Complications of left ventricular assist devices causing high urgency status on waiting list: impact on outcome after heart transplantation
Abstract
Aims
Heart transplantation (HTx) represents optimal care for advanced heart failure. Left ventricular assist devices (LVADs) are often needed as a bridge-to-transplant (BTT) therapy to support patients during the wait for a donor organ. Prolonged support increases the risk for LVAD complications that may affect the outcome after HTx.
Methods and results
A total of 342 patients undergoing HTx after LVAD as BTT in a 10-year period in two German high-volume HTx centres were retrospectively analysed. While 73 patients were transplanted without LVAD complications and with regular waiting list status (T, n = 73), the remaining 269 patients were transplanted with high urgency status (HU) and further divided with regard to the observed leading LVAD complications (infection: HU1, n = 91; thrombosis: HU2, n = 32; stroke: HU3, n = 38; right heart failure: HU4, n = 41; arrhythmia: HU5, n = 23; bleeding: HU6, n = 18; device malfunction: HU7, n = 26). Postoperative hospitalization was prolonged in patients with LVAD complications. Analyses of perioperative morbidity revealed no differences regarding primary graft dysfunction, renal failure, and neurological events except postoperative infections. Short-term survival, as well as Kaplan–Meier survival analysis, indicated comparable results between the different study groups without disadvantages for patients with LVAD complications.
Conclusions
Left ventricular assist device therapy can impair the outcome after HTx. However, the occurrence of LVAD complications may not impact on outcome after HTx. Thus, we cannot support the prioritization or discrimination of HTx candidates according to distinct mechanical circulatory support-associated complications. Future allocation strategies have to respect that device-related complications may define urgency but do not impact on the outcome after HTx.
Introduction
Orthotopic heart transplantation (HTx) provides the best long-term treatment for patients suffering from end-stage heart failure (HF).1 As the waiting lists exceed the number of performed transplantations, a left ventricular assist device (LVAD) is commonly implanted as a bridge-to-transplant (BTT) therapy to support HF patients during their time on the waiting list.2-4 Consequently, an increasing number of HF patients with LVAD are transplanted today.5 Despite tremendous improvements in recent years, median survival of LVAD patients is still limited to about 2 to 5 years, which is a distinct impairment compared with HTx.2-5 A variety of different complications are reported in relation to LVAD therapy, with device infection, thromboembolism, and device malfunction as the most severe.6 In the Eurotransplant region, patients on LVAD support can only be listed with high urgency (HU) status if severe device complications occur. In contrast, LVAD patients are automatically listed with high priority in the USA, leading to significantly shorter support duration before HTx.3, 4 While global registry data do not suggest an impact of preoperative LVAD support on outcome after HTx, European data indicate significant impairment for LVAD patients.5, 7-9 In particular, in a previous published study of a comparable cohort of patients with and without previous LVAD support, we could show that LVAD patients suffered from impaired survival compared with primary transplanted ones.9 This discrepancy to the global registry data may be caused by a higher prevalence of patients with severe LVAD complications due to longer pre-HTx support time in the Eurotransplant region.3-5, 7-9 Patients with severe complications are more compromised while undergoing HTx, which increases the risk of perioperative morbidity and mortality.7, 10-12
This study aims to investigate the impact of the distinct LVAD complication categories accepted by the Eurotransplant audit committee for HU status on the outcome after HTx. We analysed perioperative morbidity and mortality, as well as survival. In addition, the outcome data of HU-status patients with HTx after LVAD complications were compared with regular T-status patients undergoing HTx after LVAD therapy without complications.
Methods
Ethics
The study followed the principles of the Declaration of Helsinki and was approved by the local ethics committees. All patients gave their informed consent for the scientific use of anonymized patient data prior to inclusion in the study.
Patients and study design
All patients (n = 384) with preoperative LVAD support undergoing orthotopic HTx in either one of two German high-volume cardiac surgery centres between January 2010 and April 2020 were retrospectively reviewed and analysed (Figure 1). A total of 342 adult patients were included and divided according to whether they were transplanted with regular waiting list status (T, n = 73) or with HU status (n = 269) due to LVAD complications. Patients were assigned to different study groups according to the observed leading LVAD complication: device infection excluding sole driveline infection (HU1, n = 91), pump thrombosis (HU2, n = 32), stroke/intracerebral bleeding (HU3, n = 38), right HF (HU4, n = 41), malignant arrhythmia (HU5, n = 23), other severe bleeding complications (HU6, n = 18), or device malfunction including levitation error, tear of inflow cannula, technical failure, and driveline defects (HU7, n = 26). The remaining n = 42 patients were excluded from the study. Thirty-three of those were infants, and nine patients suffered from multiple complications (e.g. aortic valve regurgitation in combination left ventricular aneurysm).
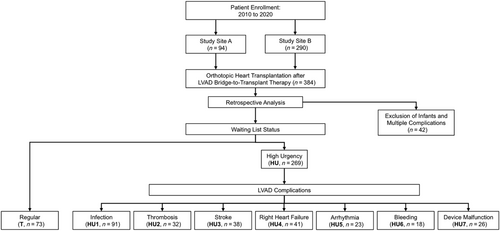
Study objectives and follow-up period
Patient and donor characteristics were evaluated and compared between the eight groups. Perioperative complications, as well as perioperative morbidity and mortality, were analysed. The follow-up examinations of the study cohort were performed on a regular basis every 3 to 6 months, with a mean follow-up period of 1270 ± 1080 days and a maximum follow-up of 3772 days.
Surgical procedure and perioperative management
Patients were either transplanted in orthotopic bicaval or Shumway technique. Anastomoses were sutured in the following order: left atrium, C. cava inferior, V. cava superior, pulmonary artery, and aorta. Immunosuppression regime consisted of a combination of tacrolimus, mycophenolate mofetil, and prednisolone. The transplant procedure and perioperative management was similar in both participating centres.
Statistics
Statistics were performed using SPSS Statistics 26 (IBM Corporation, Armonk, NY) and Prism 6 (GraphPad Software, La Jolla, CA). If possible, for dichotomous variables, two-tailed Fisher–Freeman–Halton tests were used. Because of the complexity of the calculation of eight groups, two-tailed χ2 tests were performed in cases where the software could not calculate the Fisher–Freeman–Halton test. Results of these cases are indicated throughout the manuscript. Continuous results were analysed by Kruskal–Wallis tests. All results are displayed in the corresponding tables. For statistically significant results (P < 0.05), additional post hoc analyses by Fisher's exact test and Bonferroni correction were calculated and are displayed in Supporting Information, Appendix S1. For survival analyses, Kaplan–Meier method and comparison by Mantel–Cox log-rank test were used. All results are displayed as mean with standard deviation and as a percentage of the whole.
Results
Recipient data
During the 10-year study period, a total of 342 LVAD patients who underwent HTx were included and divided into different groups differentiating their preoperative regular ‘T’ waiting list status or their ‘HU’ status justifying LVAD complication. In accordance with the study design, none of the T patients but all patients in the HU groups were listed with HU status (Table 1). There were no differences regarding the time on HU listing status before the HTx between the different HU groups. Patients in T and HU6 were significantly older than those in HU2, HU4, and HU7. Gender of the recipients was also not equally distributed throughout the different groups, with a proportion of male patients of up to 100% in HU7, but only 67.1% in the T group. In general, HU-status patients with LVAD complications were more likely to be male than regular T-status LVAD patients without complications. Consequently, height, weight, and body mass index were lower in the T group compared with the HU groups. However, calculated predicted heart mass mismatch showed no relevant differences between the different study groups. LVAD support duration ranged between 477 ± 450 (HU5) and 930 ± 502 days (HU7), reaching statistical significance. Most common implanted LVAD in all groups was Medtronic HeartWare™ HVAD™, followed by Abbott's HeartMate 2™ and HeartMate 3™ devices. Implanted devices were not distributed equally between the different groups. There were no significant differences regarding the incidence of diabetes of the recipients. As expected by the study protocol, patients of the T group had higher haemoglobin concentrations as the HU patients. Patients listed with HU status due to right HF suffered from higher bilirubin and liver enzymes and patients with pump thrombosis from higher lactate dehydrogenase values.
| Recipient variables | T | HU1 | HU2 | HU3 | HU4 | HU5 | HU6 | HU7 | P value |
|---|---|---|---|---|---|---|---|---|---|
| (n = 73) | (n = 91) | (n = 32) | (n = 38) | (n = 41) | (n = 23) | (n = 18) | (n = 26) | ||
| High urgency listing status, n (%) | 0 (0.0) | 91 (100.0) | 32 (100.0) | 38 (100.0) | 41 (100.0) | 23 (100.0) | 18 (100.0) | 26 (100.0) | <0.01 |
| Days on high urgency status | 100 ± 73 | 101 ± 76 | 123 ± 86 | 83 ± 68 | 98 ± 65 | 71 ± 51 | 122 ± 98 | 0.05 | |
| Age, y | 55.3 ± 12.7 | 50.6 ± 12.3 | 46.5 ± 13.2 | 51.4 ± 11.7 | 49.3 ± 13.4 | 51.8 ± 8.1 | 57.3 ± 8.2 | 48.7 ± 13.7 | <0.01 |
| Male gender, n (%) | 49 (67.1) | 84 (92.3) | 24 (75.0) | 31 (81.6) | 33 (80.5) | 11 (91.3) | 16 (88.9) | 26 (100.0) | <0.011 |
| Height, cm | 172 ± 8 | 178 ± 9 | 176 ± 8 | 178 ± 8 | 176 ± 10 | 180 ± 5 | 178 ± 8 | 179 ± 6 | <0.01 |
| Weight, kg | 74.0 ± 15.0 | 85.2 ± 17.8 | 83.7 ± 13.5 | 78.2 ± 13.4 | 80.2 ± 13.4 | 84.5 ± 12.3 | 89.8 ± 18.2 | 90.2 ± 13.4 | <0.01 |
| Body mass index, kg/m2 | 25.0 ± 4.5 | 26.7 ± 5.0 | 26.9 ± 3.6 | 24.5 ± 3.4 | 25.7 ± 3.6 | 26.0 ± 3.5 | 28.0 ± 4.3 | 28.1 ± 3.9 | <0.01 |
| Ventricular assist device | |||||||||
| Support duration, d | 612 ± 599 | 752 ± 750 | 660 ± 441 | 804 ± 513 | 568 ± 481 | 477 ± 450 | 638 ± 538 | 930 ± 502 | <0.01 |
| Abbott HeartMate 2™ | 11 (15.1) | 21 (23.1) | 8 (25.0) | 4 (10.5) | 12 (29.3) | 3 (13.0) | 2 (11.1) | 9 (34.6) | 0.14 |
| Abbott HeartMate 3™ | 10 (13.7) | 8 (8.8) | 0 (0.0) | 0 (0.0) | 4 (9.8) | 2 (8.7) | 5 (27.8) | 0 (0.0) | <0.01 |
| Medtronic HeartWare™ HVAD™ | 49 (67.1) | 45 (49.5) | 15 (46.9) | 25 (65.8) | 24 (58.5) | 12 (52.2) | 10 (55.6) | 7 (26.9) | 0.021 |
| Biventricular assistance, n (%) | 3 (4.1) | 14 (15.4) | 7 (21.9) | 6 (15.8) | 1 (2.4) | 3 (13.0) | 1 (5.6) | 4 (15.4) | 0.03 |
| Other | 0 (0.0) | 3 (3.3) | 2 (6.3) | 3 (7.9) | 0 (0.0) | 3 (13.0) | 0 (0.0) | 6 (23.1) | <0.01 |
| Risk factors | |||||||||
| Diabetes, n (%) | 14 (19.2) | 14 (15.4) | 4 (12.5) | 3 (7.9) | 4 (9.8) | 5 (21.7) | 3 (16.7) | 1 (3.8) | 0.42 |
| Nicotine abuse, n (%) | 14 (19.2) | 27 (29.7) | 15 (46.9) | 10 (26.3) | 9 (22.0) | 10 (43.5) | 7 (38.9) | 8 (30.8) | 0.081 |
| Preoperative laboratory values | |||||||||
| Haemoglobin, g/dL | 12.5 ± 2.3 | 10.6 ± 1.7 | 10.7 ± 2.0 | 11.6 ± 1.8 | 9.9 ± 1.3 | 10.6 ± 1.6 | 10.1 ± 2.3 | 10.8 ± 1.9 | <0.01 |
| Creatinine, mg/dL | 1.45 ± 1.24 | 1.35 ± 0.58 | 1.15 ± 0.33 | 1.22 ± 0.37 | 1.45 ± 0.71 | 1.34 ± 0.63 | 1.53 ± 0.60 | 1.56 ± 0.89 | 0.53 |
| Glomerular filtration rate, mL/min | 66.1 ± 27.9 | 65.2 ± 36.9 | 65.0 ± 23.1 | 73.7 ± 28.5 | 51.9 ± 28.6 | 64.7 ± 30.1 | 53.0 ± 23.4 | 71.1 ± 34.7 | 0.02 |
| Bilirubin, mg/dL | 0.80 ± 0.61 | 0.74 ± 0.50 | 0.87 ± 0.54 | 0.65 ± 0.33 | 1.24 ± 0.98 | 0.82 ± 0.69 | 0.57 ± 0.34 | 0.65 ± 0.35 | <0.01 |
| Lactate dehydrogenase, U/L | 335 ± 268 | 418 ± 458 | 569 ± 388 | 344 ± 294 | 390 ± 281 | 324 ± 233 | 296 ± 126 | 340 ± 240 | 0.05 |
| Aspertate aminotransferase, U/L | 40 ± 69 | 39 ± 31 | 40 ± 21 | 30 ± 15 | 47 ± 54 | 36 ± 25 | 31 ± 20 | 29 ± 15 | 0.19 |
| C-reactive protein, mg/dL | 2.45 ± 1.62 | 2.36 ± 4.05 | 2.86 ± 3.85 | 1.62 ± 2.97 | 3.57 ± 3.36 | 2.72 ± 4.09 | 0.92 ± 1.02 | 2.06 ± 1.64 | 0.01 |
| International normalized ratio, /1 | 2.14 ± 0.86 | 2.49 ± 0.56 | 2.70 ± 0.67 | 2.53 ± 0.58 | 2.06 ± 0.77 | 2.08 ± 0.75 | 2.26 ± 0.82 | 2.82 ± 0.76 | <0.01 |
| Partial thromboplastin time, s | 36 ± 9 | 40 ± 13 | 41 ± 16 | 40 ± 11 | 44 ± 11 | 41 ± 14 | 37 ± 11 | 38 ± 11 | <0.01 |
- Regular waiting list status: T, n = 73; LVAD complications causing high urgency status: infection: HU1, n = 91; thrombosis: HU2, n = 32; stroke: HU3, n = 38; right heart failure: HU4, n = 41; arrhythmia: HU5, n = 23; bleeding: HU69, n = 18; device malfunction: HU7, n = 26. Significant results of post-hoc pairwise comparison are displayed in Appendix 1.1 Because of the complexity of the calculation, Fisher-Freeman-Halton test could not be calculated by the software, and two-tailed chi-square tests were performed instead.
Donor data
Characteristics of the donors are displayed in Table 2. In general, donor data were comparable between the eight different study groups. In particular, incidence and potential duration of previous cardiopulmonary resuscitation and peak catecholamine levels showed minor differences. Because of the differences in gender, height, and weight of the allocated recipients, there were also some differences between the donors in order to avoid donor–recipient mismatches.
| Donor variables | T | HU1 | HU2 | HU3 | HU4 | HU5 | HU6 | HU7 | P value |
|---|---|---|---|---|---|---|---|---|---|
| (n = 73) | (n = 91) | (n = 32) | (n = 38) | (n = 41) | (n = 23) | (n = 18) | (n = 26) | ||
| Age, y | 46.3 ± 12.4 | 342.1 ± 12.5 | 40.2 ± 12.6 | 42.6 ± 11.6 | 42.3 ± 12.1 | 43.6 ± 10.2 | 45.0 ± 13.6 | 42.2 ± 13.8 | 0.28 |
| Male gender, n (%) | 23 (31.5) | 60 (65.9) | 17 (53.1) | 21 (55.3) | 21 (51.2) | 17 (73.9) | 9 (50.0) | 20 (76.9) | <0.011 |
| Height, cm | 170 ± 8 | 177 ± 8 | 175 ± 9 | 174 ± 8 | 174 ± 9 | 178 ± 8 | 175 ± 10 | 178 ± 9 | <0.01 |
| Weight, kg | 75.9 ± 14.1 | 80.4 ± 13.4 | 80.7 ± 14.4 | 77.0 ± 12.2 | 81.4 ± 15.2 | 81.5 ± 11.2 | 85.5 ± 21.3 | 82.6 ± 12.7 | 0.08 |
| Body mass index, kg/m2 | 26.0 ± 4.0 | 25.8 ± 4.3 | 26.3 ± 4.5 | 25.4 ± 3.9 | 26.7 ± 3.6 | 25.9 ± 3.9 | 27.9 ± 6.4 | 26.1 ± 3.6 | 0.67 |
| Donor CPR, n (%) | 12 (16.4) | 21 (23.3) | 5 (16.1) | 5 (13.2) | 12 (29.3) | 8 (34.8) | 6 (33.3) | 4 (16.0) | 0.25 |
| CPR duration, min | 12.9 ± 13.9 | 18.4 ± 18.4 | 20.2 ± 22.8 | 33.8 ± 16.4 | 19.2 ± 14.3 | 16.5 ± 8.8 | 29.6 ± 26.7 | 19.5 ± 14.6 | 0.45 |
| Peak catecholamines | |||||||||
| Dobutamine, μg/kg/min | 0.50 ± 1.55 | 0.32 ± 1.47 | 0.68 ± 2.62 | 0.49 ± 1.58 | 0.21 ± 0.78 | 0.0 ± 0.0 | 0.16 ± 0.58 | 0.0 ± 0.0 | 0.43 |
| Norepinephrine, μg/kg/min | 0.19 ± 0.21 | 0.20 ± 0.37 | 0.11 ± 0.13 | 0.13 ± 0.12 | 0.17 ± 0.27 | 0.19 ± 0.24 | 0.30 ± 0.72 | 0.08 ± 0.10 | 0.11 |
- Regular waiting list status: T, n = 73; LVAD complications causing high urgency status: infection: HU1, n = 91; thrombosis: HU2, n = 32; stroke: HU3, n = 38; right heart failure: HU4, n = 41; arrhythmia: HU5, n = 23; bleeding: HU69, n = 18; device malfunction: HU7, n = 26. Significant results of post-hoc pairwise comparison are displayed in Appendix 1. CPR, cardiopulmonary resuscitation.1 Because of the complexity of the calculation, Fisher-Freeman-Halton test could not be calculated by the software, and two-tailed chi-square tests were performed instead.
Perioperative morbidity and mortality
Graft ischaemia was significantly shorter in the T group compared with the HU groups. This was caused by shorter transport times and therefore limited to the cold ischaemia time (Table 3). Duration of mechanical ventilation and stay on intensive and intermediate care units showed no differences. In contrast, post hoc pairwise comparison revealed that the duration of the postoperative hospital stay was significantly shorter in T patients compared with each of the seven different HU groups, but showed no differences between the individual HU groups themselves.
| Recipient outcome | T | HU1 | HU2 | HU3 | HU4 | HU5 | HU6 | HU7 | P value |
|---|---|---|---|---|---|---|---|---|---|
| (n = 73) | (n = 91) | (n = 32) | (n = 38) | (n = 41) | (n = 23) | (n = 18) | (n = 26) | ||
| Predicted heart mass mismatch, % | −5.58 ± 15.93 | −8.47 ± 16.38 | −7.21 ± 18.29 | −6.83 ± 12.80 | −4.57 ± 13.98 | −5.99 ± 15.47 | −11.72 ± 16.66 | −10.01 ± 16.29 | 0.57 |
| Total ischaemic time, min | 221 ± 49 | 243 ± 55 | 239 ± 45 | 250 ± 46 | 240 ± 37 | 235 ± 35 | 255 ± 61 | 249 ± 42 | <0.01 |
| Cold ischaemic time, min | 161 ± 48 | 186 ± 52 | 183 ± 39 | 185 ± 39 | 180 ± 32 | 179 ± 29 | 193 ± 58 | 190 ± 31 | <0.01 |
| Warm ischaemic time, min | 60.6 ± 16.8 | 58.1 ± 15.3 | 58.0 ± 13.6 | 62.5 ± 25.5 | 58.6 ± 16.9 | 56.4 ± 16.4 | 63.1 ± 19.7 | 58.7 ± 16.3 | 0.95 |
| Total postoperative hospital stay, d | 50 ± 42 | 121 ± 88 | 125 ± 100 | 126 ± 80 | 142 ± 85 | 150 ± 93 | 119 ± 84 | 119 ± 105 | <0.01 |
| Mechanical ventilation, h | 169 ± 243 | 238 ± 426 | 355 ± 746 | 72 ± 103 | 237 ± 491 | 130 ± 196 | 242 ± 472 | 357 ± 786 | 0.39 |
| ICU/IMC stay, d | 18.9 ± 30.5 | 15.2 ± 18.4 | 25.5 ± 40.3 | 9.8 ± 14.5 | 26.0 ± 43.7 | 14.0 ± 21.9 | 21.9 ± 31.4 | 17.9 ± 32.4 | 0.15 |
| Primary graft dysfunction | |||||||||
| Requiring va-ECMO, n (%) | 17 (23.3) | 24 (26.4) | 7 (21.9) | 8 (21.1) | 10 (24.4) | 5 (21.7) | 3 (16.7) | 3 (11.5) | 0.90 |
| Support duration, d | 9.4 ± 6.0 | 11.8 ± 8.5 | 31.1 ± 48.6 | 7.3 ± 4.5 | 4.0 ± 2.8 | 8.6 ± 9.9 | 11.0 ± 2.0 | 8.3 ± 8.7 | 0.07 |
| Died on support, n (%) | 6 (35.3) | 6 (25.0) | 3 (42.9) | 4 (50.0) | 4 (40.0) | 2 (50.0) | 1 (33.3) | 0 (0.0) | 0.82 |
| Morbidity | |||||||||
| Delayed chest closure, n (%) | 13 (17.8) | 20 (22.0) | 7 (21.9) | 5 (13.2) | 12 (29.3) | 6 (26.1) | 5 (27.8) | 4 (15.4) | 0.65 |
| Re-exploration for bleeding, n (%) | 31 (42.5) | 30 (33.0) | 10 (31.3) | 13 (34.2) | 16 (39.0) | 8 (34.7) | 7 (30.4) | 11 (42.3) | 0.89 |
| Haemodialysis, n (%) | 40 (54.8) | 57 (62.6) | 23 (71.9) | 20 (52.6) | 29 (70.7) | 16 (69.6) | 11 (61.1) | 18 (69.2) | 0.47 |
| Neurological events, n (%) | 11 (15.1) | 15 (16.5) | 3 (9.4) | 7 (18.4) | 7 (17.1) | 4 (17.4) | 4 (22.2) | 3 (11.5) | 0.94 |
| Rejection > °1R, n (%) | 19 (26.0) | 35 (38.5) | 12 (37.5) | 15 (39.5) | 18 (43.9) | 9 (39.1) | 4 (22.2) | 12 (46.2) | 0.37 |
| Severe infection, n (%) | 9 (12.3) | 15 (16.5) | 1 (3.1) | 0 (0.0) | 1 (2.4) | 1 (4.3) | 3 (16.7) | 3 (11.5) | 0.02 |
| Survival | |||||||||
| 30-day survival, n (%) | 63/71 (88.7) | 85/91 (93.4) | 30/32 (93.8) | 33/38 (86.8) | 37/41 (90.2) | 21/23 (91.3) | 17/18 (94.4) | 26/26 (100.0) | 0.63 |
| 1-year survival, n (%) | 48/66 (72.7) | 68/88 (77.3) | 23/32 (71.9) | 26/37 (70.3) | 30/40 (75.0) | 16/22 (72.7) | 14/16 (87.5) | 18/26 (69.2) | 0.91 |
| 3-year survival, n (%) | 34/53 (62.2) | 38/60 (63.3) | 17/26 (65.4) | 20/32 (62.5) | 21/34 (61.8) | 10/17 (58.8) | 7/11 (63.6) | 17/26 (65.4) | 1.00 |
- Regular waiting list status: T, n = 73; LVAD complications causing high urgency status: infection: HU1, n = 91; thrombosis: HU2, n = 32; stroke: HU3, n = 38; right heart failure: HU4, n = 41; arrhythmia: HU5, n = 23; bleeding: HU69, n = 18; device malfunction: HU7, n = 26. As not all included patients of a study groups reached all the displayed time periods of the survival analyses, numbers of the group sizes at the different follow-up dates are listed in the table. Significant results of post-hoc pairwise comparison are displayed in Appendix 1. ICU, intensive care unit; IMC, intermediate care unit; va-ECMO, veno-arterial extracorporeal membrane oxygenation.
Postoperative mechanical assistance because of primary graft dysfunction was needed in 12% to 26% of all cases and did not show any differences between the different groups. In addition, support duration and mortality during mechanical assistance did not differ either.
Analyses of severe perioperative complications revealed no differences between the study groups regarding neurological complications like stroke and transient ischaemic attacks, acute kidney injury requiring haemodialysis, early graft rejection (>°1R), and delayed chest closure and thoracic re-exploration for severe bleeding complications. In contrast to that, severe infections defined as sepsis, pneumonia, or infective wound healing disorders during the postoperative stay after HTx were the most likely in T, HU1, and HU6 patients.
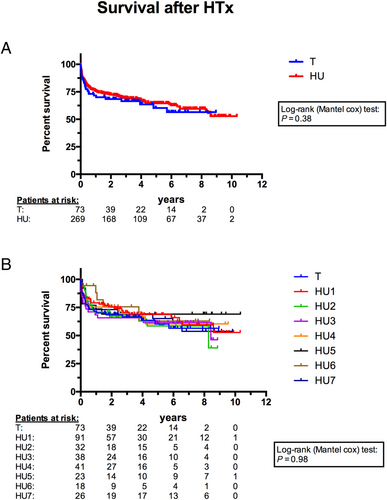
The 30-day survival was about 90% in all groups and showed no intergroup differences. Follow-up after 1 and 3 years again revealed no significant differences between the eight different study groups (P = 0.91 and P = 1.0, respectively). In particular, there was no disadvantage for patients with HTx after LVAD complications (HU1–7) compared with those without (T). Additionally, the different kinds of observed LVAD complications did not affect the outcome either.
Survival analyses
The mean follow-up period was 1270 ± 1080 days and the maximum follow-up was 3772 days for the whole cohort. Figure 2 displays the Kaplan–Meier curves for the T group and all patients with device-related complications causing HU status. Figure 2 displays the curves for all eight study groups focussing on the impact of different types of complications. A total of 122 patients (T, n = 26; HU1, n = 30; HU2, n = 14; HU3, n = 15; HU4, n = 14; HU5, n = 7; HU6, n = 5; HU7, n = 11) died during the follow-up period. The remaining patients (n = 220) were censored on the date last seen. In line with the short-term results, survival analyses did not find any significant differences between the groups.
Discussion
This two-centre approach aims to investigate the impact of different LVAD complications on the outcome after HTx. We analysed 342 consecutive patients who underwent HTx after BTT-LVAD implantation. While 73 regular T-status patients were transplanted without preoperative HU status justifying LVAD complication, the remaining 269 patients suffered from either one or more of seven different complications and were transplanted in HU status. Considering our previously published data, we could show that LVAD support generally affects the outcome after HTx, but the previous occurrence of LVAD device complications does not further impair the long-term results after transplantation.9
In the present study, we retrospectively compared patients without LVAD complications with seven different groups defined by those life-threatening LVAD complication categories accepted for HU status by the Eurotransplant audit committee. In line with previous literature, device infection (HU1) was the most common LVAD complication at the time of HTx.3, 4 About 80% of the transplanted LVAD patients suffered preoperatively from ‘HU’ status justifying LVAD complications, which is a much higher incidence when compared with data previously reported.3, 4, 7, 12 This might be caused by the relatively long LVAD support duration in our cohort, with a minimum mean support of 1.3 years (subgroup with arrhythmia) and a maximum mean support of 2.5 years (subgroup with device malfunction).7, 8 Most likely, the dramatic donor organ shortage in Germany is the reason for both the prolonged LVAD support durations and, thereby, the cumulative increase in LVAD complication incidence in transplanted LVAD patients. Based on the different prioritization of LVAD patients on the waiting list for HTx in Europe and the USA and the small likelihood of a prompt HTx, Reineke and Mohacsi13 even postulate that, in Germany, the decision to implant an LVAD nearly equals the concept of destination therapy. In fact, the latest consensus statement of the European Association for Cardio-Thoracic Surgery suggests no longer differentiating between a BTT or destination therapy indication for durable mechanical circulatory support (MCS) implantation.14-20 In the majority of HU subgroups, support duration exceeded the mean waiting time of the T group, which indicates that the prevalence of LVAD complications increases with the support duration. With the current Eurotransplant regulations, only one out of five LVAD patients with BTT could be transplanted before the occurrence of severe device-related complications in our cohort.
The baseline characteristics of the recipients showed no differences regarding the incidence of diabetes as a factor for impaired prognosis, but for kidney function and demographics.15, 16 Interestingly, patients of the T group were much more likely to be female and older than most of the HU patients. While data by Ahmed et al.17 indicated no correlation between gender and the incidence of perioperative complications of the implantation of modern continuous-flow LVAD, American data by DeFilippis et al.18 and Gruen et al.19 reported impaired results with increased device-related complications and decreased survival for female patients with CF-LVAD support compared with male ones. Therefore, our results could be caused by the fact that body weight and height of female patients increase the chance of an organ offer without HU status by rescue allocation of the Eurotransplant allocation protocol.20 This might be responsible for the differences regarding sex, height, body weight, and body mass index between the T and the HU groups, as well as for the support duration of the recipients. In line with this, Hsich et al.21 reported similarly positive effects of female gender for non-HU listed HF patients and Chen et al.22 for lower body weight on the waiting list in the United Network for Organ Sharing (UNOS) area. Nonetheless, we did not observe differences in the predicted heart mass mismatch as highlighted by the International Society for Heart and Lung Transplantation as an important predictor for donor and recipient size match.23
Cold ischaemic times were significantly shorter in the T group than in any of the HU groups. This was caused by shorter transport times, which is again the consequence of the Eurotransplant allocation procedure, with rescue offers for T patients being offered locally to centres nearby the donor's hospital.20 However, the warm ischaemic times, which are known to significantly impact on impaired graft function and increased perioperative morbidity and mortality, did not differ between the eight study groups.24 While mechanical ventilation and intensive/intermediate care unit stay showed no significant differences, there was a tremendous reduction in total postoperative hospital stay for the T group compared with the HU groups. The hospital stay is a relevant parameter for both postoperative patient recovery and the socio-economic impact of the treatment.25, 26 HTx patients with preoperative LVAD complications may experience a prolonged postoperative recovery phase compared with those without complications. This, however, will require more detailed investigations.
The majority of the most common perioperative morbidity was comparable between the different groups. In particular, neurological events such as transient ischaemic attacks or even strokes, which are one of the most severe complications after HTx, were distributed equally between the different groups. Nonetheless, we observed significant differences with regard to the postoperative incidence of severe infective diseases, such as sepsis, pneumonia, or infective wound healing disorders. The incidence was the highest in patients with LVAD infections and LVAD bleeding complications and T patients. While a correlation between LVAD infections and postoperative infective constellations might be mediated by systemic inflammation, a correlation with bleeding events and T group is not completely understood at present.10, 27 We can only suggest a role of the acquired von Willebrand syndrome, frequently observed in patients with prolonged durable MCS.28
Primary graft dysfunction is a common problem in HTx and associated with impaired survival.29 In our cohort, we did not see any differences with regard to the incidence of primary graft dysfunction that could eventually impact the survival rates of the different patient groups studied. Survival analyses with Kaplan–Meier method indicated comparable results for T patients, as well as for patients with primarily underlying causes for granted HU status. One could speculate whether the incidence of primary graft dysfunction after HTx relates to previous LVAD therapy in general, but this requires comparison with non-LVAD HTx patients, not performed herein.
The most important finding of this present investigation is the fact that we did not observe any significant alterations of the postoperative survival regarding particular types of LVAD complications. These results have to be particularly acknowledged in view of designing novel allocation policies and implementation of benefit scores (e.g. IMPACT score).30 As our data showed, there are no reasons for the prioritization or discrimination of specific kinds of LVAD complications. LVAD therapy-associated complications may very well define urgency, but they obviously do not impact on perspective transplant benefit. In line with this, Healy et al.31 also reported no differences in the postoperative survival after HTx between patients with previous device-related complications and stable LVAD patients in the UNOS area. However, the different allocation policies allowing stable LVAD patients to be listed in UNOS status A1 for discretionary 30 days during the reported study periods limit the comparability to our data.
Compared with global registry data, survival after HTx was herein clearly inferior in all groups. This most probably relates to the previously discussed relatively long LVAD support times of HTx candidates in the Eurotransplant region, the distinct regional allocation policies, and ultimately, to the dramatically low organ donation rates within the distinct Eurotransplant region.2-4, 7, 8, 13 Compared with our previously published single-centre data on the impact of LVAD therapy on the outcome after HTx, we found similar results for the LVAD patients in this two-centre approach.9 As a consequence, outcome after HTx in LVAD patients may not be affected by the occurrence of device-related complications but is endangered simply due to the prior implantation of a durable MCS device.9
These results do also impact the ethical concerns of transplant allocation policies that need to balance the principles of equity, justice, beneficence, and utility in organ distribution.32 For an allocation procedure focussing on a benefit principle, it is essential to identify patients with the highest urge but also the highest potential for long-term survival.33
Limitations
Although we included about 350 LVAD patients from two different experienced centres, the large variations in the nature of complications on LVAD support led to relatively small subgroup sizes. Therefore, we were not able to perform propensity score matching, which causes unbalanced group sizes and differences regarding baseline parameters of the recipients, such as gender, height, and body weight, implanted pump types, as well as the donor parameters. In addition, for most of the patients, the follow-up period was relatively small, which impacts on the statistical power of the survival analyses and potentially overestimated the mortality due to the disproportionately high mortality in the first year after HTx. Nevertheless, we were able to present various new data that may help to improve the current therapy regimen for LVAD-supported HTx candidates in the future.
Conclusions
Heart transplantation remains the gold standard treatment option for end-stage HF patients, yielding good long-term outcome, superior to durable MCS therapy, but survival after HTx is impaired in LVAD patients. Therefore, HTx candidates with durable MCS have to be carefully selected with respect to the net benefit of transplanted organs. The presented data show that LVAD-associated complications may not affect outcomes after HTx. Thus, we cannot support the prioritization or discrimination of HTx candidates according to distinct MCS-associated complications. Future allocation strategies have to respect that device-related complications may define urgency but do not impact on the outcome after HTx. Rather, the presence of a durable MCS device may per se negatively affect outcomes.
Acknowledgements
Sarah L. Kirkby, as a native English speaker, has corrected the manuscript for the written language.
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
None declared.
Funding
The authors did not receive any funding for this study.




