Ventricular assist device for a coronavirus disease 2019-affected heart
Abstract
Coronavirus disease 2019 (COVID-19) is challenging the care for cardiovascular patients, resulting in serious consequences with increasing mortality in pre-diseased heart failure patients. In the current state of the pandemic, the physiopathology of COVID-19 affecting pre-diseased hearts and the management of terminal heart failure in COVID-19 patients remain unclear. We outline the findings of a young COVID-19 patient suffering from idiopathic cardiomyopathy who was treated for acute multi-organ failure and required cardiac surgery with implantation of a temporary right ventricular and durable left ventricular assist device (LVAD). For deeper translational insights, we used in-depth tissue analysis by electron and light sheet fluorescence microscopy revealing evidence for spatial distribution of severe acute respiratory syndrome coronavirus 2 in the heart. This indicates that in-depth analysis may represent a valuable tool in understanding indistinct clinical cases. We conclude that COVID-19 directly affects pre-diseased hearts, but the consequences can be treated successfully with LVAD implantation.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection affects the cardiovascular system, contributing to acute coronary syndromes, arrhythmia, myocarditis, and heart failure.1, 2 We report a coronavirus disease 2019 (COVID-19)-induced cardiogenic shock in a young patient with a history of cardiomyopathy with reduced left ventricular ejection fraction (LVEF) who was treated by emergency surgery and left ventricular assist device (LVAD) implantation. This first case of a young pre-diseased patient unquestionably shows that COVID-19 directly affects pre-diseased hearts but can be treated successfully with LVAD implantation.
Case report
Clinical manifestation and evolution
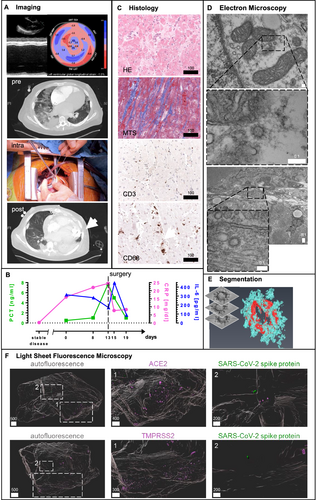
A 30-year-old male patient with a history of idiopathic cardiomyopathy with reduced LVEF was referred to our emergency department with clinical signs of respiratory failure. He presented with fever, tachypnoea, and laboratory tests indicating sepsis. Initial N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was 71 022 pg/mL, C-reactive protein was 16.1 mg/dL, interleukin 6 (IL-6) was 282 pg/mL, and procalcitonin (PCT) was 0.5 ng/mL (Figure 1 S1). During his repeated outpatient surveillance visits before, he had never presented with signs of decompensation or exertional dyspnoea and always presented with a stable disease with a 3D LVEF of 23%. Endomyocardial biopsy performed 5 months before the acute episode did not show acute inflammatory involvement nor signs for amyloidosis or M. Fabry. Initial real-time qRT-PCR testing as well as further real-time qRT-PCR and IgG-antibody tests for SARS-CoV-2 were positive during the entire hospitalization (Table 1). The chain of infection was not investigated.
| Day of hospitalization | Method | Sample origin | Result |
|---|---|---|---|
| 0 | SARS-CoV-2-RNA RT-PCR | Nasal swab testing | Positive |
| 1 | SARS-CoV-2-RNA RT-PCR | Bronchoalveolar lavage | Positive |
| 8 | SARS-CoV-2-RNA RT-PCR | Sputum | Positive |
| 10 | SARS-CoV-2-RNA RT-PCR | Bronchoalveolar lavage | Positive |
| 20 | SARS-CoV-2-RNA RT-PCR | Bronchoalveolar lavage | Positive |
| 31 | Anti-SARS-CoV-2-IgG | Blood sample | Positive |
- Testing was positive during the whole hospitalization period. Indicated are days of hospitalization, method, and origin of testing and results.
Respiratory and low-output heart failure required early oral intubation and implantation of a veno-arterial extracorporeal membrane oxygenation (VA-ECMO) system directly at admission. Echocardiography further confirmed terminal heart failure and reduced contractility [severely impaired systolic LV function (3D LVEF 6%, global longitudinal strain −1.0%), LV dilatation (LV end-diastolic diameter 8.5 cm, E-point septal separation distance 3.6 cm, LV end-diastolic volume 400 mL), impaired right ventricular function (tricuspid annular plane excursion 9 mm), RV dilatation (RV end-diastolic diameter basal 6.4 cm), and severe functional mitral regurgitation]. Later, the partial recovery of respiratory functions and the VA-ECMO allowed for respirator weaning. Owing to prolonged haemodynamic instability and cardiogenic shock, without realistic option for VA-ECMO weaning, the emergency heart failure board decided for cardiac surgery based on the patient's and patient's family consent. Nevertheless, owing to prolonged haemodynamic instability and cardiogenic shock, the emergency heart failure board decided for cardiac surgery based on the patient's consent. At that time, NT-proBNP was 163 837 pg/mL, C-reactive protein raised to 24.5 mg/dL, IL-6 to 124 pg/mL, and PCT to 7.73 ng/mL (Table S1). The patient was referred to heart surgery with LVAD as therapeutic bridge-to-transplant option, percutaneous temporary right ventricular assist device (RVAD) implantation,3 and tricuspid repair on Hospital Day 13. Hereafter, weaning from the RVAD was successful 2 weeks after implantation. Imaging confirmed successful recovery from COVID-19-associated sepsis and cardiogenic shock (Figure 1). The patient was further initially treated with convalescent serum of COVID-19 patients for 5 days. Initial angiotensin-converting enzyme (ACE) inhibitor treatment (ramipril 5 mg twice daily) was discontinued at the time of hospital admission.
Surgical myocardial biopsy
Because SARS-CoV-2 may directly affect pre-diseased hearts,4 a heart sample obtained during surgery was used for evaluation. Conventional, immunohistochemical, transmission electron, and light sheet fluorescence microscopy (LSFM) were performed on a heart sample obtained during cardiac surgery. Histology and transmission electron microscopy (TEM) confirmed moderate dilated cardiomyopathy but no lymphocytic myocarditis (Figure 1C and Figure S4). As by real-time qRT-PCR, as reported in other patients with fatal outcomes from COVID-19,4, 5 SARS-CoV-2-RNA was not detected within the myocardium, and TEM was used as it might discover coronavirus particles in the hearts of patients.6 We found structures with high similarities to SARS virus within the patient's heart cells (Figure 1). We did not find viral factories indicating virus replication, but multivesicular bodies, which are similar in structure (Figure S5). We 3D-analysed expression levels of the ACE2 receptor and transmembrane protease, Serin 2 (TMPRSS2), potentially required for cardial viral uptake.7 LSFM8 could show focal ACE2 and TMPRSS2 expression (Figure 1). In-depth analysis showed a spatial overlap of ACE2 expression with SARS-CoV-2 spike protein staining performed with specific antibodies, further implicating the presence of SARS-CoV-2 in the patient's heart (Figure 1). Similar findings could be obtained using SARS-CoV-2 nucleocapsid protein staining (Figure S1).
Discussion
While the management of patients on durable LVAD support with COVID-19 infection has been reported and discussed,9-11 we here report, to the best of our knowledge, the first case of a young COVID-19 patient with acute heart failure being recommendation adherent12, 13 treated successfully with LVAD implantation.
A biventricular assist device would have been an alternative approach, but regarding the underlying cardiomyopathy, this episode of life-threatening decompensation was rather acute on chronic. Another approach would have been the implantation of a total artificial heart (TAH). Owing to the patient's status, the results of a TAH procedure were regarded as unfavourable in the patient as surgical trauma would have been unequally larger than LVAD surgery. Within our heart failure heart team, we assessed this patient to be a good candidate for durable LVAD support with temporary groin-free RVAD,3 assuming the acute COVID-19 infection to be one major and reversible issue impairing the RV. However, the high haemocompatibility of the HeartMate 3 device remains outstanding. Still, there is evidence of (pro-)coagulopathy in florid COVID-19 infection. Hence, we decided to use this specific LVAD, which has proven its very low incidence of pump thrombosis, even in patients with deranged coagulation system.
It is unknown if SARS-CoV-2 replicates within the cardiomyocyte resulting in viral myocarditis or if COVID-19 affects pre-diseased hearts.6, 14 While we found no evidence for active viral replication within the cardiomyocyte or for fulminant myocarditis, we found indications for SARS-CoV-2 in the patient's heart that could have been affected the pre-diseased heart. The viral antigen's focal distribution might explain negative real-time qRT-PCR results in individual samples, underscoring the importance of microscopy-based diagnostics and warranting further studies for heart-organotropism in COVID-19 patients.15 With the benefit of the analysis of the spatial antigen distribution, LSFM may serve as a valuable tool in evaluating difficult or indistinct clinical cases, supporting and exceeding traditional methods. Even if we could not completely specify if the viral organotropism or the COVID-19 induced sepsis finally lead to the peracute life-threatening heart failure exacerbation, this unique report presents that a severe COVID-19 case with indications for SARS-CoV-2 in the heart was and can be treated successfully by LVAD implantation.
The study complies with the Declaration of Helsinki and was approved by the local ethics committee of the Medical Faculty, University of Duisburg-Essen, Germany (Ethics Approval Nr. 19-8982-BO for the LSFM imaging study). The patient was included in the Essen University CovCor Registry (NCT04327479, 20-9213-BO). Written informed consent was obtained from the patient.
Acknowledgement
Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




