Impact of QRS morphology on response to conduction system pacing after atrioventricular junction ablation
Abstract
Aims
His–Purkinje conduction system pacing (HPCSP) utilizing His (HBP) or left bundle branch pacing (LBBP) in patients with atrial fibrillation (AF) and wide QRS duration has not been well studied. We assessed the benefit of left bundle branch block (LBBB) correction during HPCSP in AF patients undergoing atrioventricular junction (AVJ) ablation with LBBB, compared with those with narrow QRS duration.
Methods and results
This is an observational study in consecutive patients with typical LBBB or narrow QRS duration in whom we attempted HPCSP after AVJ ablation for refractory AF with a left ventricular ejection fraction (LVEF) ≤ 50%. Echocardiographic responses and clinical outcomes were assessed at baseline and during 1 year of follow-up. A total of 178 patients were enrolled, of which 170 achieved AVJ ablation + permanent HPCSP (age 69.3 ± 10.1 years; LVEF 34.3 ± 7.7%), 133 (78.2%) patients had a narrow QRS duration, and 37 (21.2%) had an LBBB. The QRS duration changed from a baseline of 159.7 ± 16.6 ms to a paced QRS duration of 110.4 ± 12.7 ms in the LBBB cohort and from 95.6 ± 10.4 to 100.8 ± 14.5 ms (both P < 0.001) in the narrow QRS cohort after AVJ ablation and pacing. Compared with the narrow QRS cohort, the LBBB cohort showed a greater absolute increase in LVEF (+22.3% vs. +14.2%, P < 0.001), higher super responder rate (71.4% vs. 49.2%, P = 0.011), and greater New York Heart Association (NYHA) class improvement (−1.9 vs. −1.4, P < 0.001) at 1 year.
Conclusion
Patients with LBBB have greater improvement in LVEF and NYHA class function than patients with narrow QRS from HPCSP after AVJ ablation.
Introduction
The benefit of cardiac resynchronization therapy (CRT) with biventricular pacing (BVP) in heart failure is strongly dependent on QRS morphology and duration in sinus rhythm. Patients with significant QRS prolongation (>150 ms) and left bundle branch block (LBBB) benefit more from BVP-CRT compared with those with non-LBBB,1 while patients with a narrow QRS duration do not demonstrate improved clinical outcomes with BVP.2 However, the benefits of BVP-CRT after atrioventricular (AV) junction (AVJ) ablation are significantly less in patients with atrial fibrillation (AF) compared with sinus rhythm,3 which may be related to non-physiological ventricular pacing and loss fusion of native right ventricular activation.4, 5
In such situations, CRT utilizing His–Purkinje conduction system pacing (HPCSP) offers greater advantages compared with BVP,6 especially following AV node ablation.7 It has demonstrated the feasibility and effectiveness of HPCSP in those with narrow QRS who undergo AVJ ablation for refractory AF.8, 9 However, few studies have compared the response to HPCSP in patients with a wide QRS duration. In the present study, we assessed the benefit of LBBB correction by HPCSP in AF patients undergoing AVJ ablation, compared with those with a narrow QRS duration.
Methods
Study population
This was a single-centre, observational study. From August 2012 to August 2019, patients who met the following criteria were consecutively enrolled: (1) persistent or long-standing persistent AF patients who had symptomatic heart failure with left ventricular (LV) ejection fraction (LVEF) ≤ 50% even after ventricular rate control with medication; (2) the intrinsic QRS morphology was narrow (≤120 ms) or typical LBBB, which strictly met Strauss criteria10; (3) agreed to undergo AVJ ablation and permanent His (HBP) or left bundle branch pacing (LBBP); (4) and patients were at least 18 years old, not pregnant, and had a life expectancy of more than 12 months. Beginning in June 2017, LBBP was offered as either a first-line treatment or a rescue strategy to failed HBP. The operators discussed the benefits, risks, and alternatives to conduction system pacing before obtaining consent. The study protocol was approved by the Institutional Review Board of The First Affiliated Hospital of Wenzhou Medical University. All patients gave written informed consent.
Pacemaker implantation and atrioventricular node ablation
The implantation procedures of HBP and LBBP were described in our previous studies.11-13 Successful HBP in narrow QRS was defined as the same QRS morphology as the native QRS; successful correction of LBBB was defined by a paced electrocardiogram morphology without LBBB.11 LBB capture was confirmed by our previously reported criteria for LBB conduction system pacing.12, 14
We performed AVJ ablation after successful HBP/LBBP at the same procedure.6 An 8.5-Fr non-deflectable sheath (SR0 or SL1; St Jude Medical) or a deflectable sheath (Agilis; Abbott Electrophysiology) was inserted through the femoral vein to the AVJ keeping more than 8 mm distance from the distal pacing electrode. Complete AV block was achieved by radiofrequency ablation using a quadripolar 7-Fr 4 mm tip ablation catheter (Therapy Cool Flex, St. Jude Medical Inc or Celsius, Biosense Webster Inc). If the HBP threshold increased by >1 V/0.5 ms, the pacing lead was repositioned. The success of AVJ ablation was evident with complete AV block.
All patients had single site pacing using HBP or LBBP lead. The lower pacing rate was initially set at 80 bpm; the rate was decreased to 70 bpm at 1 to 3 months after the procedure according to patient cardiac function and echocardiography measurements. When a right ventricular or LV lead was implanted, it was programmed to pace with a long delay so that pacing would occur only if there was loss of capture from the HBP/LBBP lead; programming as back-up pacing was based on whether HBP/LBBP pacing alone ensured patient safety.
Data collection and clinical follow-up
Primary endpoint was the evaluation of LVEF estimated using Simpsons' method. Echocardiograms were assessed by two experienced echocardiographers blinded to study design. Secondary endpoints were the evaluation of a composite clinical endpoint (all-cause mortality, heart transplantation, or hospitalization for heart failure) during follow-up from computer-based patient records or during a follow-up patient visit. The diagnosis of heart failure required signs and symptoms consistent with congestive heart failure that was responsive to intravenous diuretic or inotropic therapy.
Patients received guideline-directed medical therapy and were assessed at outpatient visits 6 months and then annually. Echocardiographic parameters such as LV end-systolic volume (LVESV) and LV end-diastolic volume, mitral and tricuspid valve regurgitation graded (severity level 0–3), brain natriuretic peptide (BNP) levels, New York Heart Association (NYHA) functional class, and cardiothoracic ratio from chest X-ray were collected.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation or median (interquartile range). Paired t-test or Wilcoxon signed-ranks test were used to compare the differences between two time points during the follow-up. Categorical data were described as number (%), and χ2 test or Fisher's exact test was used to examine the above-mentioned differences. Logistic regression analysis was used to assess for predictors of super response. Kaplan–Meier curves were constructed to compare event-free survival for the composited clinical endpoint between the two groups. The log-rank test was used to determine significance. Significance was defined as P < 0.05. The data managements and analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA).
Result
Baseline characteristics
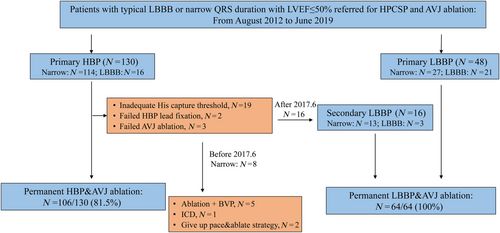
Figure 1 shows a total of 178 patients who had attempted AVJ ablation and HPCSP and 170 who received permanent HPCSP (106 HBP and 64 LBBP) after successful AVJ ablation. A total of 133 (78.2%) patients had QRS ≤ 120 ms, and 37 (21.8%) had LBBB. The baseline characteristics are shown in Table 1. Patient characteristics were similar between the two groups. HBP was more often performed in patients with a narrow QRS duration, compared with those with LBBB (69.9% vs. 35.1%, P < 0.001).
| Total | Narrow QRS | LBBB | P value | |
|---|---|---|---|---|
| N | 170 | 133 | 37 | |
| Age, y, mean ± SD | 69.3 ± 10.1 | 69.0 ± 10.1 | 70.5 ± 10.1 | 0.407 |
| Male, n (%) | 103 (60.6%) | 86 (64.7%) | 17 (45.9%) | 0.039 |
| HBP | 106 (62.4%) | 93 (69.9%) | 13 (35.1%) | <0.001 |
| LBBP | 64 (37.6%) | 40 (30.1%) | 24 (64.9%) | <0.001 |
| BMI | 24.0 ± 3.2 | 23.9 ± 3.4 | 24.4 ± 2.7 | 0.434 |
| Hypertension, n (%) | 102 (60.0%) | 78 (58.6%) | 24 (64.9%) | 0.495 |
| Diabetes, n (%) | 43 (25.3%) | 33 (24.8%) | 10 (27.0%) | 0.784 |
| Renal dysfunction, n (%) | 42 (24.7%) | 35 (26.3%) | 7 (18.9%) | 0.356 |
| ICM, n (%) | 22 (12.9%) | 17 (12.8%) | 5 (13.5%) | 0.907 |
| PCI | 23 (13.5%) | 16 (12.0%) | 7 (18.9%) | 0.279 |
| Intrinsic QRS duration, ms | 109.9 ± 30.1 | 95.6 ± 10.4 | 161.3 ± 19.4 | <0.001 |
| Intrinsic heart rate, bpm | 90.8 ± 17.9 | 91.1 ± 18.1 | 89.7 ± 17.2 | 0.675 |
| Baseline LVEF, % | 34.3 ± 7.7 | 34.7 ± 7.9 | 32.9 ± 6.8 | 0.195 |
| NYHA functional class | 2.9 ± 0.6 | 2.9 ± 0.6 | 3.0 ± 0.5 | 0.23 |
| Medicine | ||||
| Beta-blockers | 147 (86.5%) | 115 (86.5%) | 32 (86.5%) | 0.997 |
| ACE inhibitors/ARB | 135 (79.4%) | 102 (76.7%) | 33 (89.2%) | 0.096 |
| Diuretics | 157 (92.4%) | 122 (91.7%) | 35 (94.6%) | 0.562 |
| Aldosterone antagonist | 146 (85.9%) | 114 (85.7%) | 32 (86.5%) | 0.905 |
| Digoxin | 49 (28.8%) | 37 (27.8%) | 12 (32.4%) | 0.584 |
| Device type | 0.135 | |||
| Dual chamber PM, n (%) | 39 (22.9%) | 35 (26.3%) | 4 (10.8%) | |
| CRT-P, n (%) | 42 (24.7%) | 32 (24.1%) | 10 (27.0%) | |
| CRT-D, n (%) | 89 (52.4%) | 66 (49.6%) | 23 (62.2%) |
- ACE angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CRT, cardiac resynchronization therapy; HBP, His bundle pacing; ICM, ischaemic cardiomyopathy; LBBP, left bundle branch pacing; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
QRS duration and pacing parameters
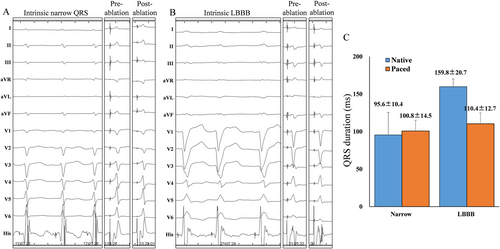
In the LBBB cohort, 76.9% (10/13) achieved selective HBP and 54.1% (13/24) achieved selective LBBP, while in narrow QRSd, the selective HBP and LBBP rates were 87.1% (81/93) and 67.5% (27/40), respectively. Figure 2 shows that the paced QRSd in patients with narrow QRS duration was 100.8 ± 14.5 ms similar to the intrinsic one (95.6 ± 10.4 ms), while there is a mean reduction of 50.4 ± 21.6 ms in LBBB patients with HPCSP. Also, a greater reduction in QRSd was observed in LBBB patients in both HBP and LBBP (Table 2).
| Narrow | LBBB | |||||
|---|---|---|---|---|---|---|
| HBP | LBBP | P | HBP | LBBP | P | |
| Intrinsic QRSd | 95.3 ± 10.6 | 96.3 ± 10 | 0.626 | 169.5 ± 22.4 | 156.9 ± 16.3 | 0.056 |
| Paced QRSd | 95.8 ± 13.6 | 112.3 ± 9 | <0.001 | 103.8 ± 13 | 114.1 ± 10.7 | <0.001 |
| Threshold | 0.93 ± 0.67 | 0.42 ± 0.13 | <0.001 | 1.6 ± 0.85 | 0.54 ± 0.17 | <0.001 |
| R amplitude | 2.7 ± 2.4 | 12 ± 5.3 | <0.001 | 4.2 ± 5.1 | 10.1 ± 5.3 | <0.001 |
- HBP, His bundle pacing; LBBB, left bundle branch block; LBBP, left bundle branch pacing.
The pacing threshold and R-wave amplitude are shown in Table 2. Compared with HBP, LBBP had lower conduction capture threshold in narrow QRSd cohort and lower LBBB correction threshold in LBBB cohort. The mean R-wave amplitude in the LBBP group was 12 ± 5.3 and 10.1 ± 5.3 mV in narrow QRSd and LBBB cohort, both higher than that in the HBP group (2.7 ± 2.4 and 4.2 ± 5.1 mV).
Echocardiographic and clinical outcomes in patients
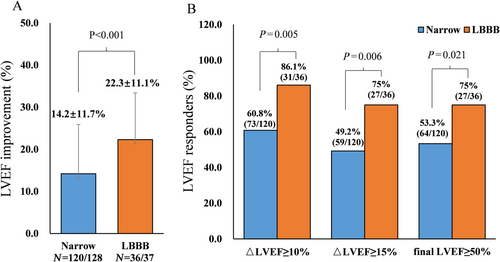
Of 170 patients, 156 finished 1-year follow-up with five deaths and nine lost to follow-up. The changes in LVEF at 12 months grouped by QRS morphology are shown in Figure 3. For the LBBB cohort, there was a 22.3% mean increase in LVEF, whereas in the narrow QRS cohort, this increase was only 14.2% (P < 0.001). The super responder rate (defined as absolute LVEF increase ≥15%) at 1-year follow-up was greater in the LBBB group (75.0%, 27/36) than in the narrow QRSd group (49.2%, 59/120). Rates of normalization of LVEF at 1 year were 75.0% (27/36) and 53.3% (64/120) in LBBB and normal QRS groups, respectively (P = 0.021). As shown in Supporting Information, Figure S1, HBP resulted in an increase in LVEF of +15.6% and +23.2% in patients with narrow QRS and LBBB, while the increases were 13.2% and 22.7% during LBBP. HBP shows a slightly absolute higher but not statistically significant improvement in LVEF.
Both of the groups demonstrated significant improvements in NYHA functional class, BNP levels, cardiothoracic ratio, and LVESV when compared with the baseline, while patients with intrinsic LBBB showed significantly greater improvements in LVESV and LV end-diastolic volume, NYHA class, and BNP and a trend towards improvements in left atrial size, tricuspid regurgitation, and cardiothoracic ratio compared with the narrow QRS duration group (Table 3).
| Narrow, N = 120 | LBBB, N = 36 | P values | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 year | Change | Baseline | 1 year | Change | ||
| LVEF, % | 35 ± 8 | 49.1 ± 13.2** | 14.2 ± 11.7 | 33.1 ± 6.7 | 55.4 ± 10.3** | 22.3 ± 11.1 | <0.001 |
| LVEDV, mL | 152.5 ± 50.6 | 129.1 ± 53.2** | −22.8 ± 51.2 | 173.9 ± 70.8 | 131.2 ± 54.1** | −44.7 ± 49.2 | 0.028 |
| LVESV, mL | 99.7 ± 40.8 | 72.5 ± 48.6** | −27.5 ± 40.0 | 113 ± 55.7 | 61.7 ± 33.2** | −52.6 ± 46.8 | 0.002 |
| LA, mm | 52.6 ± 5.9 | 50.8 ± 6.1* | −1.7 ± 4.4 | 50.5 ± 7.3 | 48.3 ± 8.0 | −2.2 ± 4.2 | 0.522 |
| MR | 1.90 ± 0.85 | 1.28 ± 0.80** | −0.60 ± 0.83 | 1.74 ± 0.90 | 1.20 ± 0.67** | −0.58 ± 0.91 | 0.903 |
| TR | 1.63 ± 0.89 | 1.16 ± 0.87** | −0.41 ± 0.98 | 1.76 ± 0.84 | 1.05 ± 0.71** | −0.65 ± 1.03 | 0.23 |
| NYHA class | 2.8 ± 0.6 | 1.5 ± 0.7** | −1.4 ± 0.8 | 3.0 ± 0.5 | 1.1 ± 0.3** | −1.9 ± 0.6 | <0.001 |
| BNP, pg/dL | 507 (236–1016) | 251 (134–482)** | −177 (−574 to 66) | 939 (452 to 1677) | 248 (108–514)* | −668 (−1457 to 139) | <0.001 |
| CTR, % | 64.3 ± 6.1 | 61.1 ± 6.4** | −3.1 ± 6.4 | 64.9 ± 5.1 | 59.9 ± 6.8** | −4.8 ± 5.4 | 0.203 |
- BNP, brain natriuretic peptide; CTR, cardiothoracic ratio; LA, left atrial size; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MR, mitral valve regurgitation; NYHA, New York Heart Association; TR, tricuspid valve regurgitation.
- P, comparison between the change of baseline and 1-year follow-up between two groups.
- * P < 0.05, compared with baseline.
- ** P < 0.01, compared with baseline.
Multivariate logistics regression analysis showed that intrinsic LBBB, chronic kidney disease, and faster intrinsic ventricular rate were associated with the rate of super LVEF response, with odds ratio (95% confidence interval) of 3.93 (1.54–10.07), 0.33 (0.14–0.76), and 1.02 (1–1.05), respectively (Table 4).
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| LBBB/narrow | 3.1 | 1.35–7.15 | 0.008 | 3.93 | 1.54–10.07 | 0.004 |
| Age | 1.01 | 0.97–1.03 | 0.994 | 1.01 | 0.97–1.05 | 0.77 |
| HBP/LBBP | 0.93 | 0.49–1.78 | 0.836 | 0.54 | 0.25–1.18 | 0.122 |
| Diabetes | 0.82 | 0.39–1.72 | 0.597 | 0.72 | 0.31–1.67 | 0.446 |
| CKD | 0.43 | 0.2–0.91 | 0.028 | 0.33 | 0.14–0.76 | 0.01 |
| LVEF | 0.98 | 0.94–1.03 | 0.432 | 0.99 | 0.94–1.04 | 0.595 |
| ICM | 0.79 | 0.3–2.12 | 0.642 | 0.77 | 0.26–2.31 | 0.641 |
| Hypertension | 1.06 | 0.55–2.03 | 0.861 | 1.16 | 0.56–2.4 | 0.698 |
| NYHA | 0.93 | 0.53–1.61 | 0.785 | 0.67 | 0.36–1.27 | 0.221 |
| BMI | 1.05 | 0.95–1.17 | 0.319 | 1.08 | 0.96–1.21 | 0.205 |
| Intrinsic rate | 1.01 | 1–1.03 | 0.124 | 1.02 | 1–1.05 | 0.025 |
- BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; HBP, His bundle pacing; HR, hazard ratio; ICM, ischaemic cardiomyopathy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention.
Clinical events and lead complications
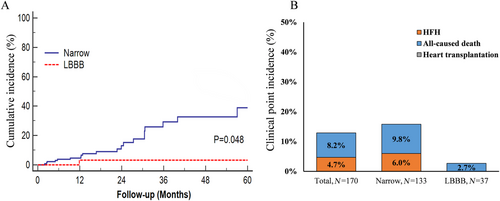
We evaluated the commonly used composite endpoint of hospitalization for heart failure, all-cause mortality, or heart transplantation. Figure 4 displays Kaplan–Meier survival curves for the composite endpoint stratified according to the two groups (log-rank test P = 0.002). During a median follow-up of 18.9 (11.9, 30.5) months, a total of 22 subjects (12.9%) experienced the composite outcome with 14 deaths (8.2%) and eight heart failure hospitalizations (4.7%). The LBBB group patients had a significantly lower risk of the composite endpoint than patients with a narrow QRS duration (2.7% vs. 15.8%).
In the narrow QRSd cohort, four had an increase in His bundle capture threshold >1.5 V/0.5 ms (two at 6 months, one at 3 years, and one at 5 years) and one had a pocket infection; all received an HBP lead. In the LBBB cohort, one had an increase in LBB capture threshold >1.5 V/0.5 ms; however, the LV septal capture remained low at 0.75 V/0.5 ms. No LV perforation was observed during the study (either clinically or by echocardiography).
Discussion
Major findings
- Atrioventricular junction ablation + HPCSP was effective and safe in permanent AF patients who had symptomatic heart failure with LVEF ≤ 50%.
- Compared with patients with a narrow QRS, patients with intrinsic LBBB were more likely to have an improvement in LVEF and reverse LV remodelling and had a lower composite clinical event rate.
- Left bundle branch pacing showed similar outcomes to patients with HBP, but with lower pacing thresholds and higher R-wave amplitude.
Pacing and atrioventricular junction ablation for patients with symptomatic atrial fibrillation
Atrioventricular junction ablation is performed in patients with symptomatic, drug-refractory, long-standing persistent AF as part of an ‘ablate and pace’ strategy.6 The APAF-CRT and other studies have demonstrated that BVP combined with AVJ ablation can be the mainstay of pacing and ablation therapy in patients who have decreased ejection fraction or clinical heart failure by reducing the negative haemodynamic effects of right ventricular apical pacing and underlying rapid AF.15, 16 The benefits of BVP are limited by non-responders and patients with suboptimal clinical outcomes, which may in part be due to the fact that BVP does not completely reverse electrical dyssynchrony, but rather decreases its magnitude, limiting its application, especially in patients with narrow QRS duration or non-LBBB.5 Fusion with intrinsic conduction by optimized AV intervals plays an important role in determining the benefit of BVP-CRT.4 However, in AF patients who received AVJ ablation, programming to obtain an adequate BVP-CRT pacing percentage (>98%) and fusion with intrinsic conduction are two factors that work in opposite directions in patients. A recent meta-analysis including 1256 CRT-AF recipients (QRS duration ≥ 120 ms, LVEF ≤ 35%) identified mild improvements in LVEF by AVJ ablation of only 9.7% (95% confidence interval: 8.32–11.08).17 These findings may explain why the indication for BVP + AVJ ablation in AF patients is only a Class IIa/b in the present guidelines, even for those with intrinsic LBBB.18
We hypothesized that patients with wide QRS duration benefit more from pace and ablate for AF than those with narrow QRS, as is also true in sinus rhythm.19 However, BVP after AVJ ablation was mainly performed in a heterogeneous AF populations with wide QRS and depressed systolic function.20 Post-hoc analyses of small subpopulations have been unable to find differences in outcome between patients with narrow QRS and those with wide QRS.21
His-Purkinje conduction system pacing can result in a normal, rapid conduction and delivers more effective ventricular resynchronization than BVP. Previous studies have demonstrated that HPCSP could preserve/restore cardiac synchrony in patients with both narrow and wide QRS in sinus rhythm.11, 22-25 Fusion with intrinsic ventricular activation seems impossible in patients with AVJ ablation. However, unlike BVP-CRT, HPCSP-CRT could achieve optimal LV synchronization theoretically by single point pacing in patients with narrow or typical LBBB, regardless of whether ablate AVJ or not (Figure 2). This may explain why patients with AVJ ablation can still benefit greatly from HPCSP-CRT. HBP after AVJ ablation was first reported as safe and effective in patients with chronic AF and dilated cardiomyopathy and normal ventricular activation (i.e. QRS < 120 ms) by Deshmukh et al., with improvement in the LVEF from 18.2% to 28.6%.8 Our previous study also demonstrated the benefit of HPCSP and AVJ ablation in patients with long-standing persistent AF and heart failure who had an indication for implantable cardioverter-defibrillator implantation (QRS < 130 ms).9
The benefit of HPCSP-CRT in patients with AF and wide QRS duration has not yet been well established. For the first time, we compared the effect of HPCSP after AVJ ablation for AF between patients with typical LBBB and narrow QRS duration. As expected, the LBBB cohort showed a more pronounced reduction in QRS duration during HPCSP-CRT. Compared with the narrow QRSd cohort, HPCSP in the LBBB cohort delivered significantly greater improvement in LEVF and higher LVEF super responder rate. The magnitude of response in the LBBB group suggests benefit from LV resynchronization by HPCSP-CRT, in addition to rate and rhythm control by AVJ ablation. The magnitude of response with HPCSP-CRT was the same as in the MADIT-CRT study19: the LBBB patients treated with CRT-D experienced a hazard ratio of 0.47 (P < 0.001), decreasing the incidence of heart failure events or death compared with those treated with implantable cardioverter-defibrillator only. However, the respective hazard ratios were 0.99 (P = 0.969) in right bundle branch block patients and a hazard ratio of 1.44 (P = 0.143) in intraventricular conduction delay patients. Besides QRS morphology, a higher intrinsic ventricular rate was also associated with a higher chance of LVEF response, indicating additional benefit from slowing and regularization of the ventricular rate by AVJ ablation.
We performed a sub-analysis to assess the underlying differences between HBP and LBBP. The magnitude of LVEF improvement in HBP and LBBP was similar. LBBP may offer a better safety margin in patients who are pacemaker dependent, avoiding the potential risk of rising capture thresholds seen in some patients with HBP.6 We could find that with introduction of LBBP, the final success rate of permanent physiological pacing utilizing HBP or LBBP after AVN ablation increased to approximately 100% after June 2017 (Figure 1). Intraseptal LBBP not only captured the proximal left bundle branch but also offers the ability to pace the LV septum with a low pacing threshold and higher R-wave amplitudes, which may make it a preferable first choice for pacing after AVJ ablation.6
Limitation
This is a non-randomized, real-life observational study in a relatively small population, with its inherent limitations. Ideally, our findings should be confirmed in large randomized trials.
While the study included consecutive patients, the majority of patients had non-ischaemic cardiomyopathy and the results cannot be generalized to patients with ischaemic cardiomyopathy. This may be particularly important with respect to the high response rate in LBBB patients with HPCSP.
We did not assess the effectiveness of HPCSP after AVJ ablation in patients with intrinsic right bundle branch block and non-specific intraventricular conduction delay. We compared the different response rate between patients with intrinsic narrow QRSd and typical LBBB, and further comparison in patients with LBBB correction by HPCSP vs. those without LBBB correction should be investigated.
Conclusion
In this observational study with patients received pacing and ablation strategy for AF, we identified that AF patients with intrinsic LBBB derive more favourable clinical benefit from HPCSP after AVJ ablation than those with QRS ≤ 120 ms.
Conflict of interest
None declared.
Funding
This study was funded by the Natural Science for Youth Foundation, grant/award number 81900345; Major Project of the Science and Technology of Wenzhou, grant/award number ZS2017010; and the Key Research and Development Program of Zhejiang, grant/award number 2019C03012.




