Prevalence and prognostic value of the coexistence of anaemia and frailty in older patients with heart failure
Abstract
Aims
There have been no investigations of the prevalence and clinical implications of coexistence of anaemia and frailty in older patients hospitalized with heart failure (HF) despite their association with adverse health outcomes. The present study was performed to determine the prevalence and prognostic value of the coexistence of anaemia and frailty in hospitalized older patients with HF.
Methods and results
We performed post hoc analysis of consecutive hospitalized HF patients ≥65 years old enrolled in the FRAGILE-HF, which was the prospective, multicentre, observational study. Anaemia was defined as haemoglobin < 13 g/dL in men and <12 g/dL in women, and frailty was evaluated according to the Fried phenotype model. The study endpoint was all-cause mortality. Of the total of 1332 patients, 1217 (median age, 81 years; 57.4% male) were included in the present study. The rates of anaemia and frailty in the study population were 65.7% and 57.0%, respectively. The patients were classified into the non-anaemia/non-frail group (16.6%), anaemia/non-frail group (26.4%), non-anaemia/frail group (17.7%), and anaemia/frail group (39.3%). A total of 144 patients died during 1 year of follow-up. In multivariate analyses, only the anaemia/frail group showed a significant association with elevated mortality rate (adjusted hazard ratio, 1.94; 95% confidence interval, 1.02–3.70; P = 0.043), compared with the non-anaemia/non-frail group after adjusting for other covariates.
Conclusions
Coexistence of anaemia and frailty are prevalent in hospitalized older patients with HF, and it has a negative impact on mortality.
Introduction
Heart failure (HF) is a global public health concern estimated to affect 26 million people worldwide, and its prevalence is increasing with the aging of the population.1 HF adversely affects both mortality and quality of life, as well as increasing medical costs.2 Although drugs directly targeting aspects of the pathophysiology of HF are essential for treatment, it is also necessary to recognize and appropriately manage comorbidities to improve quality of life and prognosis.
Frailty consisting of reduced physiological reserve and vulnerability to external stressors in geriatric patients is a high-priority issue in cardiovascular medicine.3 Anaemia and frailty are conditions commonly encountered in hospitalized older patients with HF and are associated with poorer clinical status and higher risk of death.4-6 Anaemia and frailty have been shown to share a pathophysiology associated with chronic inflammatory processes, and coexistence of both conditions often come up on the agenda in the field of gerontology.7, 8 In healthy individuals, reduced oxygen delivery is compensated by increases in both heart rate and stroke volume9; however, these mechanisms already impaired in patients with HF. Consequently, anaemia reduces tissue oxygenation and impairs muscle performance in HF patients, thus increasing fatigue, cognitive decline, and weakening muscle strength.4, 7 As these factors contribute to frailty syndrome, many HF patients have both anaemia and frailty, leading to a vicious cycle of physiological decline.5, 10 Despite the burden imposed on healthcare systems worldwide by the coexistence of anaemia and frailty in older patients with HF, there have been no previous investigations of the prevalence rate of coexisting anaemia and frailty and the impact on mortality in this population.
The present study was therefore designed to investigate the prevalence and prognostic impact of the coexistence of anaemia and frailty in hospitalized older patients with HF based on data from the FRAGILE-HF (Prevalence and Prognostic Value of Physical and Social Frailty in Geriatric Patients Hospitalized for Heart Failure) cohort study.
Methods
Study design and patient population
This was a secondary analysis of the FRAGILE-HF study, a prospective, multicentre, observational study performed in 15 hospitals in Japan, as reported previously.11 Briefly, all consecutive patients ≥65 years old hospitalized for the first time due to decompensation of HF and capable of ambulation at discharge between September 2016 to March 2018 were evaluated for eligibility. The Framingham criteria were used for diagnosis of decompensation of HF. Patients with previous heart transplantation or left ventricular assist device implantation, those receiving either chronic peritoneal dialysis or haemodialysis, those with acute myocarditis, and those with brain natriuretic peptide (BNP) level <100 pg/mL or N-terminal-proBNP level < 300 pg/mL at admission, as well as those for whom these data were not available were excluded from the present study.
The study was performed in accordance with the tenets of the Declaration of Helsinki and Japanese Ethical Guideline for Medical and Health Research Involving Human Subjects and was approved by the Ethics Committee of each participating hospital. All participants were free to opt out of the study at any time. Study information, which included the study objectives, inclusion and exclusion criteria, and the names of participating institutes, were published in the University Hospital Information Network (UMIN-CTR, unique identifier: UMIN000023929) prior to enrolment of the first patient.
Data collection and definitions
Baseline physical findings, blood samples, echocardiography, and medications were obtained before discharge for all patients with haemodynamic stability. A diagnosis of anaemia was made based on haemoglobin < 13 g/dL for men and <12 g/dL for women in accordance with the criteria of the World Health Organization.12 The formula of the Japanese Society of Nephrology was used to determine the estimated glomerular filtration rate (eGFR),13 and eGFR <60 mL/min/1.73 m2 was used as a criterion for a diagnosis of renal dysfunction.
Frailty and both physical and cognitive function were evaluated by a trained personnel. Frailty status was evaluated using the Fried phenotype model,14 with patients considered to be frail if they had three or more of the following components: weakness (hand grip strength), slowness (gait speed), weight loss, exhaustion, and low physical activity. The questionnaires used to check and diagnose frailty were published in detail previously.11
The Short Physical Performance Battery (SPPB), which consists of three components (standing balance, usual gait speed, repeated chair stands), was applied according to established methods,15 with SPPB scores ranging from 0 to 12, that is 0–4 points for each component (0 = worst; 12 = best). The 6 min walking distance was determined according to the guidelines of the American Thoracic Society.16 Briefly, the patients were instructed to cover as much distance as possible within the allotted time using assistive devices if necessary. Cognitive function was evaluated using Mini-Cog, which is a composite of a three-item recall test and clock-drawing test.17 Instructions were provided in accordance with the Mini-Cog© website (https://mini-cog.com), and scores were given on a 5-point scale (0 = worst; 5 = best), and a score of <3 was considered abnormal.
The endpoint of this study was all-cause mortality, and the time to the endpoint was calculated as the number of days from the date of discharge to the date of the event.
Statistical analysis
Continuous variables with a normal distribution are presented as the means ± SD, while variables with a non-normal distribution are presented as the median and interquartile range. Categorical variables are expressed as numbers and percentages. The cohort was divided into four groups: (i) non-anaemia/non-frail group; (ii) anaemia/non-frail group; (iii) non-anaemia/frail group; and (iv) anaemia/frail group. Differences between groups were evaluated by one-way analysis of variance or the Kruskal–Wallis test for continuous variables, and χ2 or Fisher's exact test for dichotomous variables, as appropriate.
Survival was evaluated using the Kaplan–Meier survival method and compared using log-rank statistics. For analysis of all-cause mortality, we used the Meta-analysis Global Group in Chronic HF (MAGGIC) risk score and (log-transformed) BNP levels at discharge as adjustment variables in a multivariable prognostic model because MAGGIC score is a well validated risk score for Japanese HF patients.18
Multiple imputation was used to take into account the missing covariate data, excluding anaemia and frailty status, to construct multivariable Cox regression models. We created 20 datasets using a chained-equations procedure.19 Parameter estimates were obtained for each dataset and subsequently combined to produce an integrated result using the method described by Barnard and Rubin.20
A two-tailed P < 0.05 was taken to indicate statistical significance in all analyses. Statistical analyses were performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0, URL http://www.R-project.org).
Results
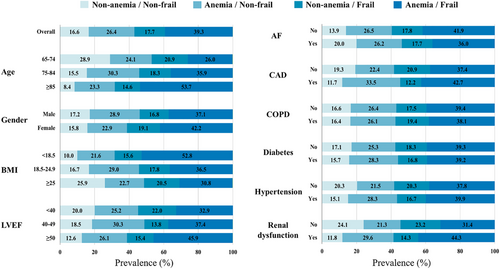
A total of 1332 hospitalized HF patients ≥65 years old were registered in the FRAGILE-HF study during the study period. After excluding 115 patients due to missing data regarding anaemia or frailty status, the remaining 1217 patients were included in the analysis (Table 1). The median age of the study population was 81 years, and 57.4% were male participants. The prevalence rates of anaemia and frailty were 65.7% (799/1217 patients) and 57.0% (694/1217 patients), respectively, and both increased with age (Supporting Information, Figure S1). Patients with anaemia had a significantly higher rate of frail compared with those without anaemia (59.8% vs. 51.7%, respectively, P = 0.007). The study population was classified into four groups as follows: non-anaemia/non-frail group, 202 (16.6%) patients; anaemia/non-frail group, 321 (26.4%) patients; non-anaemia/frail group, 216 (17.7%) patients; and anaemia/frail group, 478 (39.3%) patients. More than half of the patients aged ≥85 years and with body mass index <18.5 kg/m2 were positive for both anaemia and frailty (Figure 1). The anaemia/frail group was associated with older age, more severe symptoms, lower body mass index, lower diastolic blood pressure, higher left ventricular ejection fraction, lower proportion of atrial fibrillation, higher proportion of renal dysfunction, less prescription of beta blockers and mineralocorticoid receptor antagonists, and higher MAGGIC risk score than the other groups. In addition, the anaemia/frail group had lower haemoglobin, haematocrit, albumin and eGFR, and higher creatinine, blood urea nitrogen, and brain natriuretic peptide level at discharge than the other groups.
| Characteristic | Overall | Non-anaemia/Non-frail | Anaemia/Non-frail | Non-anaemia/Frail | Anaemia/Frail | P value |
|---|---|---|---|---|---|---|
| (n = 1217) | (n = 202; 17%) | (n = 321; 26%) | (n = 216; 18%) | (n = 478; 39%) | ||
| Age (years) | 81 [74, 86] | 76 [70, 82] | 80 [75, 85] | 79 [73, 85] | 84 [78, 88] | <0.001 |
| Age group (%) | <0.001 | |||||
| 65–74 | 311 (25.6) | 90 (44.6) | 75 (23.4) | 65 (30.1) | 81 (16.9) | |
| 75–84 | 502 (41.2) | 78 (38.6) | 152 (47.4) | 92 (42.6) | 180 (37.7) | |
| ≥85 | 404 (33.2) | 34 (16.8) | 94 (29.3) | 59 (27.3) | 217 (45.4) | |
| Male (%) | 698 (57.4) | 120 (59.4) | 202 (62.9) | 117 (54.2) | 259 (54.2) | 0.063 |
| Living alone (%) | 254 (20.9) | 49 (24.3) | 63 (19.6) | 36 (16.7) | 106 (22.2) | 0.212 |
| NYHA Class III/IV (%) | 168 (13.8) | 15 (7.4) | 36 (11.2) | 29 (13.4) | 88 (18.4) | 0.001 |
| BMI (kg/m2) | 21.4 ± 3.8 | 22.5 ± 3.9 | 21.5 ± 3.2 | 22.0 ± 4.5 | 20.6 ± 3.7 | <0.001 |
| BMI group (%) | <0.001 | |||||
| <18.5 | 269 (22.1) | 27 (13.4) | 58 (18.1) | 42 (19.4) | 142 (29.8) | |
| 18.5–24.9 | 762 (62.7) | 127 (62.9) | 221 (68.8) | 136 (63.0) | 278 (58.3) | |
| ≥25 | 185 (15.2) | 48 (23.8) | 42 (13.1) | 38 (17.6) | 57 (11.9) | |
| SBP (mmHg) | 114 ± 17 | 112 ± 15 | 116 ± 18 | 110 ± 16 | 114 ± 17 | <0.001 |
| DBP (mmHg) | 62 ± 11 | 64 ± 10 | 62 ± 11 | 64 ± 11 | 60 ± 11 | <0.001 |
| Heart rate (beats/min) | 71 ± 14 | 71 ± 15 | 69 ± 13 | 72 ± 16 | 71 ± 14 | 0.054 |
| LVEF (%) | 46 ± 17 | 43 ± 16 | 46 ± 16 | 42 ± 18 | 48 ± 17 | <0.001 |
| LVEF group (%) | <0.001 | |||||
| <40 | 496 (41.2) | 99 (49.5) | 109 (50.7) | 125 (39.3) | 163 (34.5) | |
| 40–49 | 195 (16.2) | 36 (18.0) | 59 (18.6) | 27 (12.6) | 73 (15.5) | |
| ≥50 | 514 (42.7) | 65 (32.5) | 134 (42.1) | 79 (36.7) | 236 (50.0) | |
| HF duration >18 months (%) | 487 (40.1) | 67 (33.2) | 79 (36.7) | 140 (43.6) | 201 (42.1) | 0.057 |
| Prior HF admission (%) | 501 (41.2) | 61 (30.2) | 148 (46.1) | 80 (37.0) | 212 (44.4) | 0.001 |
| Current smoker (%) | 116 (9.5) | 20 (9.9) | 29 (9.0) | 22 (10.2) | 45 (9.4) | 0.971 |
| Comorbidities (%) | ||||||
| Atrial fibrillation | 541 (44.5) | 108 (53.5) | 142 (44.2) | 96 (44.4) | 195 (40.8) | 0.026 |
| Coronary artery disease | 436 (35.8) | 51 (25.2) | 146 (45.5) | 53 (24.5) | 186 (38.9) | <0.001 |
| COPD | 134 (11.0) | 22 (10.9) | 35 (10.9) | 26 (12.0) | 51 (10.7) | 0.961 |
| Diabetes | 434 (35.7) | 68 (33.7) | 123 (38.3) | 73 (33.8) | 170 (35.6) | 0.644 |
| Hypertension | 868 (71.3) | 131 (64.9) | 246 (76.6) | 145 (67.1) | 346 (72.4) | 0.014 |
| Renal dysfunction | 743 (61.1) | 88 (43.6) | 220 (68.5) | 106 (49.1) | 329 (68.8) | <0.001 |
| History of cancer | 176 (14.5) | 17 (8.4) | 49 (15.3) | 30 (13.9) | 80 (16.7) | 0.042 |
| Medications (%) | ||||||
| ACE-I/ARB | 821 (67.5) | 147 (72.8) | 214 (66.7) | 152 (70.4) | 308 (64.4) | 0.138 |
| Beta-blocker | 899 (73.9) | 161 (79.7) | 236 (73.5) | 178 (82.4) | 324 (67.8) | <0.001 |
| MRA | 603 (49.5) | 120 (59.4) | 146 (45.5) | 125 (57.9) | 212 (44.4) | <0.001 |
| Aspirin | 424 (34.8) | 46 (22.8) | 143 (44.5) | 57 (26.4) | 178 (37.2) | <0.001 |
| Thienopyridine | 192 (15.8) | 29 (14.4) | 53 (16.5) | 23 (10.6) | 87 (18.2) | 0.078 |
| Warfarin | 281 (23.1) | 36 (17.8) | 106 (33.0) | 32 (14.8) | 107 (22.4) | <0.001 |
| DOAC | 401 (32.9) | 89 (44.1) | 75 (23.4) | 102 (47.2) | 135 (28.2) | <0.001 |
| Laboratory data at discharge | ||||||
| Haemoglobin (g/dL) | 11.8 ± 2.0 | 14.0 ± 1.3 | 10.8 ± 1.2 | 13.9 ± 1.3 | 10.6 ± 1.2 | <0.001 |
| Haematocrit (%) | 36 ± 6 | 42 ± 4 | 33 ± 4 | 42 ± 4 | 33 ± 4 | <0.001 |
| Albumin (g/dL) | 3.5 ± 0.5 | 3.7 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.4 | 3.3 ± 0.4 | <0.001 |
| Creatinine (mg/dL) | 1.39 ± 0.82 | 1.13 ± 0.42 | 1.54 ± 0.98 | 1.19 ± 0.78 | 1.48 ± 0.82 | <0.001 |
| eGFR (mL/min/1.73 m2) | 53 ± 22 | 62 ± 20 | 49 ± 22 | 60 ± 20 | 49 ± 22 | <0.001 |
| BUN (mg/dL) | 26 [19.80, 36] | 23.30 [19, 30.73] | 27 [19.70, 36] | 25.65 [19, 35] | 28 [20.30, 39] | <0.001 |
| Sodium (mEq/L) | 139 ± 4 | 139 ± 3 | 139 ± 4 | 139 ± 4 | 139 ± 4 | 0.020 |
| BNP (pg/mL) | 281 [139, 498] | 215 [109, 360] | 290 [148, 498] | 248 [136, 457] | 323 [153, 573] | 0.001 |
| MAGGIC risk score | 26 [23, 30] | 23 [19.50, 27] | 26 [23, 30] | 25 [22, 29] | 28 [24, 31] | <0.001 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure.
- Values are expressed as means ± SD, n (%), or median (interquartile range).
As shown in Table 2, the mean handgrip strength and gait speed for the whole study population were 20.1 ± 7.9 kg and 0.79 ± 0.30 m/s, respectively. The median SPPB score was 9 points, and lower extremity strength (chair stand test) was especially impaired, with 18% of the total cohort lacking the leg strength to stand from a seated position without using their arms even once. The mean 6MWD was 253 ± 126 m, and most patients (80.4%) had 6MWD < 400 m. Patients with anaemia had a significantly shorter 6MWD compared with those without anaemia (237 ± 121 m vs. 284 ± 129 m, respectively, P < 0.001), and patients with frailty also had a significantly shorter 6MWD compared with those without frailty (221 ± 121 m vs. 295 ± 120 m, respectively, P < 0.001). Patients in the anaemia/frail group showed greater impairment of physical function than the other groups. The median Mini-Cog score was 3 points, and 36.9% of the patients had scores <3 points. Patients with anaemia and/or frailty had a higher proportion of cognitive abnormalities compared with those without anaemia and frailty.
| Component | Overall | Non-anaemia/Non-frail | Anaemia/Non-frail | Non-anaemia/Frail | Anaemia/Frail | P value |
|---|---|---|---|---|---|---|
| (n = 1217) | (n = 202; 17%) | (n = 321; 26%) | (n = 216; 18%) | (n = 478; 39%) | ||
| Frailty components (%) | ||||||
| Weakness | 922 (75.8) | 88 (43.6) | 201 (62.6) | 188 (87.0) | 445 (93.3) | <0.001 |
| Slowness | 430 (35.3) | 20 (9.9) | 43 (13.4) | 103 (47.7) | 264 (55.2) | <0.001 |
| Weight loss | 689 (56.6) | 78 (38.6) | 104 (32.4) | 167 (77.3) | 340 (71.1) | <0.001 |
| Exhaustion | 632 (51.9) | 69 (34.2) | 74 (23.1) | 162 (75.0) | 327 (68.4) | <0.001 |
| Low physical activity | 628 (51.6) | 66 (32.7) | 72 (22.4) | 154 (71.3) | 336 (70.3) | <0.001 |
| Physical function | ||||||
| Handgrip strength (kg) | 20.1 ± 7.9 | 24.7 ± 8.2 | 21.9 ± 7.7 | 19.5 ± 7.6 | 17.1 ± 6.5 | <0.001 |
| Gait speed (m/s) | 0.79 ± 0.30 | 0.96 ± 0.28 | 0.88 ± 0.26 | 0.74 ± 0.30 | 0.67 ± 0.29 | <0.001 |
| SPPB (point) | 9 [6, 11] | 11 [9, 12] | 10 [8, 12] | 8 [5, 11] | 7 [5, 10] | <0.001 |
| Standing balance (point) | 4 [2, 4] | 4 [3, 4] | 4 [3, 4] | 4 [2, 4] | 3 [2, 4] | <0.001 |
| Gait speed (point) | 3 [2, 4] | 4 [3, 4] | 4 [3, 4] | 3 [2, 4] | 2 [2, 4] | <0.001 |
| Chair stands (point) | 2 [1, 4] | 4 [2, 4] | 3 [2, 4] | 2 [1, 4] | 1 [0, 3] | <0.001 |
| 6 min walking distance (m) | 253 ± 126 | 319 ± 121 | 280 ± 117 | 250 ± 128 | 207 ± 115 | <0.001 |
| <300 (%) | 699 (57.4) | 74 (36.7) | 155 (48.3) | 127 (58.8) | 343 (71.8) | <0.001 |
| <400 (%) | 979 (80.4) | 146 (72.3) | 252 (78.5) | 173 (80.1) | 408 (85.4) | <0.001 |
| Cognitive function | ||||||
| Mini-Cog, point | 3 [2, 5] | 4 [2, 5] | 3 [2, 5] | 3 [2, 5] | 3 [2, 4] | <0.001 |
| <3 (%) | 449 (36.9) | 51 (25.2) | 119 (37.1) | 84 (38.9) | 195 (40.8) | 0.002 |
- SPPB, short physical performance battery.
- Values are expressed as means ± SD, n (%), or median (interquartile range).
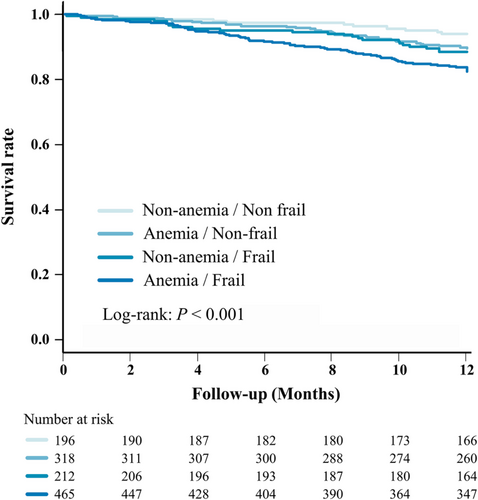
A total of 144 deaths occurred in the whole study population during 1 year of follow-up, and Kaplan–Meier curve analysis indicated that the incidence of all-cause mortality was higher in the anaemia/frail group than the other groups (Figure 2). Table 3 shows the results of Cox regression analyses for all-cause death. After adjusting for other covariates, adjusted models showed that only the anaemia/frail group was associated with higher mortality (hazard ratio 1.94;95% confidence interval, 1.02–3.70; P = 0.043) compared with the non-anaemia/non-frail group.
| Group | Unadjusted Cox model | Adjusted Cox model | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Non-anaemia/Non-frail | 1.00 | [Reference] | 1.00 | [Reference] | ||||
| Anaemia/Non-frail | 1.80 | 0.91 | 3.57 | 0.093 | 1.21 | 0.60 | 2.42 | 0.593 |
| Non-anaemia/Frail | 2.00 | 0.97 | 4.10 | 0.059 | 1.69 | 0.82 | 3.46 | 0.155 |
| Anaemia/Frail | 3.16 | 1.68 | 5.94 | <0.001 | 1.94 | 1.02 | 3.70 | 0.043 |
- BNP, brain natriuretic peptide; CI, confidence interval; MAGGIC, Meta-analysis Global Group in Chronic Heart Failure.
- Adjusted variables: MAGGIC risk score and log-transformed BNP.
Discussion
This study was performed to examine the prevalence of anaemia and frailty and the coexistence of both conditions in a large cohort of hospitalized older HF patients from the FRAGILE-HF cohort study. More than 80% of the patients included in the analysis had either anaemia or frailty, and one third of the patients showed both conditions, particularly associated with more advanced age and lower body mass index. The mortality rate was higher among patients with both anaemia and frailty, even after adjusting for known risk factors. To our knowledge, this is the first report that the combination of anaemia and frailty has prognostic significance in hospitalized older HF patients and may have important implications for risk stratification and management of these two important comorbidities.
The reported prevalence rates of anaemia in HF patients vary over a wide range from 17% to 70%, which may be due to differences in the definition of anaemia and characteristics of the patient populations between studies.4 The prevalence rate of anaemia of 65.7% in the present study was consistent with the ADHERE and ATTEND acute HF registries, which indicated that more than half of all hospitalized HF patients are anemic.21, 22 The reported prevalence of frailty in HF varies widely from 19% to 77%,23 likely due to differences in definitions of frailty and in characteristics of the study populations between studies. The rate of frailty was 57.0% in the present study, which was similar to the prevalence of ~50% among hospitalized HF patients reported previously.23 The prevalence of frailty in hospitalized patients with HF is higher than in patients with stable HF,24 because the symptoms of HF overlap with exhaustion, and hospitalization itself causes weakness and slowness. In addition, patients who are hospitalized in severe condition are more likely to have weakness and slowness, and the hospital environment itself leads to inactivity. Although fluid retention can cause weight gain in patients with HF, the progression of HF often leads to malnutrition, resulting in weight loss.25 It is important to understand the condition of each component of frailty to improve the effectiveness of interventions.
More than 80% of hospitalized HF patients ≥65 years old had anaemia and/or frailty in the present study, which was a markedly high proportion compared with the rate of 57.6% reported previously in patients ≥80 years old with acute coronary syndromes.26 Anaemia can be induced by HF via a number of related pathophysiological mechanisms, especially functional or absolute iron deficiency and erythropoietin synthesis and response.10 In addition, HF can result in frailty due to coordinated multisystem dysfunction as a result of its systemic nature, including systemic inflammation, high comorbidity burden, advanced age, and skeletal muscle abnormalities.5 The results of the present study indicated that the coexistence of both anaemia and frailty was associated with reduced functional status and elevated mortality rate, consistent with a previous study in community-dwelling older adults.27 Impairments in physical function were broad and severe, involving all SPPB components (balance, mobility, and strength) and 6MWD (endurance), among patients with both anaemia and frailty. Similarly to a previous study, lower extremity strength was especially impaired among each component of the SPPB,24 and the profoundly reduced 6MWD in the patients with both anaemia and frailty was comparable with that in patients with advanced HF awaiting left ventricular assist device implantation.28 In addition, handgrip strength and gait speed are not only used to assess frailty, but are also used to define sarcopenia, which is considered an important factor in the frailty cycle.3 International consensus on sarcopenia defines a person with sarcopenia who walks <400 m during a 6 min walk as ‘sarcopenia with limited mobility,’29 and more than 80% of the patients had 6MWD < 400 m in the present study. These conditions may be related to low physical and social activities after discharge, leading to social frailty and poor prognosis. Cognitive function was lower in patients with anaemia and/or frailty than in those without anaemia and frailty, but there was no apparent effect of the combination of anaemia and frailty on cognitive function. As the present study included patients who were able to walk at discharge, patients with relatively preserved cognitive function could have been included, and the range of the Mini-Cog score was narrow (5-point scale). These factors may have reduced the statistical power to detect such associations.
Planning of medical care and improvement of the prognosis of vulnerable populations require accurate risk stratification related to acute HF. The accurate assessment of both anaemia and frailty status in hospitalized older HF patients is important because both of these conditions are not only indicators of the severity of disease but may also be treatable and thus lead to improvement of the overall clinical outcome.5 Iron deficiency, which is an independent predictor of reduced exercise capacity, quality of life, and survival, is recognized as a key treatable cause of anaemia in HF patients.30, 31 FAIR-HF and CONFIRM-HF showed that intravenous iron therapy improved exercise tolerance and quality of life in patients with HF,32, 33 and the European Society of Cardiology guidelines have given a class IIa recommendation for iron deficiency correction by intravenous ferric carboxymaltose.34 Although the role of intravenous iron therapy in acute HF is not yet clear, a new trial, AFFIRM-AHF, is currently underway with the composite of recurrent HF hospitalizations and cardiovascular mortality as the primary outcome.35 On the other hand, outpatient cardiac rehabilitation participation was associated with reduced risks of all-cause death and HF rehospitalization in frail patients with HF.36 A new trial, the REHAB-HF study, is currently underway, and this pilot study has shown that rehabilitation therapy beginning in the hospital improved 6MWD and SPPB score, with the strongest trend seen with chair stands, in hospitalized older patients with HF, and the change in SPPB score was strongly related to all-cause rehospitalization.37
Tissue oxygenation is decreased by anaemia and hypoxia, which may lead to functional impairment of muscle. A previous longitudinal study showed that anaemia preceded the occurrence of frailty,38 and a meta-analysis indicated that the odds of frailty are more than doubled by the presence of anaemia in older individuals.39 Although the rate of frailty was significantly higher in patients with than without anaemia in the present study (59.8% vs. 51.7%, respectively), there was no apparent difference in these rates. As HF itself can cause anaemia and frailty, HF patients may show an attenuated relation between these two conditions. On the other hand, the rate of coexisting anaemia and frailty increased markedly with age in the present study. Anaemia and frailty share pathophysiological mechanisms related to chronic inflammatory processes induced by immunosenescence-associated changes and oxidative stress.7, 8 Metabolism and skeletal muscle mass are adversely affected by systemic inflammation, resulting in sarcopenia and cachexia.40 Cachexia is a generalized wasting process occurring at rates of 5–15% in HF patients, especially in cases of more advanced disease.34 Although body mass index is inversely associated with risk of HF, patients with low body mass index have poorer prognosis once HF has been established in what is referred to as the ‘obesity paradox.’34 Patients in the present study with body mass index <18.5 kg/m2 had a greater likelihood of having both anaemia and frailty, and therefore, careful assessment and discussion are required in the clinical management of such patients. In addition, patients with anaemia and/or frailty were more likely to have history of cancer in the present study. Cancer investigations are needed for such patients because prior cancer history was associated with high cardiac event and mortality rates in hospitalized patients with HF.41 Further detailed studies are required to address these issues and to guide future clinical decision making for the treatment of older HF patients.
Study limitations
The present study had several limitations. First, iron indices were not available in this study to allow us to determine the causes of anaemia, and data were not available regarding whether patients received intravenous iron supplementation and were administered erythropoiesis-stimulating agents, folate, and vitamin B12. However, iron supplementation has not been confirmed to reduce all-cause mortality rates in HF populations.42 As our endpoint was all-cause mortality, this may have had only a limited impact on the results and conclusions of our study. Second, frailty was defined based on questionnaires, which may be susceptible to recall bias, although such questionnaires have been widely applied in population studies. In addition, we did not exclude patients with cognitive impairment from the present study, and this may have impacted the results. Third, we evaluated frailty status only once before discharge, and no information was obtained regarding changes in frailty status, which have the potential of dynamic variability after changes in medical therapy or programs of rehabilitation.43 Fourth, unadjusted and unmeasured factors, such as multiple comorbidities, polypharmacy, and changes in baseline variables, all of which may have an effect on mortality, leave residual bias, and the results must be replicated in future studies. Finally, this study was conducted mostly in Japanese HF patients, and further studies in other populations are required to validate the prognostic value of combined anaemia and frailty.
Conclusions
Both anaemia and frailty have highly prevalence rates in hospitalized older patients with HF, and the coexistence of both conditions was shown to adversely affect mortality rate in this population. On the basis of their high prevalence rate and clinical impact in older HF patients, both anaemia and frailty may be important targets for therapy in these patients.
Conflict of interest
Dr Yuya Matsue is affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi and received an honorarium from Otsuka Pharmaceutical Co. Dr Takatoshi Kasai is affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi. The other authors have nothing to declare.
Funding
The FRAGILE-HF study was supported by Novartis Pharma Research Grants, a Japan Heart Foundation Research Grant, and the Japan Society for the Promotion of Science Grant-in-Aid (JSPS KAKENHI, Grant Number 19K11424).




