Crosstalk between the activated Slit2–Robo1 pathway and TGF-β1 signalling promotes cardiac fibrosis
Abstract
Aims
Previous reports indicated that the Slit2–Robo signalling pathway is involved in embryonic heart development and fibrosis in other solid organs, but its function in adult cardiac fibrosis has not been investigated. Here, we investigate the role of the Slit2–Robo1 signalling pathway in cardiac fibrosis.
Methods and results
The right atrial tissue samples were obtained from patients with valvular heart disease complicated by atrial fibrillation during heart valve surgery and from healthy heart donors. The fibrotic animal model is created by performing transverse aortic constriction (TAC) surgery. The Robo1, Slit2, TGF-β1, and collagen I expression levels in human and animal samples were evaluated by immunohistochemistry and western blot analysis. Echocardiography measured the changes in heart size and cardiac functions of animals. Angiotensin II (Ang II), Slit2-siRNA, TGF-β1-siRNA, recombinant Slit2, and recombinant TGF-β1 were transfected to cardiac fibroblasts (CFs) respectively to observe their effects on collagen I expression level. The right atrial appendage of patients with valvular heart disease complicated by atrial fibrillation found significantly up-regulated Slit2, Robo1, TGF-β1, and collagen I expression levels. TAC surgery leads to heart enlargement, cardiac fibrosis, and up-regulation of Slit2, Robo1, TGF-β1, and collagen I expression levels in animal model. Robo1 antagonist R5 and TGF-β1 antagonist SB431542 suppressed cardiac fibrosis in TAC mice. Treatment with 100 nM Ang II in CFs caused significantly increased Slit2, Robo1, Smad2/3, TGF-β1, collagen I, PI3K, and Akt expression levels. Transfecting Slit2-siRNA and TGF-β1-siRNA, respectively, into rat CFs significantly down-regulated Smad2/3 and collagen I expression, inhibiting the effects of Ang II. Recombinant Slit2 activated the TGF-β1/Smad signalling pathway in CFs and up-regulated Periostin, Robo1, and collagen I expression.
Conclusions
The Slit2–Robo1 signalling pathway interfered with the TGF-β1/Smad pathway and promoted cardiac fibrosis. Blockade of Slit2–Robo1 might be a new treatment for cardiac fibrosis.
Introduction
Heart failure (HF) remains a major cause of mortality and poor quality of life and has become an increasing threat to global health.1 During HF, multiple pathogenic factors can exacerbate adverse cardiac remodelling and fibrosis, leading to further cardiac contractile function loss and arrhythmias.2 Therefore, understanding the currently understudied molecular mechanism of cardiac fibrosis is key to discovering potential treatments for many cardiac diseases.
The pathogenesis of cardiac fibrosis includes disruption of normal myocardial construction and abnormal extracellular matrix (ECM) accumulation.3 The key pathophysiological basis is the feedback cycle between HF and continuous differentiation of cardiac fibroblasts (CFs) into cardiac myofibroblasts,4 which have a potent ability to divide and cause ECM overproduction.5, 6 This activation is mediated by the renin–angiotensin system and numerous neurohumoral factors, including angiotensin II (Ang II) and transforming growth factor β1 (TGF-β1).3, 5 Ang II promotes the release of catecholamine and aldosterone, up-regulates the expression of TGF-β1 in fibroblasts, and is a strong promoting factor for cardiac fibrosis. It is also widely used in vitro to activate CFs or to induce cardiac fibrosis in animal models.7 Markedly activated TGF-β1 interacts with renin–angiotensin system and mediates fibroblast activation and proliferation and is also the most effective fibrosis-promoting cytokine.8, 9 Together with Smad2/3, an essential component of the intracellular TGF-β1 signalling pathway, activation of the TGF-β1/Smad signalling pathway can facilitate fibrosis by stimulating collagen synthesis while inhibiting ECM degradation.10 Although this signalling pathway has been extensively studied recently, effective preventive measures and treatments for cardiac fibrosis are still highly unsatisfactory.
Recent studies have shown that the intensity and duration of fibrosis-promoting signals are regulated by a cascade of multiple molecular interactions rather than an isolated pathway.11, 12 In our preliminary clinical study, we observed increased Slit2 expression levels and collagen deposition, accompanied by activation of TGF-β1 signalling, in the right atrial appendage of patients with valvular heart disease complicated by atrial fibrillation (AF), the most common form of arrhythmia. The Slit2 protein has been studied in lung and liver fibrosis, but its role in cardiac fibrosis is not established. Current lung research suggests that fibroblasts secrete Slit2, which inhibits the proliferation and differentiation of bleomycin-induced lung fibroblasts, and that exogenous Slit2 in the lung reduces fibrosis formation.13 In contrast to Slit2's inhibitory function in the lung, another study demonstrated that in an animal model of liver fibrosis, the serum concentrations of Slit2 and Robo1 were significantly increased via a mechanism possibly related to the key TGF-β1–Smad pathway.14 The Slit gene encodes glycoprotein ligands for the Robo family of immunoglobulin receptors; the Slit2–Robo1 signalling pathway has been reported to mediate some congenital heart diseases.15 However, no reports have addressed the function of the Slit2–Robo1 signalling pathway in the adult animal heart. After reviewing the findings of previous clinical studies and reports, we speculated that the Slit2–Robo1 pathway plays a critical role in adult cardiac fibrosis, participating in the complex fibrosis-promoting pathway.
Material and methods
Human atrium tissue sample collection
A cohort of patients with clinically diagnosed valvular heart disease complicated by AF was evaluated based on their medical records and 12-lead electrocardiogram findings. Right atrial tissue samples were obtained from five patients during heart valve replacement surgery, and another five right atrial tissue samples were obtained from healthy heart donors without diagnosed AF. The tissue samples were obtained at the time of surgery and divided into two parts: one was immediately stored in liquid nitrogen for western blot analysis; the other was fixed with 4% paraformaldehyde for immunohistochemistry.
All clinical samples were obtained from The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. The investigation complied with the principles of human tissue use outlined in the Declaration of Helsinki and was approved by the Human Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. Informed consent was obtained from all participants.
Animal model of transverse aortic constriction
Male C57BL mice (8–10 weeks old, 25–30 g, n = 30) were anaesthetised by intraperitoneal injection of xylazine (5 mg/kg) and ketamine (100 mg/kg) and were then subjected to transverse aortic constriction (TAC, n = 24) surgery or sham surgery (n = 6). The surgery was performed as follows16: under anaesthesia, a skin incision was made at the level of the second intercostal space, 2 mm to the left of the sternum. Then, the aortic arch was exposed by careful blunt dissection of the intercostal muscles, thymus, and other tissues. A 27G needle was placed at the telocentric end of the aortic arch and used to place a 7-0 silk ligature around the aorta. After removing the needle, we achieved a 0.4 mm diameter coarctation of the aorta. The sham surgery was the same procedure without aortic ligature.
The 24 animals that received TAC surgery were divided into four groups (n = 6). For 6 weeks after surgery, each group received different weekly intraperitoneal injections: (i) TAC + R5 group (40 mg/kg R5), (ii) TAC + SB431542 group (5 mg/kg SB431542), (iii) TAC + R5 + SB431542 group (40 mg/kg R5 + 5 mg/kg SB431542), and (iv) TAC group.17, 18 Mice in the TAC and sham groups were injected with saline at a volume equal to that saline used in the other groups.
Echocardiography
Tissue Doppler imaging (Vevo 2100 high-resolution ultrasound imaging system, VisualSonics, Toronto, ON, Canada) was used to quantify heart function 6 weeks post surgery. A long-axis image was acquired at the cardiac apex. The left ventricular ejection fraction was calculated by a trace algorithm. A short-axis image was acquired at the level of the left ventricular papillary muscle adjacent to the sternum. The interventricular septal thickness at end-diastole and left ventricular posterior wall thickness were measured, and the left ventricular fractional shortening (FS (%) = [(LVIDd − LVIDs)/LVIDd × 100%]), left ventricular end-diastolic volume, and left ventricular stroke volume were calculated from the image. Ultrasound data were analysed in a double-blind manner by two ultrasound physicians. The averages of the two measurements were accepted as the final values.
Histology and immunohistochemistry
Body weights were measured 6 weeks post surgery. Then, the hearts from all mice were harvested, cleaned, and weighed. Sections were made along the short axis of the left ventricle. After fixation with 4% paraformaldehyde, Masson staining and haematoxylin–eosin (HE) staining were performed to evaluate the degree of myocardial hypertrophy and fibrosis. The remaining tissue was used for western blot analysis.
Masson staining was used to detect the expression of collagen and assess the degree of myocardial fibrosis. Slides of heart tissue were oxidized in 1% potassium permanganate solution for 5 min; bleached in oxalic acid for 1 min; stained in Celestine blue, Mayer's haematoxylin solution, and Ponceau S for 5 min each; and rinsed in 1% acetic acid water for 2 min. The slides were further differentiated in 1% phosphomolybdic acid for 5 min, stained with 1% light green or toluidine blue for 30 s, rinsed in 1% acetic acid water, and differentiated in 95% alcohol. After the aforementioned procedures, the slides were hydrated in absolute alcohol, cleared with xylene, and mounted with neutral resin. All slides were visualized with a light microscope (BX51, OLYMPUS, Tokyo, Japan). The fibrosis size was quantified using the ImageJ program.19
The HE staining process had several stages. Each section was first stained with haematoxylin buffer for 12 min, differentiated with 1% hydrochloric acid and alcohol for 20 s each, and subjected to bluing by incubation with 1% ammonia for 30 s. Each of the aforementioned steps was followed by washing with Scott blue staining solution for 1 min. Finally, the sections were stained with 0.5% eosin for 30 s and were then washed with tap water. The sections were dehydrated with ascending concentrations of ethanol (85%, 95%, and 100%). After staining was complete, all slides were sealed with neutral gum and evaluated with a light microscope.
Immunohistochemical staining was performed as follows: the sections were incubated in a humidified chamber at room temperature for 20–30 min, and goat serum was added as a blocking agent. Then, the primary antibody (30–50 μL) was added to the sections (TGF-β1, 1:100, Abcam, Cambridge, MA, USA; Slit2, 1:100, Proteintech, Rosemont, IL, USA; collagen I, 1:100, Abcam), which were then incubated at 4°C overnight. Then, a biotin-labelled secondary antibody (30–50 μL) was added to the sections at room temperature for 20 min. The sections were washed with phosphate-buffered saline, 3,3′-Diaminobenzid (DAB) colour development solution was added, and the sections were washed with distilled water. Finally, all sections were counterstained with haematoxylin for 1 min, differentiated using 0.1% hydrochloric acid alcohol for 5 s, and washed with running tap water for 20 min to allow bluing of haematoxylin in an alkaline environment. After staining was complete, the slides were evaluated under a light microscope.
Cardiac fibroblast isolation and cultivation
Cardiac fibroblasts were isolated from the ventricular tissues of male C57BL/6 mice (8–10 weeks old, 25–30 g, n = 5) obtained from the Experimental Animal Center, Sun Yat-sen University, Guangzhou, China. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). This study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. Mice were anaesthetised by intraperitoneal injection of a mixture of xylazine (5 mg/kg) and ketamine (100 mg/kg), and the ventricular tissues were harvested. Tissues were then washed with D-Hanks' solution to remove all blood components, minced into 1 mm3 pieces, and digested by adding 0.25% trypsin at 37°C twice for 10 min each and 0.01% type I collagenase at 37°C for 120–180 min to obtain cell suspensions, which were filtered through a 40 μm nylon mesh and centrifuged at 180 g for 5 min. The cell pellets were resuspended and incubated in Dulbecco's Modified Eagle Medium (DMEM)/F12 (Gibco, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Gibco) at 37°C in a humidified atmosphere of 5% CO2. Fibroblasts were identified by the differential adherence method. The adherent cells remaining after two differential adherence cultures of approximately 45 min to 1 h each were CFs.20
siRNA synthesis and transfection
The siRNA sequences targeting the mice Slit2 gene (si-Slit2) and the mice TGF-β1 gene (si-TGF-β1) and the scrambled siRNA sequence (si-Scramble) were designed and synthesized by RiboBio (Guangzhou, China). Passage 3 CFs were plated in a 6 well plate (1 × 105 cells/well) in 10% fetal bovine serum-containing medium and at 80% confluence were starved by incubation in serum-free medium for 24 h. siRNAs (50 nM) were transfected into CFs with Lipofectamine™ RNAiMAX (Invitrogen) according to the manufacturer's protocol. Cells in the 1st–6th wells received the following treatments for 24: (i) 50 nM si-Scramble, (ii) 50 nM si-Scramble and 100 nM Ang II (Sigma, North St. Indianapolis, IN, USA), (iii) 50 nM si-TGF-β1, (iv) 50 nM si-TGF-β1 and 100 nM Ang II, (v) 50 nM si-Slit2, and (vi) 50 nM si-Slit2 and 100 nM Ang II. The second dose of Ang II, at the same concentration as the first dose, was added to the media after 12 h.
Immunofluorescence staining
Cardiac fibroblasts were divided into six groups as described in the Cardiac fibroblasts isolation and cultivation section and were then fixed with 4% paraformaldehyde for 20 min. CFs were incubated with rabbit polyclonal anti-collagen I (1:100, Abcam) and anti-Vimentin (1:100, CST, Beverly, MA, USA) primary antibodies overnight at 4°C and then with the DyLight 594 AffiniPure goat anti-rabbit IgG secondary antibody (1:100, EarthOx, Millbrae, CA, USA) for 1 h at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (1 mg/mL; Solarbio, Tongzhou District, Beijing, China) for 5 min, and images were acquired using a Leica DMi8 (Buffalo Grove, IL, USA) inverted microscope (magnification: ×200).
Cell proliferation assay
Cardiac fibroblasts were seeded in 96 well plates (2 × 103 cells/well) overnight and were then divided into six groups as described previously in the Cardiac fibroblasts isolation and cultivation section. Each group comprised 6 wells and was treated as described in the siRNA synthesis and transfection section for 48 h. The degree of CF proliferation was measured using a Cell Counting Kit-8 (CCK-8; DOJINDO, Rockville, MA, USA), with CCK-8 solution (10 μL) added to each well. The plate was incubated for 4 h at 37°C. The absorbance at 450 nm was measured with a microplate reader (Thermo Scientific, Waltham, MA, USA).
RNA extraction and real-time PCR
RNA was extracted with a TaKaRa MiniBEST Universal RNA Extraction Kit (Takara, Kusatsu, Shiga, Japan) and reverse transcribed with PrimeScript™ RT Master Mix (Takara) according to the manufacturer's protocols. Real-time PCR was performed in a LightCycler 480 thermal cycler using SYBR Green PCR kits (Roche, Indianapolis, IN, USA) under the following conditions: (i) 95°C for 5 min and (ii) 34 cycles at 95°C for 10 s, 60°C for 20 s, and 72°C for 20s. Data were analysed with a Stratagene Mx3000p (Agilent Technologies, Santa Clara, CA, USA). GAPDH served as the internal control (for PCR primer sequences, see Supporting Information, Data S1).
Stimulation of cardiac fibroblasts using recombinant Slit2 and TGF-β1
Passage 3 CFs were seeded in 6 well plates and divided into the recombinant TGF-β1 (rTGF-β1), recombinant Slit2 (rSlit2), and control groups. At 60% confluence, the corresponding groups were treated with 10 mg/mL mouse rTGF-β1 (Biomart, Avenue Los Angeles, CA, USA), 100 ng/mL mouse rSlit2 (Cloud-Clone, Katy, TX, USA),21, 22 or no drug (control group). Cells were incubated for 24 h, and total protein was extracted for western blot analysis.
Western blot analysis
Homogenized myocardial tissue and CFs were lysed using radio immunoprecipitation assay buffer (CST) containing phenylmethanesulfonyl fluoride (Solarbio). Total protein (20 μg) was separated by 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking in 5% bovine serum albumin diluted with tris-buffered saline tween-20 at room temperature for 1 h, membranes were incubated overnight at 4°C with primary antibodies against TGF-β1 (1:1000, Abcam), Slit2 (1:1000, Proteintech), Robo1 (1:1000, Santa Cruz, Dallas, TX, USA), Smad2/3 (1:1000, CST), p-Smad2 (1:1000, CST), p-Smad3 (1:1000, CST), collagen I (1:1000, Abcam), PI3K (1:1000, CST), Akt (1:1000, CST), p-PI3K (1:1000, CST), p-Akt (1:1000, CST), and Periostin (1:1000, Abcam). Membranes were incubated with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5000, CST) at room temperature for 1 h. Immunoreactive bands were visualized with ECL western blotting detection reagents (Millipore). Band densities were quantified by densitometry with Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). To ensure equal loading of samples, protein expression level was normalized to GAPDH.
Statistical analysis
Data are expressed as means ± standard deviations. Differences between discrete variables were evaluated by the χ2 test; comparisons between two groups of continuous variables were evaluated by the Student's t-test; comparisons between more than two groups of continuous variables were evaluated by one-way ANOVA (SPSS Statistics 19.0, IBM, USA). Differences with P < 0.05 were considered statistically significant.
Results
Slit2–Robo1 signalling is activated in patients with cardiac fibrosis
The clinical data showed that the left and right atria of patients with valvular heart disease and AF were significantly larger than those of heart donors; in addition, the patients exhibited worsened cardiac function characterized by a significant decrease in ejection fraction (58.1 ± 7.7 vs. 69.6 ± 3.8%, P = 0.008) and higher N-terminal pro brain natriuretic peptide levels (964.3 ± 473.6 vs. 89.3 ± 50.6 pg/mL, P = 0.0016) than heart donors (Table 1). Masson staining of patient specimens revealed a more disordered arrangement and greater collagen deposition than observed in donor specimens, which did not exhibit substantial collagen deposition. HE staining showed that patients' cardiomyocytes were significantly more disordered and hypertrophied than donors' (Figure 1).
| AF patients | Healthy donors | P-value | |
|---|---|---|---|
| Age (years) | 51.8 ± 8.9 | 30.8 ± 7.3 | 0.007 |
| Gender | |||
| Female | 3 | 0 | 0.083 |
| Male | 2 | 5 | 0.257 |
| Diagnosis | |||
| MI | 4 | 0 | <0.001 |
| MS | 5 | 0 | <0.001 |
| TI | 5 | 0 | <0.001 |
| AF | 5 | 0 | <0.001 |
| Atrial diameter (mm) | |||
| RA | 67.8 ± 9.8 | 33.8 ± 1.8 | <0.001 |
| LA | 68.2 ± 25.7 | 34.6 ± 2.9 | 0.014 |
| LVEF (%) | 58.1 ± 7.7 | 69.6 ± 3.8 | 0.008 |
| NT-proBNP (pg/mL) | 964.3 ± 473.6 | 89.3 ± 50.6 | 0.0016 |
- AF, atrial fibrillation; LA, left atrial; LVEF, left ventricular ejection fraction; MI, mitral valve insufficiency; MS, mitral valve stenosis; NT-proBNP, N-terminal portion of pro-BNP; RA, right atrial; TI, tricuspid valve insufficiency.
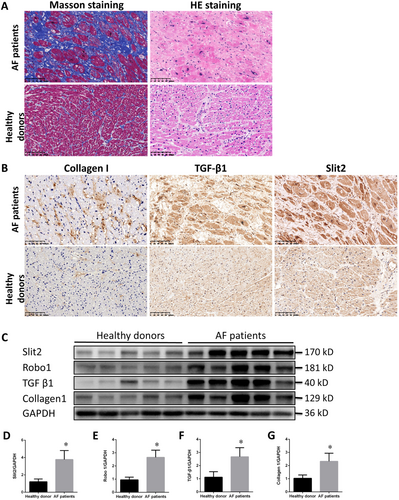
Immunocytochemistry revealed significantly increased expression levels of collagen I, TGF-β1, and Slit2 in the right atrial appendage of patients with valvular heart disease complicated by AF (Figure 1). Western blot analysis of the right atrial myocardium suggested that the protein expression of TGF-β1 and collagen I was significantly up-regulated in the patient cohort (Figure 1). Similarly, Slit2–Robo1 expression was significantly increased in heart patients, indicating the Slit2–Robo1 signalling pathway associated with cardiac fibrosis (Figure 1).
Cardiac function and remodelling
Tissue Doppler imaging revealed that TAC mice had enlarged hearts, thickened myocardia, decreased systolic function, and increased heart weight/body weight ratios compared with sham mice. Treatment with R5 (the Robo1 antagonist) or SB431542 (the TGF-β1 antagonist) partially reversed the effect of TAC surgery (Figure 2, Table 2). Masson staining showed a significant proliferation of the fibrous myocardial connective tissue in the TAC group compared with the sham group (Figure 2). In the sham group, the Slit2, Robo1, and collagen I expression levels were low, and the TGF-β1 level was high. The Slit2, Robo1, collagen I, and TGF-β1 expression levels were increased in the TAC group (Figure 2), suggesting that TAC surgery can lead to cardiac fibrosis in mice and up-regulation of Slit2, Robo1, and TGF-β1 expression. In the SB431542 and R5 + SB431542 groups, the collagen I and TGF-β1 expression levels were lower than those of the TAC group but higher than those of the sham group, suggesting that R5 and SB431542 can alleviate the effects of TAC. The TAC + R5, TAC + SB431542, and TAC + R5 + SB431542 groups had lower Robo1 expression levels than the TAC group, although the difference was statistically insignificant, and significantly higher Robo1 expression levels than the sham group. The R5 group had the highest Slit2 expression level, which was significantly higher than that in the TAC group (P < 0.05). Further experiments might be needed to address this observation.
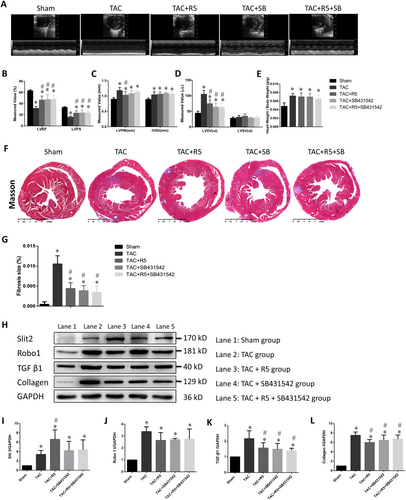
| Parameter | Sham | TAC | TAC + R5 | TAC + SB431542 | TAC + R5 + SB431542 |
|---|---|---|---|---|---|
| LVPW (mm) | 0.90 ± 0.05 | 1.18 ± 0.10* | 1.03 ± 0.12*, ** | 1.08 ± 0.09* | 1.14 ± 0.06* |
| IVSD (mm) | 0.89 ± 0.04 | 1.04 ± 0.10* | 1.06 ± 0.07* | 1.09 ± 0.03* | 1.07 ± 0.07* |
| LVDV (μL) | 44.28 ± 6.40 | 104.80 ± 12.57*, ** | 74.71 ± 12.46*, ** | 63.82 ± 13.00*, ** | 63.58 ± 14.40*, ** |
| LVSV (μL) | 28.00 ± 3.94 | 31.42 ± 5.20 | 34.82 ± 7.10 | 29.28 ± 1.66 | 30.07 ± 5.53 |
| EF (%) | 63.27 ± 1.59 | 31.80 ± 3.50*, ** | 46.66 ± 6.98*, ** | 47.58 ± 8.79*, ** | 48.10 ± 6.67*, ** |
| FS (%) | 33.36 ± 1.18 | 15.97 ± 1.83*, ** | 23.19 ± 4.22*, ** | 23.66 ± 4.98*, ** | 23.89 ± 3.94*, ** |
- EF, ejection fraction; FS, fractional shortening; IVSD, interventricular septal thickness at end-diastole; LVDV, left ventricular end-diastolic volume; LVPW, left ventricular posterior wall thickness; LVSV, left ventricular stroke volume; TAC, transverse aortic constriction.
- * P < 0.05 vs. the sham control group.
- ** P < 0.05 vs. the TAC control group.
Slit2 knockdown inhibits Ang II-induced collagen I production and cardiac fibroblasts proliferation
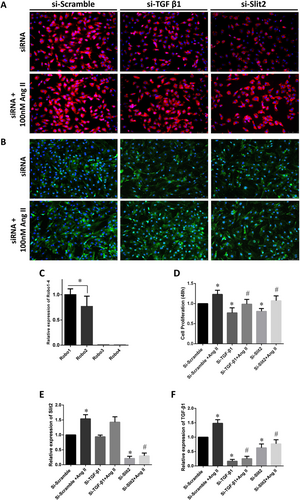
The positive control group was treated with Ang II, a common activator of CFs, to establish an in vitro cellular model of cardiac fibrosis.10 The immunofluorescence staining results showed that si-Slit2 and si-TGF-β1 significantly inhibited Ang II-induced collagen I and Vimentin overexpression, which was obvious in the si-Scramble group (Figure 3). Separate measurements of the Slit2 ligand subtypes Robo1–4 in CFs demonstrated that the Robo1 expression level was significantly higher than that of Robo2 (P < 0.05); however, almost no expression of Robo3 or Robo4 was detected (Figure 3). The CCK-8 assay results showed that siRNA transfection significantly inhibited Ang II-induced proliferation of CFs (Figure 3). The real-time PCR results confirmed that Ang II up-regulated the expression of Slit2 and TGF-β1 mRNA. However, this effect was dramatically inhibited by both si-Slit2 and si-TGF-β1 (Figure 3). si-Slit2 down-regulated TGF-β1 mRNA expression, but si-TGF-β1 did not significantly impact Slit2 mRNA expression (Figure 3).
The Slit2–Robo1 signalling pathway is involved in cardiac fibrosis
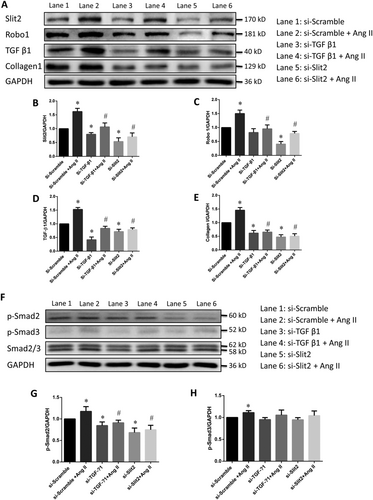
Angiotensin II stimulation increased the Slit2, Robo1, TGF-β1, and collagen I protein expression levels in CFs compared with those in the si-Scramble group, which served as the negative control group and baseline for western blot analysis (Figure 4). Transfection of si-Slit2 or si-TGF-β1 reduced the protein expression levels of Slit2, Robo1, TGF-β1, and collagen I in CFs to very low levels; re-addition of Ang II after the first transfection only partially restored the expression levels (Figure 4). Previous studies revealed that Ang II regulates cardiac fibrosis via the TGF-β1–Smad signalling pathway.9, 10 In this study, siRNA transfection significantly inhibited Smad2 phosphorylation, but neither si-Slit2 nor si-TGF-β1 impacted Smad3 phosphorylation (Figure 4).
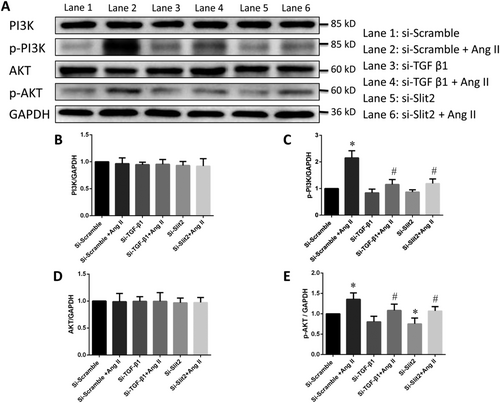
The PI3K/Akt pathway is involved in regulating cardiac fibroblast activity via Slit2–Robo1 signalling
The PI3K/Akt signalling pathway plays an important role in the modulation of cardiac fibrosis.23 We performed western blotting to determine the phosphorylation levels of PI3K and Akt and found that Ang II significantly increased the phosphorylation of PI3K and Akt, but this effect was effectively inhibited by transfection with si-TGF-β1 or si-Slit2 (Figure 5). However, the levels of total PI3K and Akt did not differ significantly (Figure 5).
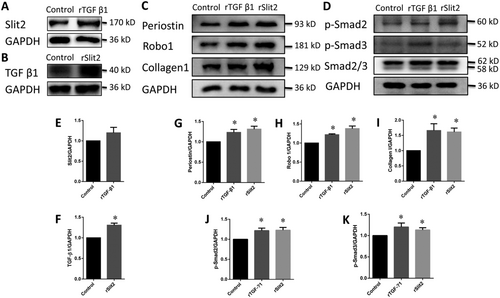
Recombinant Slit2 activates the Smad signalling pathway and up-regulates Periostin expression in cardiac fibroblasts
After treatment with rTGF-β1, the Slit2 expression level in CFs increased non-significantly (Figure 6). However, the TGF-β1 expression level in CFs was increased significantly by rSlit2 (Figure 6). Both rSlit2 and rTGF-β1 promoted Smad2 and Smad3 phosphorylation and significantly up-regulated Periostin, Robo1, and collagen I expressions (Figure 6).
Discussion
The Slit2–Robo1 signalling pathway has been reported to participate in many physiological processes, including axon guidance, neuronal migration, and leucocyte chemotaxis.24-27 Previous studies have shown that activation of Slit2–Robo1 signalling promotes liver fibrosis.14 Our clinical research suggested that Slit2–Robo1 signalling is also activated in cardiac fibrosis, suggesting that it may play a role in cardiac fibrosis.
Atrial fibrillation, the most common clinical arrhythmia, correlates with poor cardiac electrical and structural remodelling.28 The consequence of AF includes structural remodelling of the atrium and ventricle, which reciprocally promotes AF development.29 Previous studies have reported that TGF-β1 may play an important role in AF occurrence and progression.24, 27 TGF-β1 is the most potent profibrotic cytokine and is a key mediator of fibroblast activation and fibrosis in heart diseases.30 Increasing expression of TGF-β1 in atrial tissue is associated with increased susceptibility to atrial fibrosis and AF.31 Activation of the TGF-β1/α-SMA/collagen I profibrotic pathway by Galectin-3 in fibroblasts contributes actively to atrial fibrosis in both patients and experimental AF models.32 Therefore, we used TGF-β1 and collagen I as control molecules to observe the degree of cardiac fibrosis/CF activation in clinical specimens and CFs in vitro.
The expression levels of TGF-β1 and collagen I, as well as those of Slit2 and Robo1, were significantly higher in the right atrial appendages of AF patients than in those of healthy donors, indicating fibrosis in the right atrial appendage of patients. The results indicating Slit2–Robo1 pathway activation also suggested the correlation of this pathway with cardiac fibrosis. TAC leads to cardiac hypertrophy and cardiac fibrosis in mice.16 The reduced expression of TGF-β1 and collagen I in the SB431542 and R5 + SB431542 groups and alleviation of myocardial remodelling and fibrosis suggest that suppressing the Robo1 and/or the TGF-β1–Smad signalling pathway can lead to suppression of cardiac fibrosis. The high expression level of Ang II in atrial tissue of patients with valvular heart disease can significantly up-regulate TGF-β1 expression and promote cardiac fibrosis.33, 34 Here, adding Ang II to CF culture medium led to significant increases in TGF-β1, Smad2/3, collagen I, Slit2, and Robo1 expressions. Transfection of si-Slit2 inhibited TGF-β1, Smad2/3, Robo1, and collagen I expressions and effectively inhibited the effects of Ang II on TGF-β1, Smad2/3, and collagen I expressions. Both si-Slit2 and si-TGF-β1 significantly inhibited Ang II-induced collagen I expression. These results strongly suggest that Slit2–Robo1 signalling is involved in cardiac fibrosis and is closely related to the Ang II/TGF-β1–Smad2/3 pathway.
Previous studies have revealed that aspects of profibrotic processes, especially the regulation of the profibrotic signal intensity and duration, are controlled by a network cascade of multiple molecular interactions rather than an isolated pathway.35, 36 Our experiments reconfirmed that the cardiac fibrosis process relies on complex molecular regulatory networks. Interestingly, the effect of si-Slit2 transfection on TGF-β1 expression in CFs was equivalent to that of direct transfection of si-TGF-β1. The effect of Slit2 knockdown on the p-Smad2 and collagen I levels was stronger than that of TGF-β1 knockdown. These results suggest that Slit2 is located upstream of TGF-β1 in the Ang II-induced cardiac fibrosis molecular network. However, TGF-β1 knockdown also slightly down-regulated Slit2 expression, but the effect was less significant, indicating the possible existence of a feedback mechanism between Slit2 and TGF-β1 network. Independent treatment of CFs with rSlit2 and rTGF-β1 revealed that rSlit2 significantly promotes TGF-β1 synthesis, although rTGF-β1 did not significantly affect Slit2 synthesis. This result indicated that Slit2 is upstream of TGF-β1 in the CF activation pathway.
Here, Ang II treatment activated the PI3K/Akt pathway in CFs, whereas Slit2 or TGF-β1 knockdown inhibited PI3K/Akt signalling. The PI3K/Akt signalling pathway plays an important role in the regulation of cardiac fibrosis.23 The PI3K inhibitor NVP-BEZ235 can block BMI1-induced cardiac fibrosis.37 Fusaric acid protects against HF by preventing cardiac fibrosis via modulation of the TGF-β1–Smad and PI3K/Akt signalling pathways.38 Chang et al. reported that the PI3K/Akt pathway promotes liver fibrosis after activation of Slit2–Robo1 signalling.14 rSlit2 promotes liver fibrosis by activating the PI3K/Akt signalling pathway, whereas addition of the PI3K/Akt antagonist LY294002 inhibits this effect.22 These findings imply that the Slit2–Robo1, TGF-β1–Smad, and PI3K/Akt pathways might be involved in fibrosis regulation. Our results suggest that the PI3K/Akt signalling pathway is involved with the Slit2–Robo1 and TGF-β1–Smad pathways in cardiac fibrosis. However, the specific molecular mechanisms remain to be further studied.
As a non-structural component of the ECM, Periostin cooperates with TGF-β signalling, mediates cell–matrix signal transduction, and plays an important role in fibrosis in both the heart and other organs.39, 40 In healthy tissue, the expression of Periostin is low, but in damaged hearts or blood vessels, cardiac myofibroblasts and vascular smooth muscle cells are activated, leading to synthesis and release of Periostin, which mediates the reconstruction of cardiac interstitial tissue.39, 41 Our study revealed that both rSlit2 and rTGF-β1 can promote Smad2/3 phosphorylation and significantly up-regulate collagen I, Robo1, and Periostin synthesis. These results demonstrated that Periostin participates in the cardiac fibrosis pathways mediated by Slit2–Robo1 signalling.
In summary, our results showed that Slit2–Robo1 signalling is activated in fibrotic heart tissues, acting as part of the cardiac fibrosis-promoting component. The exact molecular mechanism underlying the role of the Slit2–Robo1 pathway in cardiac fibrosis, especially its relationship with TGF-β1 and Periostin, needs further exploration. Slit2 exerts its profibrotic effects at least partially via activation of TGF-β1–Smad, PI3K/Akt, and Periostin signalling. Hence, blockade of Slit2–Robo1 signalling may offer a novel therapeutic approach for preventing cardiac fibrosis.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the Science and Technology Planning Project of Guangdong Province (2014A020215007 and 2015B010125001) and the Science and Technology Program of Guangzhou City (201803010077).
Author contributions
Y.L., Z.Y., J.Y., and X.X. were involved in conception and design, performance of experiments, data analysis and interpretation and manuscript writing; X.X., J.Y., and C.L. were involved in the development of methodology; X.D. and Q.S. were involved in the data acquisition; Y.T. was involved in the histology and immunohistochemistry assay; Y.L., J.Y., and S.Y. were involved in study supervision and final approval of the manuscript.




