3], P (DAOHHCTR > DAOHUC): mean [95% confidence interval].
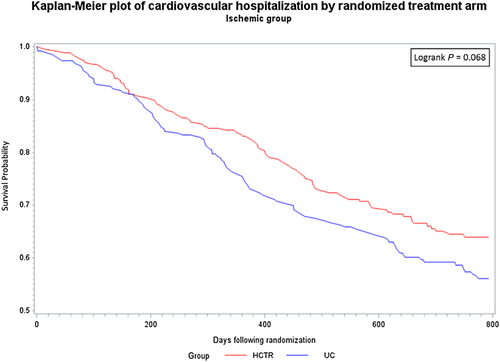
There were no statistically significant differences between probability of CV hospitalization rate over the entire observation period between the HCTR and UC groups for either aetiology. Similarly, there were no statistically significant differences between probability of CV mortality rate between the HCTR and UC groups or between the aetiologies over the entire observation period (Figures 2-5).
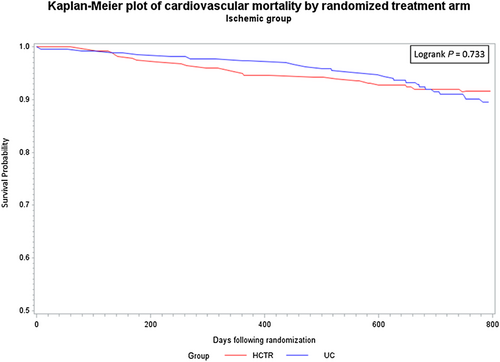
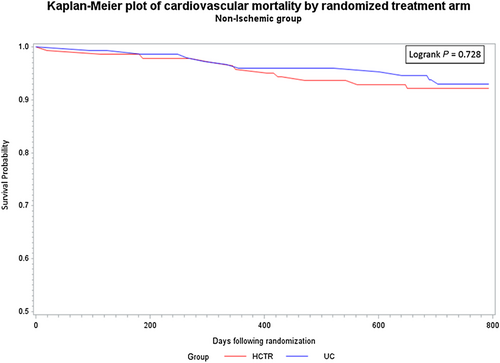
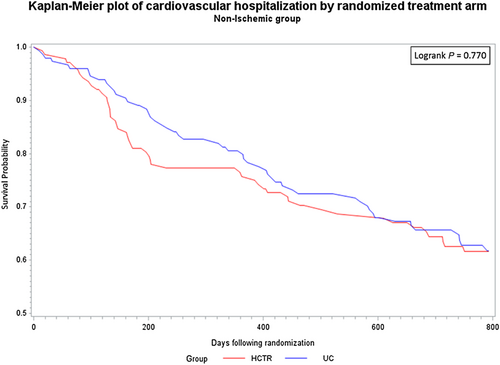
In Cox proportional hazards regression analysis, treatment was not independently associated with the secondary outcomes.For all-cause mortality, adjusted for age, baseline LVEF, and severity of depression, the adjusted hazard ratio for HCTR vs UC groups was 0.90 ( 95% CI, 0.54–1.51) for IS aetiology and 1.42 (95% CI, 0.69–2.94) for NIS aetiology, and there was no interaction between the groups and aetiology (p=0.316).
Tertiary outcome measures
After adjustments for age, baseline LVEF, and severity of depression traits, differences between HCTR and UC in terms of change in 6MWT (Δ6MWT) distance and change in CPET time after the 9 weeks were statistically significant in the IS arm, but not in the NIS arm. After 9 weeks, change in 6MWT distance from baseline was 34.6 [26.8–42.4] m in the IS-HCTR group and 17.9 [10.2–25.6] m in the IS-UC group (P = 0.015). Change in CPET time was 51.2 [39.7–62.6] s in the IS-HCTR group and 15.9 [4.5–27.2] s in the IS-UC group (P < 0.001). There were no differences between the aetiologies in the effect of HCTR and UC on other continuous outcomes from baseline to 9 weeks (Table 3). The corresponding P-values for the heterogeneity of effect between aetiologies were all above 0.05. The analyses performed without adjustments are presented in Supporting Information, Tables S3–S6.
| Ischaemic aetiology group | Non-ischaemic aetiology group | P-value interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Δ9 week value–baseline value Means [95% CI] | Difference [95% CI] | P | Δ9 week value–baseline value Means [95% CI] | Difference [95% CI] | P | ||||
| IS-HCTR | IS-UC | NIS-HCTR | NIS-UC | ||||||
| 6MWTD (m) | 34.6 [26.8; 42.4] | 17.9 [10.2; 25.6] | 16.7 [2.36; 31.0] | 0.015 | 37.0 [26.1; 47.9] | 28.6 [18.2; 39.2] | 8.4 [−11.2; 27.9] | 0.688 | 0.377 |
| CPET time (s) | 51.2 [39.7; 62.6] | 15.9 [4.5; 27.2] | 35.3 [14.3; 56.3] | <0.001 | 47.8 [31.9; 63.8] | 21.3 [6.0; 36.6] | 26.5 [−2.0; 55.2] | 0.079 | 0.528 |
| Peak VO2 (mL/kg/min) | 1.05 [0.51; 1.59] | 0.21 [−0.33; 0.74] | 0.84 [−0.15; 1.84] | 0.127 | 0.75 [−0.01; 1.51] | −0.08 [−0.80; 0.65] | 0.83 [−0.53; 2.18] | 0.393 | 0.979 |
| RER | 0.036 [0.023; 0.050] | 0.012 [−0.001; 0.025] | 0.024 [−0.001; 0.049] | 0.058 | 0.016 [−0.003; 0.035] | −0.015 [−0.033; 0.003] | 0.031 [−0.003; 0.064] | 0.088 | 0.685 |
| Logarithm NT-proBNP | −0.007 [−0.080; 0.067] | −0.040 [−0.113; 0.033] | 0.033 [−0.101; 0.169] | 0.918 | −0.047 [−0.150; 0.057] | −0.084 [−0.182; 0.014] | 0.037 [−0.146; 0.222] | 0.952 | 0.963 |
| LVEF (%) | 1.75 [1.15; 2.36] | 0.67 [0.06; 1.29] | 1.08 [−0.05; 2.20] | 0.066 | 1.70 [0.82; 2.57] | 1.29 [0.49; 2.08] | 0.41 [−1.12; 1.93] | 0.902 | 0.364 |
- 6MWTD, 6 min walk test distance; CI, confidence interval; CPET, cardiopulmonary exercise test; IS-HCTR, patients with ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; IS-UC, patients with ischaemic aetiology of heart failure randomized to the usual care group; LVEF, left ventricular ejection fraction; NIS-HCTR, patients with non-ischaemic aetiology of heart failure randomized to the hybrid comprehensive telerehabilitation group; NIS-UC, patients with non-ischaemic aetiology of heart failure randomized to the usual care group; NT-proBNP, N-terminal pro-brain natriuretic peptide; Peak VO2, peak oxygen uptake; RER, respiratory exchange ratio.
Discussion
The TELEREH-HF study demonstrated that the positive effects of the 9 week HCTR in HF patients did not lead to an increase in the number of days alive and out of the hospital and did not reduce mortality and hospitalization over the 14–26 months of follow-up.16 In this subanalysis of the TELEREH-HF trial, it was shown that HF aetiology did not influence the primary and secondary outcome measures in terms of improving prognosis. However, concerning tertiary outcomes, HCTR improved functional status alone in patients with IS HF aetiology; however, the magnitude of the changes in the clinical and functional statuses of HF patients did not differ between the IS and NIS groups. The clinical outcomes of the present study are consistent with those of other studies, despite differences in organizational models of telerehabilitation, modalities of monitoring, frequencies of exercise training, and the data transfer in specific centres.21, 22 It should be noted that an IS HF aetiology identifies patients with more severely impaired exercise capacity.23
In the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, which is a randomized controlled trial evaluating an exercise training programme plus UC vs. UC alone in HF subjects with reduced left ventricular function, there was no interaction between HF aetiology and treatment for the primary outcomes, CV mortality, CV hospitalization, CV mortality, or HF hospitalization. There was a significant interaction between aetiology and treatment for mortality; however, the interaction was no longer significant when adjustments were made with HF-ACTION adjustment model predictors. In the HF-ACTION study, the modest clinical benefit derived from supervised ET followed by the maintenance of home-based exercise, which was identified within the overall HF-ACTION cohort, was not significantly different between aetiologies (IS vs. NIS).24 However, the HF-ACTION and TELEREH-HF trials are different in terms of rehabilitation methodology (i.e. the new model of HCTR used in this study) and the supervision of home training (remote and unsupervised training in HF-ACTION vs. remotely supervised training in TELEREH-HF). The follow-up protocol in HF-ACTION was such that patients returned for facility-based training sessions every 3 months, whereas in the TELEREH-HF study, there were no such booster visits.15 The findings of the TELEREH-HF study should be considered a confirmation of the ‘Exercise training meta-analysis of trials in patients with chronic heart failure’ (ExTraMATCH) analysis, which showed no significant interaction between the effects of HCTR and aetiology because the adjusted analysis of ExTraMATCH did not reach significance and no other significant differences were identified for other endpoints.25 However, it should be noted that in the ExTraMATCH analysis, patients with chronic congestive HF or left ventricular dysfunction underwent exercise training in different places and using different modalities (stationary, ambulatory, and home based), unlike the TELEREH-HF trial, where only HCTR was used.
In a meta-analysis of randomized clinical trials performed by Imran et al., home-based cardiac rehabilitation and hybrid cardiac rehabilitation improved functional capacity compared with UC in patients with HF and were potentially good alternatives for patients who were ineligible for centre-based cardiac rehabilitation.26 In the INCARD (French acronym for ‘Insuffisance Cardiaque en Réadaptation Durable’) trial, which is a prospective, monocentric study, Koukoui et al. demonstrated that inpatient physical training followed by a home-based programme improved functional parameters in both IS and NIS cohorts over 24 months of follow-up.21 Arena et al. showed that in both IS and NIS patients, peak VO2 was a significant predictor of cardiac events.27 In the TELEREH-HF trial, improvement in functional status, defined as a significant increase in 6MWT distance and CPET time, was more evident in patients with an IS HF aetiology. Therefore, the results of the present study may have crucial prognostic value in the long-term observation of HF patients. Research conducted in the previous century and more recent research have demonstrated that cardiac rehabilitation in inpatient or outpatient centres may improve functional capacity, quality of life, and prognosis in patients with HF, possibly by improving the autonomic control of blood circulation and peripheral muscle blood flow, by reversing abnormalities of skeletal muscle metabolism, and by improving patients' perceptions of their quality of life and the severity of clinical symptoms.28, 29 The mechanism through which moderate exercise training improves the outcomes of patients with chronic HF has not been established, especially because in the present study, patients with an IS aetiology obtained better results than patients with an NIS aetiology. One possible explanation could be that moderate exercise training improves myocardial perfusion, and this improvement may be driven by a mechanism of vessel neoformation throughout intermittent ischaemia or by the vasodilation of pre-existing coronary vessels due to a reduction in endothelial dysfunction.23, 30, 31 One of the most notable findings of previous research concerning exercise in HF patients is that for the same level of left ventricular dysfunction, different degrees of exercise intolerance were found.32, 33 No significant changes in LVEF after exercise training were demonstrated in previous studies. Therefore, the findings of the current study are consistent with those of other studies.34, 35
Study limitations
Our study had several limitations. The main limitation was that we did not obtain detailed information on the aetiologies of the NIS group; that is, we did not determine the diagnosis in these patients by assessment of genetic test results or magnetic resonance imaging. Moreover, we did not evaluate the specific causes of HF in the patient population, which was mainly because of the several co-morbidities. Another study limitation was that only 11.5% of the participants were women; therefore, caution is required in extrapolating the study results to the female population.16
Conclusions
The trial showed no difference between HCTR and UC in the primary outcome of number of days alive and out of the hospital for either IS or NIS aetiology. Furthermore, the magnitude of changes in the clinical and functional statuses of HF patients did not differ by aetiology. In IS patients, HCTR might have beneficial effects on 6MWT distance and CPET time after 9 weeks; however, the effect was not statistically significantly different from that observed in NIS patients.
Acknowledgements
The authors thank all the medical and technology members of the TELEREH-HF team.
Conflict of interest
All authors declare that they were supported by the National Centre for Research and Development, Warsaw, Poland, grant number STRATEGMED1/233547/13/NCBR/2015.
Funding
This study was supported by the National Centre for Research and Development, Warsaw, Poland (grant number STRATEGMED1/233547/13/NCBR/2015).




