Incremental prognostic value of cardiac metaiodobenzylguanidine imaging over the co-morbid burden in acute decompensated heart failure
Abstract
Aims
Co-morbidities are associated with poor clinical outcomes in patients with chronic heart failure, while cardiac iodine-123 (I-123) metaiodobenzylguanidine (MIBG) imaging provides prognostic information in such patients. We sought to prospectively investigate the incremental prognostic value of cardiac MIBG imaging over the co-morbid burden, in patients admitted for acute decompensated heart failure (ADHF).
Methods and results
In 433 consecutive ADHF patients with survival to discharge, we measured the co-morbidity using age-adjusted Charlson co-morbidity index (ACCI), commonly employed to evaluate a weighted and scored co-morbid condition, adding additional points for age. In cardiac MIBG imaging, the cardiac MIBG heart-to-mediastinum ratio (late HMR) was measured on the delayed image. Over a follow-up period of 2.9 ± 1.5 years, 160 patients had a cardiac event (a composite of cardiac death and unplanned hospitalization for worsening heart failure). Patients with high ACCI (≥6: median value) had a significantly greater risk of a cardiac event. In multivariate Cox analysis, the ACCI and late HMR were significantly and independently associated with a cardiac event. In both high and low ACCI subgroups (ACCI ≥ 6 and <6, respectively), patients with low late HMR had a significantly greater risk of a cardiac event (high ACCI: 51% vs. 34% P = 0.0026, adjusted HR 1.74 [1.21–2.51]; low ACCI: 34% vs. 17%, P = 0.0228, adjusted HR 2.19 [1.10–4.37]).
Conclusions
Cardiac MIBG imaging could provide additional prognostic information over ACCI, which was also promoted to be a useful risk model, in patients admitted for ADHF.
Introduction
The prevalence of heart failure (HF) has increased dramatically over the past decades. One of the main causes is an increase in the number of elderly patients,1-3 because the general population continues to age rapidly. In addition, although the improved treatment of acute coronary syndrome and valvular diseases is extending life expectancy, earlier diagnosis and better treatment of HF are also leading to progressive aging of the HF population.2, 4 The elderly with HF often suffer from multiple co-existent diseases and/or conditions (co-morbidities). Co-morbidities are strongly associated with poor clinical outcomes in patients with chronic HF, although most existing reviews or reports focus on individual diseases or syndromes.1-5 Age-adjusted Charlson co-morbidity index (ACCI), which is a validated instrument to assess co-morbidity and has been shown to predict prognosis in a variety of cardiovascular patient groups,6-9 has recently been used as a robust prognostic model in chronic HF patients.3, 10-12 However, the long-term prognostic significance of ACCI remains to be fully clarified in patients with acute decompensated HF (ADHF).
In HF, abnormalities in cardiac autonomic control characterized by sympathetic overactivity are strongly associated with poor clinical outcomes.13 Cardiac iodine-123 (I-123) metaiodobenzylguanidine (MIBG) imaging is known to estimate cardiac adrenergic nerve activity and provides prognostic information on patients with chronic HF.14-20 Moreover, we have recently reported the prognostic value of cardiac MIBG imaging in patients admitted for ADHF.21
The aim of this study was to evaluate the incremental prognostic value of cardiac MIBG imaging over the co-morbid burden in case of usefulness of ACCI in patients admitted for ADHF.
Methods
Subjects
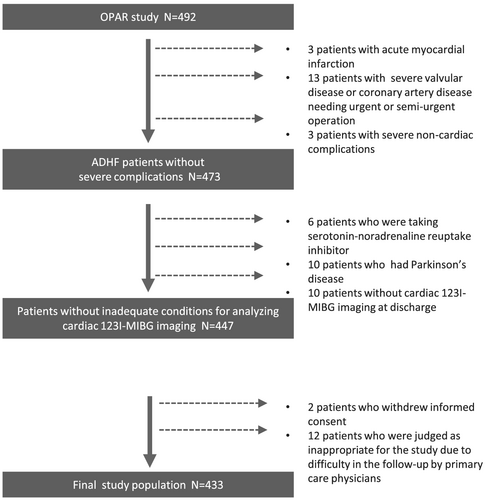
We analysed data from Osaka Prefectural Acute Heart Failure Syndrome Registry (UMIN 000015246), which was a single-centre, observational, prospective cohort study.22, 23 We enrolled 492 consecutive patients who were admitted for ADHF with survival to discharge between October 2011 and November 2016. We ascertained the presence of ADHF by clinical signs and symptoms based on the Framingham Heart Failure criteria. Patients were excluded from this study if they had severe valvular disease or coronary artery disease requiring urgent or semi-urgent operation (n = 13), complicating acute coronary syndrome (n = 3), or severe non-cardiac complications (n = 3). Patients were also excluded because they withdrew informed consent (n = 2). Patients were judged as inappropriate for the study due to difficulties of the follow-up by primary care physicians (n = 12) were also excluded. Patients who did not receive cardiac 123I-MIBG imaging at discharge (n = 10) were also excluded. Patients who were taking serotonin-noradrenaline reuptake inhibitor (SNRI) (n = 6) and who had Parkinson's disease (n = 10) were also excluded because these factors interfere with cardiac 123I-MIBG uptake. After excluding these patients, we studied 433 ADHF patients (Figure 1). All patients gave written informed consent for the participation in this study, which was approved by the Osaka General Medical Center's Review Committee. The investigation conformed with the principles outlined in the Declaration of Helsinki.
Data collection
All patients underwent echocardiography and venous blood sampling at discharge. In echocardiography, left ventricular end-diastolic (LVDd), end-systolic dimensions (LVDs), and left atrial dimension (LAD) were measured by two-dimensional echocardiography using the standard technique. Left ventricular ejection fraction (LVEF) was measured by the modified Simpson's method. Blood samples for the assessment of complete blood count, haemoglobin, serum sodium, chloride, potassium, creatinine, blood urea nitrogen (BUN), albumin, uric acid, C-reactive protein (CRP), and plasma B-type natriuretic peptide (BNP) level were obtained at discharge.
Evaluation of non-cardiac co-morbidity
The co-morbid conditions of patients at discharge was evaluated using ACCI by a clinician who was blinded to the clinical status of the patient. ACCI contains 19 medical conditions: myocardial infarction, congestive HF, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, gastrointestinal ulcer disease, mild or moderate to severe liver disease, diabetes mellitus with or without its complications, moderate or severe renal disease, any tumour, leukaemia, lymphoma, secondary metastasis, and acquired immunodeficiency syndrome. These medical conditions are weighted and scored, and additional points are assigned per decade of age over 40 years.24, 25 ACCI values were derived for each patient based on chart review and assigned according to baseline clinical information.
Cardiac metaiodobenzylguanidine imaging
At discharge, all patients underwent myocardial imaging with 123I-MIBG (MyoMIBG-I 123 injection; FUJIFILM Toyama Chemical, Tokyo, Japan) using a conventional rotating gamma camera (BrightView; Philips, Amsterdam, The Netherlands) equipped with a low-energy type, cardiac high-resolution collimator. An 111 MBq dose of I-123 MIBG was injected intravenously at rest after an overnight fast. Initial and delayed image acquisitions were performed in the anterior chest view at 20 and 200 min after isotope injection as previously described.14, 15 A clinician who was unaware of the clinical status of the patients assessed the cardiac MIBG uptake. The MIBG heart-to-mediastinum ratio (HMR) was determined by dividing the counts/pixel in a visually drawn heart region of interest by the counts/pixel in an upper mediastinum region of interest. Background subtraction was performed with the upper mediastinal region of interest. After considering the radioactive decay of I-123, the cardiac MIBG washout rate (WR) was calculated from the initial and delayed images.
Clinical outcome
After discharge, all patients were followed up by the HF team at our institution. Survival data were obtained by physicians of the patients making direct contact with them in a hospital outpatient setting, by a telephone interview with the family of the patients, or by a mail by dedicated coordinators and investigators. The primary endpoint of this study was a cardiac event, defined as a composite of cardiac death and unplanned hospitalization for worsening HF.
Statistics
All continuous variables were expressed as mean (±standard deviation, SD) or median (25–75 percentiles) as appropriate, and categorical variables were expressed as percentage. Univariate differences in baseline variables between patients with and without cardiac events were evaluated using a χ2 test for categorical variables or a two-sample t-tests for continuous variables. A Mann–Whitney U-test was also used to compare differences in data that was not normally distributed. The areas under the curve (AUC) of cardiac MIBG parameters were compared using a receiver-operating characteristic (ROC) curve analysis. Event-free survival rates were calculated using the Kaplan–Meier method, and differences in survival rates were compared between groups using the log-rank test. Cox proportional hazards regression models were used to identify patients at risk of a cardiac event by calculating the multivariate-adjusted hazard ratios (HR) and 95% confidence interval (CI). Multivariate analysis consisted of major confounding variables that were proposed by previous HF reports, namely sex, systolic blood pressure and heart rate at discharge, body mass index (BMI), LVEF, New York Heart association functional classification (NYHA class), history of atrial fibrillation, ischaemic origin, haemoglobin, serum sodium, BUN, albumin, and plasma BNP levels.26-30 Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) attained by the addition of late HMR to ACCI were also calculated. A P value <0.05 was considered statistically significant. MedCalc version 17.11.5-64 bit (MedCalc software bvba) and EZR version 1.03 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) was used for the statistical analysis.
Results
Comparison of baseline characteristics in patients with acute decompensated heart failure with and without cardiac events
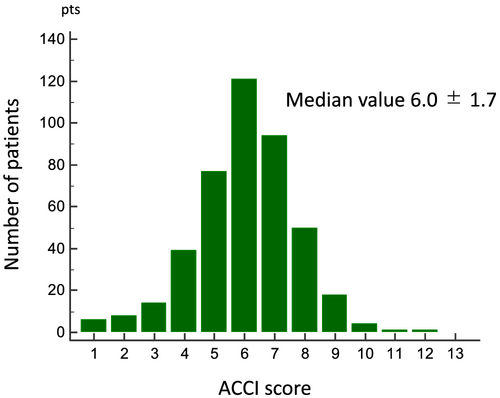
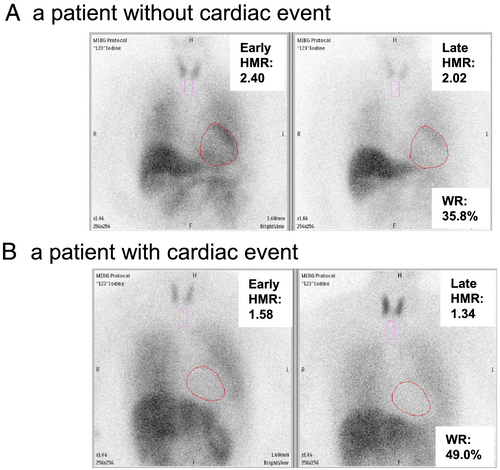
The distribution of ACCI is shown in Figure 2. During a mean follow-up period of 2.9 ± 1.5 years, 160 out of 433 ADHF patients (37%) had a cardiac event. Baseline characteristics of ADHF patients with and without cardiac events were shown in Table 1. When compared with patients without cardiac events, those with cardiac events were significantly older, had a significantly higher NYHA class and BNP level, and had lower BMI and lower haemoglobin, serum albumin, sodium, and chloride levels. Furthermore, patients with cardiac events had a significantly higher ACCI. Representative cardiac MIBG images in patients with and without cardiac events were shown in Figure 3. The early and late HMRs were significantly lower, and the WR was significantly higher in patients with cardiac events. There was no significant correlation between ACCI and either HMR or WR. When compared with non-ischaemic HF patients, late HMR were significantly lower in patients with ischaemic HF (late: 1.61 ± 0.26 vs. 1.70 ± 0.30, P = 0.0032).
| Cardiac events | P value | ||
|---|---|---|---|
| With (N = 160) | Without (N = 273) | ||
| Clinical data | |||
| Age (years) | 77 ± 11 | 72 ± 14 | 0.0002 |
| Gender (men, %) | 57 | 60 | 0.5342 |
| Body mass index | 20.8 ± 3.5 | 21.5 ± 4.8 | 0.0947 |
| Heart rate (b.p.m.) | 66 ± 11 | 66 ± 11 | 0.9424 |
| Systolic blood pressure (mmHg) | 114 ± 18 | 117 ± 18 | 0.0959 |
| NYHA class I/II/III (%) | 43/38/19 | 30/35/36 | 0.0001 |
| Prior HF hospitalization (%) | 34 | 12 | <0.0001 |
| Atrial fibrillation (%) | 50 | 45 | 0.4039 |
| Ischaemic origin (%) | 47 | 31 | 0.0006 |
| Hypertension (%) | 16 | 18 | 0.4655 |
| Co-morbidity burden (%) | |||
| Peripheral vascular disease | 26 | 25 | 0.1925 |
| Cerebrovascular disease | 23 | 26 | 0.5752 |
| Dementia | 8 | 7 | 0.6626 |
| Chronic obstructive pulmonary disease | 12 | 8 | 0.1137 |
| Connective tissue disease | 5 | 4 | 0.6403 |
| Peptic ulcer disease | 3 | 6 | 0.2542 |
| DM without complications | 0 | 16 | 0.0085 |
| DM with complications | 41 | 27 | <0.0001 |
| Moderate to severe CKD | 11 | 10 | 0.756 |
| Mild liver disease | 1 | 2 | 0.4772 |
| Moderate to severe liver disease | 1 | 0 | 0.1923 |
| Haemiplegia | 2 | 2 | 0.6423 |
| Leukaemia | 0 | 0 | ・・・ |
| Malignant lymphoma | 0 | 1 | 0.2775 |
| Tumour | 0 | 1 | 0.9161 |
| Secondary metastasis | 1 | 0 | 0.4431 |
| AIDS | 0 | 0 | ・・・ |
| ACCI (points) | 6.5 ± 1.6 | 5.8 ± 1.7 | <0.0001 |
| Medication use | |||
| ACE inhibitors/ARB (%) | 58 | 58 | 0.7878 |
| MRA (%) | 34 | 36 | 0.3652 |
| Beta-blockers (%) | 90 | 89 | 0.5751 |
| Loop diuretics (%) | 82 | 86 | 0.2837 |
| Digitalis (%) | 3 | 4 | 0.4900 |
| Echocardiography | |||
| LVEF (%) | 46 ± 15 | 46 ± 14 | 0.8738 |
| LVDd (mm) | 54 ± 10 | 53 ± 9 | 0.4118 |
| LVDs (mm) | 41 ± 12 | 40 ± 11 | 0.6558 |
| LAD (mm) | 43 ± 7 | 43 ± 7 | 0.0887 |
| Laboratory data | |||
| Haemoglobin (g/dL) | 11.1 ± 1.9 | 12.2 ± 2.3 | <0.0001 |
| Albumin (g/dL) | 3.3 ± 0.5 | 3.5 ± 0.5 | 0.0027 |
| Creatinine (mg/dL) | 1.4 (1.2, 1.5) | 1.1 (1.0, 1.1) | <0.0001 |
| BUN (mg/dL) | 30 (27, 33) | 23 (22, 25) | <0.0001 |
| Uric acid (mg/dL) | 7.3 ± 2.3 | 7.0 ± 1.9 | 0.1246 |
| Serum sodium (mEq/L) | 138 ± 4 | 138 ± 3 | 0.0086 |
| Serum potassium (mEq/L) | 4.2 ± 0.5 | 4.2 ± 0.5 | 0.2563 |
| Serum chloride (mEq/L) | 101 ± 6 | 102 ± 4 | 0.0448 |
| BNP (pg/mL) | 260 (219, 350) | 192 (164, 222) | 0.0022 |
| MIBG data | |||
| Early HMR | 1.82 ± 0.31 | 1.93 ± 0.30 | 0.0004 |
| Late HMR | 1.61 ± 0.30 | 1.73 ± 0.28 | <0.0001 |
| WR (%) | 38.5 ± 16.5 | 34.2 ± 13.8 | 0.0034 |
- ACCI, age-adjusted Charlson co-morbidity index; ACEI, angiotensin converting enzyme inhibitor; AIDS, acquired immune deficiency syndrome; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HMR, heart-to-mediastinum metaiodobenzylguanidine uptake ratio; LAD, left atrial diameter; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; WR, washout rate.
- Values are mean ± SD or %.
Prognostic analysis by age-adjusted Charlson co-morbidity index and cardiac metaiodobenzylguanidine imaging
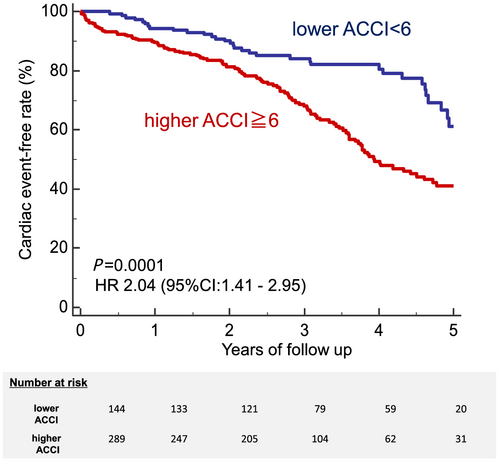
Patients with a higher ACCI (≥6: median value) had a significantly greater risk of cardiac events than those with a lower ACCI (<6) (43% vs. 26%, P = 0.0001; Figure 4). Patients with a lower early and late HMR had a significantly greater risk of cardiac events (early HMR < 1.78 determined by ROC analysis; 49% vs. 31%, P = 0.0087, late HMR < 1.56 determined by ROC analysis; 54% vs. 24%, P < 0.0001). Furthermore, a higher WR also had a significantly greater risk of cardiac events (WR > 38.4% determined by ROC analysis; 47% vs. 32%, P < 0.0001). ROC curve analysis showed that AUC of early HMR, late HMR, and WR were 0.597 (95% CI: 0.549–0.644), 0.625 (95% CI: 0.577–0.670), and 0.575 (95% CI: 0.527–0.622) respectively, and the predictive power of late HMR was superior to that of the other parameters (Figure 5).
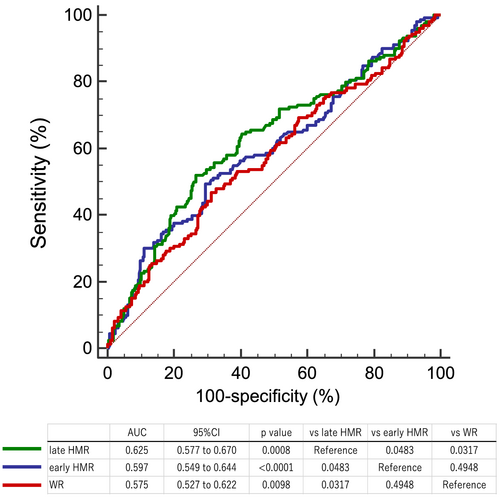
Incremental prognostic value of cardiac metaiodobenzylguanidine imaging over age-adjusted Charlson co-morbidity index
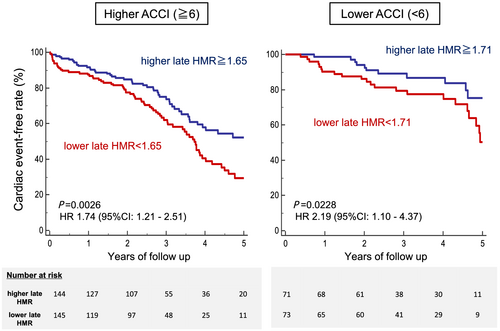
In the multivariate Cox proportional hazards analysis, both ACCI (P = 0.0015) and late HMR (P = 0.0069) were significantly and independently associated with cardiac events after adjustment for major HF confounders (Table 2). In the ACCI ≥ 6 subgroup, patients with a lower late HMR (<1.65: median value) had a significantly greater risk of a cardiac event (51% vs. 34%, P = 0.0026, HR 1.74 [1.21–2.51]). Furthermore, in the ACCI < 6 subgroup, patients with a lower late HMR (<1.71: median value) also had a significantly greater risk of a cardiac event (34% vs. 17%, P = 0.0228, adjusted HR 2.19 [1.10–4.37]; Figure 6). Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) attained by the addition of late HMR to ACCI were also statistically significant for the prediction of the cardiac events (NRI: 0.3491 [95% CI: 0.1654–0.5329], P = 0.0002; IDI: 0.0214 [0.0079–0.0348], P = 0.00185).
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| ACCI | <0.0001 | 1.3487 (1.2147–1.4974) | 0.0015 | 1.2269 (1.0817–1.3912) |
| Late HMR | <0.0001 | 0.2557 (0.1426–0.4585) | 0.0069 | 0.4244 (0.2278–0.7907) |
| BUN | <0.0001 | 1.0260 (1.0172–1.0349) | 0.0021 | 1.0169 (1.0061–1.0279) |
| Haemoglobin | <0.0001 | 0.7938 (0.7333–0.8594) | 0.0019 | 0.8349 (0.7452–0.9353) |
| NYHA class III | <0.0001 | 2.0743 (1.4983–2.8718) | 0.0396 | 1.5086 (1.0568–2.1536) |
| SBP | 0.0076 | 0.9880 (0.9792–0.9968) | 0.0011 | 0.9839 (0.9744–0.9935) |
| Log BNP | <0.0001 | 1.4633 (1.2458–1.7188) | – | – |
| Sex | 0.6222 | 1.0826 (0.7895–1.4846) | – | – |
| Body mass index | 0.0105 | 0.9485 (0.9109–0.9877) | – | – |
| Heart rate | 0.8020 | 1.0018 (0.9875–1.0164) | – | – |
| Albumin | 0.0005 | 0.5511 (0.3943–0.7702) | – | – |
| Sodium | 0.0002 | 0.9184 (0.8782–0.9605) | – | – |
| LVEF | 0.4766 | 0.9962 (0.9857–1.0068) | – | – |
| Ischaemic origin | 0.0003 | 1.7901 (1.3090–2.4480) | – | – |
| AF history | 0.2160 | 1.2170 (0.8916–1.6613) | – | – |
- ACCI, age-adjusted Charlson co-morbidity index; AF, atrial fibrillation; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; HMR, heart-to-mediastinum metaiodobenzylguanidine uptake ratio; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
Discussion
In the present study, we show the prognostic significance of co-morbidities in patients with ADHF. Furthermore, to our knowledge, this is the first prospective study to show the incremental prognostic value of cardiac MIBG imaging over the co-morbid conditions evaluated by ACCI in ADHF patients.
Co-morbid burden of patients admitted for acute decompensated heart failure
The increased population of elderly patients with HF, their higher risk mortality, and repetitive hospitalization imposes a heavy social and economic burden. Therefore, the management of older HF patients with complex co-morbidities is becoming an important topic, and prognosis prediction for such patients is an urgent issue for society. Although holistically recognizing multiple co-morbidities is necessary for the comprehensive understanding of the clinical condition of elderly HF patients, most previous studies have focused on individual diseases or syndromes.1, 31, 32 One reason for this was the presumed difficulty in evaluating the burden of co-morbidity quantitatively, and that there were few established risk models to score it. Charlson co-morbidity index (CCI) is derived from a cohort of 604 oncology patients in a single US hospital for whom the 1 year mortality was predicted in 1987, and because age is undoubtedly an important predictor of mortality, it has been revised such that for each decade over the age of 40 years, 1 point is added to the overall score of a patient.24, 25 ACCI became widely known and recently has been used as a robust prognostic model to evaluate multiple co-morbid conditions in patients with various diseases, including HF.2, 3, 10, 11, 33-35
Previous studies has showed that a non-cardiac co-morbidity burden is strongly associated with poor clinical outcomes in chronic HF.1, 31 Regarding patients with ADHF, a few studies34, 36 have shown the prognostic significance of non-cardiac co-morbidity burden. In these studies, the follow-up period was relatively short (12 months), and there was consequently no information available on the long-term prognostic significance of co-morbidity burden in ADHF patients. In our study, we demonstrate that ADHF patients with multiple co-morbidities evaluated by ACCI had a greater risk of cardiac events over a 5 year period, suggesting that ACCI is a useful long-term prognostic marker not only in chronic HF patients but also in ADHF patients.
Incremental prognostic value of cardiac metaiodobenzylguanidine imaging over age-adjusted Charlson co-morbidity index and future perspective
Cardiac MIBG imaging is a useful tool for examining the increase in sympathetic nerve activity, which is strongly associated with a poor prognosis in HF patients. Because the first report by Merlet et al., which demonstrated the prognostic value of cardiac MIBG imaging in patients with chronic HF, the predictive value of MIBG imaging has been confirmed in several studies, including our own previous work.15, 18, 37-39 In the present study, there was no significant correlation between the ACCI and cardiac MIBG parameters, although these variables were independently associated with poor outcomes. A previous report suggested that chronic obstructive pulmonary disease was associated with sympathetic nerve system dysfunction.40 However, there are no reports that describe the prognostic value of the co-morbid burden as a whole and sympathetic nerve system. ACCI includes multiple co-morbid conditions, while there is a paucity of information on the sympathetic nerve activity that is an essential factor when considering the conditions of HF patients.
In the previous reports which mainly included chronic HF patients with reduced LVEF, late HMR was significantly associated with the poor outcome independently of plasma BNP level and LVEF.18, 38 In the present study, we enrolled ADHF patients irrespectively of reduced or preserved LVEF, which resulted in that late HMR was more predictive and LVEF was not predictive. The discrepancy between the present study and previous reports might be due to the patient's characteristics (chronic HF vs. acute HF, reduced LVEF vs. non-reduced LVEF).
Limitations
There are several limitations in this study. First, because this is a single-centre study, the small and empirically selected population sample size was a major limitation. Second, the medications used during the follow-up might have affected MIBG uptake and the clinical outcome. A study with serial evaluation of cardiac MIBG might therefore be necessary. Third, we need to consider the specificity of our study patients when we generalize our results. There were few patients with tumours, secondary metastases, moderate to severe liver disease, or malignant lymphoma. Additionally, there were no patients with leukaemia or acquired immune deficiency syndrome, which score 2 and 6 points, respectively, and as such, the ACCI score may be lower than at other institutions in Japan or in non-Japanese populations. Thus, external validation would be needed to verify our results.
Conclusions
Cardiac MIBG imaging could provide additional prognostic information over the co-morbid conditions evaluated by the ACCI.
Conflict of interest
None declared.
Author contributions
Takahisa Yamada designed this study. Takahisa Yamada, Shunsuke Tamaki, and Masatake Fukunami contributed to the analysis and assisted in writing the manuscript. All remaining authors have contributed to the data collection, interpretation, and critical review of the manuscript.




