Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic
Abstract
Aims
Wild type transthyretin amyloidosis (ATTRwt) has gained interest during recent years due to better diagnostic tools and the emergence of treatment options. Little is known about the prevalence of the disease. We aimed to investigate the prevalence in a heart failure population with myocardial hypertrophy.
Methods and results
All patients with an ICD code of heart failure living within the catchment area of Umeå University hospital and intraventricular septum >14 mm were offered screening with 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) scan and a clinical work up. Out of 2238 patients with heart failure, 174 patients were found to have a septum >14 mm. Ten patients were already diagnosed with hereditary ATTR cardiomyopathy, 12 patients had ATTRwt cardiomyopathy, 12 patients had known HCM, one patient had AL amyloidosis, and four patients had already undergone a negative DPD scan (DPD uptake grade 0 and 1) within the last 3 years. This left 134 patients who we tried to contact for screening, but 48 patients had either died or declined to participate. Out of 86 screened patients, 13 had a DPD uptake of grade 2 or 3 without other amyloid disease making the total number of patients with ATTRwt in this population 25.
Conclusions
Approximately 20% of investigated patients in a cohort with heart failure and increased myocardial wall thickness has ATTRwt. Calculated for the whole population of heart failure patients, the prevalence is just over 1.1%. Comparing this number to the total population would give an estimated prevalence of 1:6000.
Background
Transthyretin (ATTR) amyloidosis has in recent years been recognized to be the most common cause of cardiac amyloidosis (CA). ATTR amyloidosis is a systemic amyloidosis that can be hereditary or acquired. Hereditary ATTR (ATTRv) is generally characterized by a wide spectrum of manifestations including peripheral polyneuropathy and gastrointestinal disturbances as well as cardiac manifestations.1, 2 The acquired form of ATTR (ATTRwt) amyloidosis is dominated by cardiac symptoms.3 The typical cardiac manifestations of both ATTRwt and ATTRv amyloidosis are increased wall thickness, restrictive left ventricular filling pattern, bradyarrhythmias, and atrial fibrillation.4
In recent years, diagnosing ATTR CA has been facilitated by the re-establishing of technetium diphosphonate bone scintigraphy, for example, 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) for the detection of cardiac ATTR.5, 6
The emergence of disease modifying treatments aimed at both ATTRv and ATTRwt amyloidosis combined with better diagnostic tools has increased the interest among physicians for these diseases.7 The disease is considered to be underdiagnosed and misdiagnosis is common. Furthermore, no prevalence data has been presented.
The aim of this study was to establish the prevalence of ATTRwt amyloidosis in an unselected heart failure population.
Material and methods
Patient selection
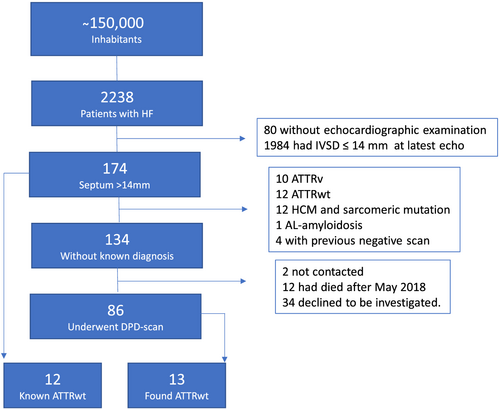
Umeå University Hospital is the sole provider of specialized heart care in an area with approximately 150 000 inhabitants. Electronic medical records at the hospital are electronic. All patients with an ICD-10 diagnosis of heart failure (I50), cardiomyopathy (I42, I43), or hypertensive heart disease (I11) set at the departments of internal Medicine or Cardiology between January 2010 and May 2018 were identified and included in the study cohort. A flow chart of the study cohort is displayed in Figure 1. Because increased cardiac wall thickness at onset of symptoms is often pronounced in this disease, we chose to focus on patients with cardiac septal wall thickness of more than 14 mm.3
All study procedures were approved of the local ethics board in Umeå. The study was performed in accordance with the 1964 Helsinki declaration. All patients with ATTRwt amyloidosis gave their consent to study participation.
Echocardiography
All patients with echocardiograms, where end diastolic interventricular septum thickness (IVSD) was reported to be >11 mm, had their last investigation re-evaluated with regards to IVSD. A total of 412 patients were re-evaluated by these criteria.
Septal thickness at end diastole was re-measured based on evaluation from parasternal long and short axis views as well as apical four chamber view. Furthermore, all patients diagnosed with ATTRwt amyloidosis underwent a new complete, echocardiographic examination. In addition to measuring IVSD, we measured left ventricular (LV) posterior wall thickness in diastole, calculated septal/posterior wall diastolic thickness ratio, LV ejection fraction using Simpsons biplane formula, global longitudinal strain (tri-plane method), and relative apical sparing from the same tri-plane view.8
3,3-Diphosphono-1,2-propanodicarboxylic acid referral and drop out
Patients with known hereditary sarcomeric hypertrophic cardiomyopathy (HCM) or CA that could explain their left ventricular (LV) hypertrophy were excluded from the screening.9
The remainder of the patients were contacted via telephone and mailed by a nurse from the cardiology outpatient clinic. All patients who were interested in further work up were referred to scintigraphy.
3,3-Diphosphono-1,2-propanodicarboxylic acid scintigraphy
All patients were scanned using a hybrid single-photon emission computed tomography (SPECT)-CT gamma camera (General Electric Medical Systems, Milwaukee, WI, USA, Infinia Hawkeye) as described by Perugini et al. using the four point grading system.5 A DPD uptake of grade two or more was considered positive. All patients with DPD uptake of grade one or higher were offered a visit to a cardiologist (BP) at the cardiology outpatient clinic.
Excluding light chain and hereditary transthyretin amyloidosis
Blood and urine samples were analysed regarding serum free light chain (FLC) abnormalities (Freelite, Binding Site reagent, reference range 0.27–1.64) and the presence of monoclonal bands. Patients with abnormalities in these analyses were carefully evaluated, and their clinical history and disease progression were reviewed to assess the probability of AL amyloidosis. Cardiac biomarkers were taken to determine disease severity. Sequencing of the TTR-gene was performed to rule out ATTRv amyloidosis.
Results
A total of 2238 patients with heart failure were alive in May 2018. Mean age was 76.5 ± 12. 7 years and 43% were women; 2158 (96%) had an echocardiographic exam available for re-evaluation with a mean EF of 44.8% ± 11.3%. Out of these, 174 patients were found to have a IVSD > 14 mm. Ten patients were already diagnosed with ATTRv cardiomyopathy, 12 patients had ATTRwt cardiomyopathy, 12 patients had known HCM (with known underlying mutation), one patient had AL amyloidosis, and four patients had already undergone clinical investigation for CA including a negative DPD scan (DPD uptake grade 0 or 1) within the last 3 years.
This left 134 patients who were contacted for screening. We were unable to reach two patients, 11 patients died prior to, and 34 patients declined or were unable to go through with the work up. This was mainly due to old age and co-morbidities with dementia being the most common cause. A total of 86 patients went through with DPD scintigraphy (Figure 1).
Fourteen of the 86 patients investigated with DPD scintigraphy had a DPD uptake of grade 2 or 3. Five patients had an uptake of 1. The rest had normal scans. All patients with DPD uptake underwent sequencing of the TTR gene and one patient was found to carry the V30M mutation endemic to northern Sweden.1 The mean duration between the first HF diagnosis and the positive DPD scintigraphy was 2 years, standard deviation of 2.8 years, range 0–8 years.
Seven of the remaining 13 patients had FLC ratio above 1.64, and three of these also had monoclonal bands in their plasma (two with IgG kappa and one IgA kappa). No patient had a monoclonal band in urine. The patients with abnormal FLC ratio all had slow disease progression prior to the work up, and none of them had nephrotic syndrome or other clinical findings suggesting AL amyloidosis. Three deaths due to non-cardiac causes (two caused by infection and one due to bowel ischemia) at very advanced age (89–91 years of age) occurred in these patients over 2 years after the work up. The patients with FLC abnormalities or DPD uptake grade 1 were old, and it was not deemed indicated or ethical to perform endomyocardial biopsies on them. According to the diagnostic criteria, certain diagnosis of ATTRwt amyloidosis was made in six of the 13 patients and a highly probable ATTRwt diagnosis in the remaining seven.
Clinical characteristics for the patients found through the screening and patients with known ATTRwt are summarized in Table 1. All patients but one had increased IVSD at the echocardiography performed after the DPD. That patient had suffered an anteroseptal myocardial infarction with subsequent thinning of the IVS. The patients were old, mean age was 84.5 years, and only one patient were below 70 years of age. They were predominately male (88%), and a majority (80%) had atrial fibrillation. The patients with IVSD > 14 mm who had a negative DPD scintigraphy had a mean age of 77 years, 51% had atrial fibrillation, 25% had a pacemaker, and 68% were men.
| Age, years, median (min–max) | 84 (58–91) |
| Male gender, n (%) | 22/25 (88.0%) |
| Previous carpal tunnel operation, n (%) | 7/25 (28%) |
| Spinal stenosis, n (%) | 4/25 (16%) |
| Cardiac pacemaker, n (%) | 5/25 (20%) |
| Diagnosis of atrial fibrillation, n (%) | 20/25 (80.0%) |
| NTproBNP, ng/L, median (min–max) | 2113 (592–29 459) |
| Hs-TroponinT, ng/L, median (min–max) | 52 (18–168) |
| NYHA-class I, n (%) | 3 (12%) |
| NYHA-class II, n (%) | 9 (36%) |
| NYHA-class III, n (%) | 13 (52%) |
| Known coronary disease, n (%) | 8 (32%) |
| Significant valve diseasea, n (%) | 2 (8%) |
| LVEF < 50%, n (%) | 11/24 (46%) |
| Interventricular septal diameterb (IVSD), mm, median (min–max) | 16.5 (12–25)2 |
| Posterior wall thickness (PWT), mm, median (min–max) | 13 (7–18) |
| IVSD/PWT median (min–max) | 1.4 (1.0–2.1) |
| Global longitudinal strain, %, median (min–max) | 10.6 (6.6–21.4) |
| Apical sparing, n (%) | 24/25 (96%) |
- a One patient with severe tricuspid regurgitation. One patient with previous AVR.
- b One patient had previous myocardial infarction with thinning of interventricular septum.
Discussion
This is, to the best of our knowledge, the first attempt so perform a popullation-based estimation of the prevalence of clinically relevant ATTRwt cardiomyopathy. We found the disease in approximately 20% of investigated patients in a cohort with HF and increased myocardial wall thickness of more than 14 mm. Although the patient recruitment in this study differs from a previous study, estimates of ATTRwt prevalence are similar.10 Gonzalez-Lopez et al. focused on patients with preserved LVEF. Interestingly, our study, focusing on patients with increased cardiac wall thickness, shows that the disease does not seem confined to heart failure with preserved ejection fraction as more than half of our patients had an ejection fraction below 50%. However, 95% of our patients had a GLS less than −17% and relative apical sparing less than 1 was present in 95%.
As shown by previous studies, the disease was predominately affecting old, male patients.3, 11 Calculated for the whole population of heart failure patients, the prevalence is just over 1.1%. Comparing this number with the total population of the Umeå area would give an estimated prevalence of 1:6000. Of the population aged over 70 (n = 21 000), we estimate the prevalence to around 1:800. Because slightly more than a third of patients with increased wall thickness and HF were unwilling or unable to undergo the screening and were also older in age, the true prevalence could be higher. Furthermore, as the patients with grade 1 DPD uptake had no signs of monoclonal disease, the finding probably represents early ATTR amyloid cardiomyopathy.
The cut off value of >14 mm might be inaccurate in finding women as women with ATTR-CA have shown to have less increased septal thickness.12 If we would have chosen a lower cut off value than 14 mm, it is highly likely that we would have found more cases and consequently a higher prevalence than we found in our study.
From our results, we do however find it to be a reasonable cut off for clinical screening, as a lower threshold would likely yield a too high proportion of unnecessary scans.
Interestingly, the prevalence of atrial fibrillation in our cohort was higher than that previously reported.3 This could be due to the higher age of our patients compared with other clinical cohorts of ATTRwt.13 The general HF population of these patients were collected from a previous study14 with a mean age of 76 years, 51% had atrial fibrillation, and 57% were men. Interestingly, the patients with septum >14 mm and a negative DPD scan were remarkably more similar to the general HF population in these aspects than the population with ATTRwt amyloidosis.
In conclusion, we found that clinically relevant ATTRwt amyloidosis was common in patients with a heart failure diagnosis and increased wall thickness. We suggest that in patients with heart failure and increased septal wall thickness with unknown origin, ATTRwt cardiomyopathy should be suspected, and DPD scintigraphy is recommended.
Limitations
Endomyocardial biopsies were not performed in this study, and in the patients with positive DPD and abnormal FLC analysis and monoclonal gammopathy, AL amyloidosis cannot be completely ruled out. However, the slow rate of disease progression and the absence of renal involvement observed in these patients are strongly suggesting ATTR amyloidosis. Furthermore, the high rate of MGUS in ATTRwt has recently been presented.15
Conflict of interest
All authors declare no conflicts of interest regarding this study.




